Evaluation of Pozzolanic and Alkali-Activated Reactivity of Low-Purity Calcium Bentonite
Abstract
1. Introduction
2. Experimental Sections
2.1. Materials
2.2. Treatment and Activity Assessment for CB
2.2.1. Thermal Treatment
2.2.2. Phase Analysis of CB
2.2.3. Zeta Potential
2.2.4. Evaluation of Pozzolanic Activity
2.3. Preparation: Strength Measurement and Characterization of AAC
2.3.1. Compressive Strength
2.3.2. Microstructural Investigation of AAC
3. Results and Discussion
3.1. Thermodynamic Changes of CB
3.2. Crystal Phase Change of CB
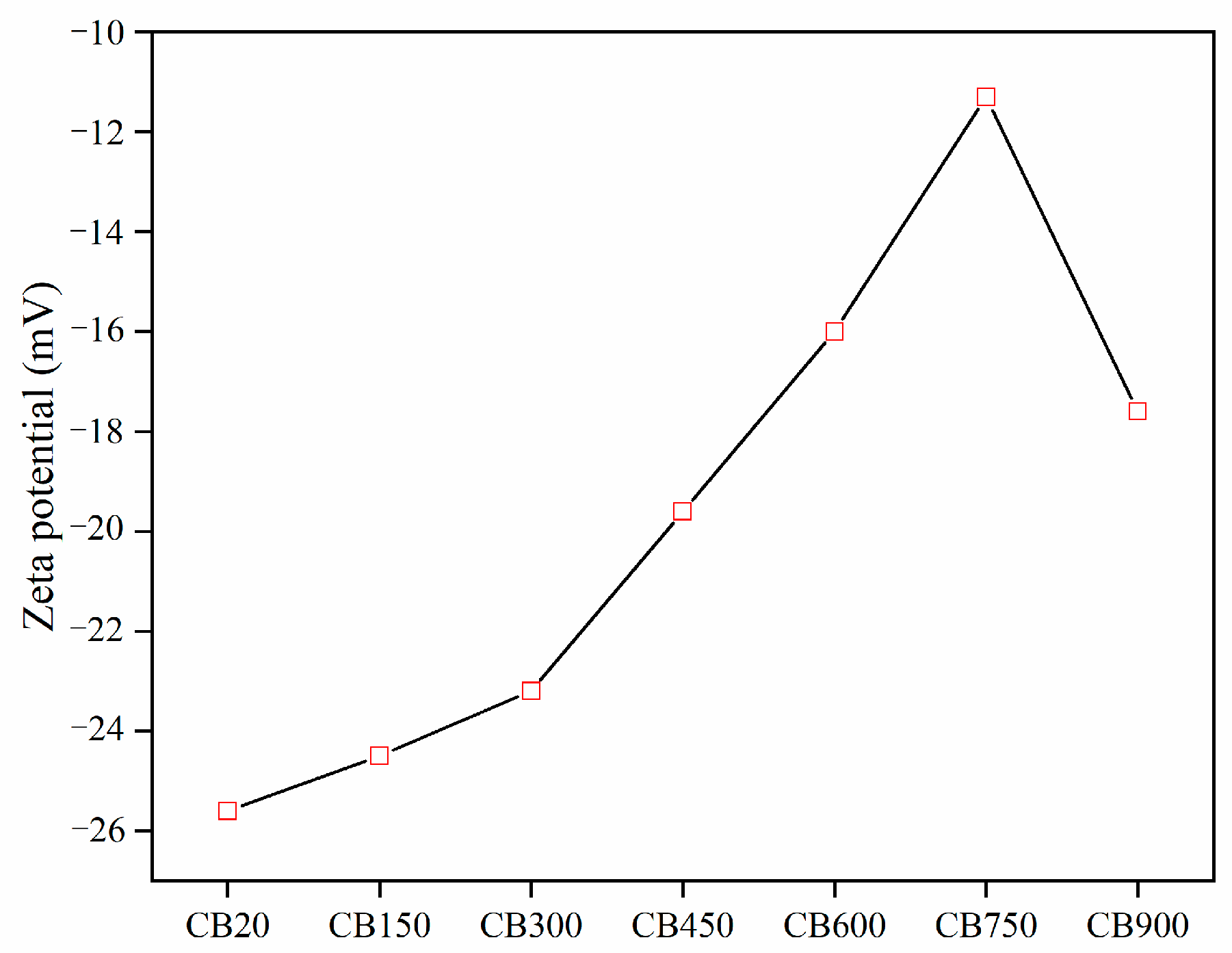
3.3. Zeta Potential of CB
3.4. SAI Analysis
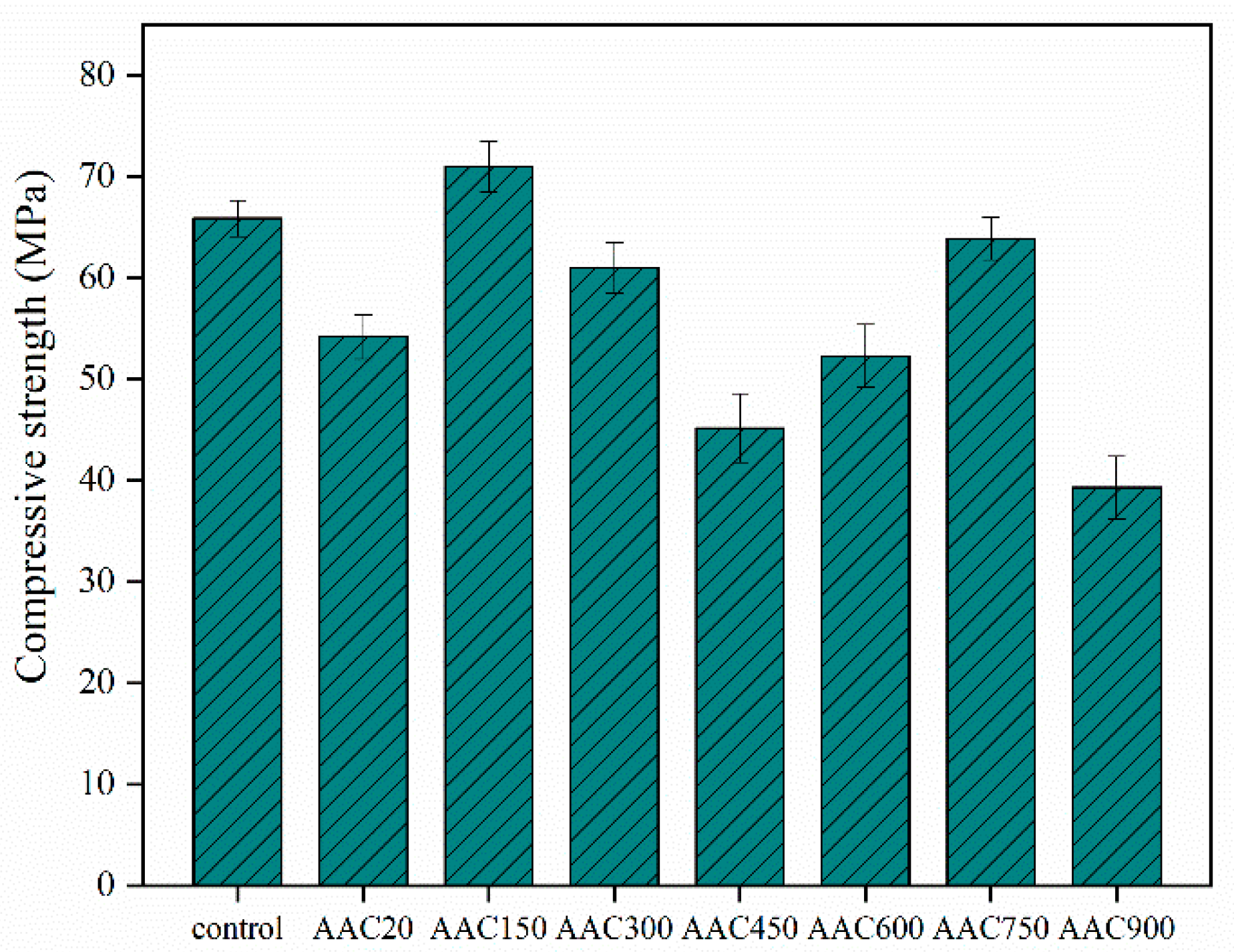
3.5. Compressive Strength of AAC
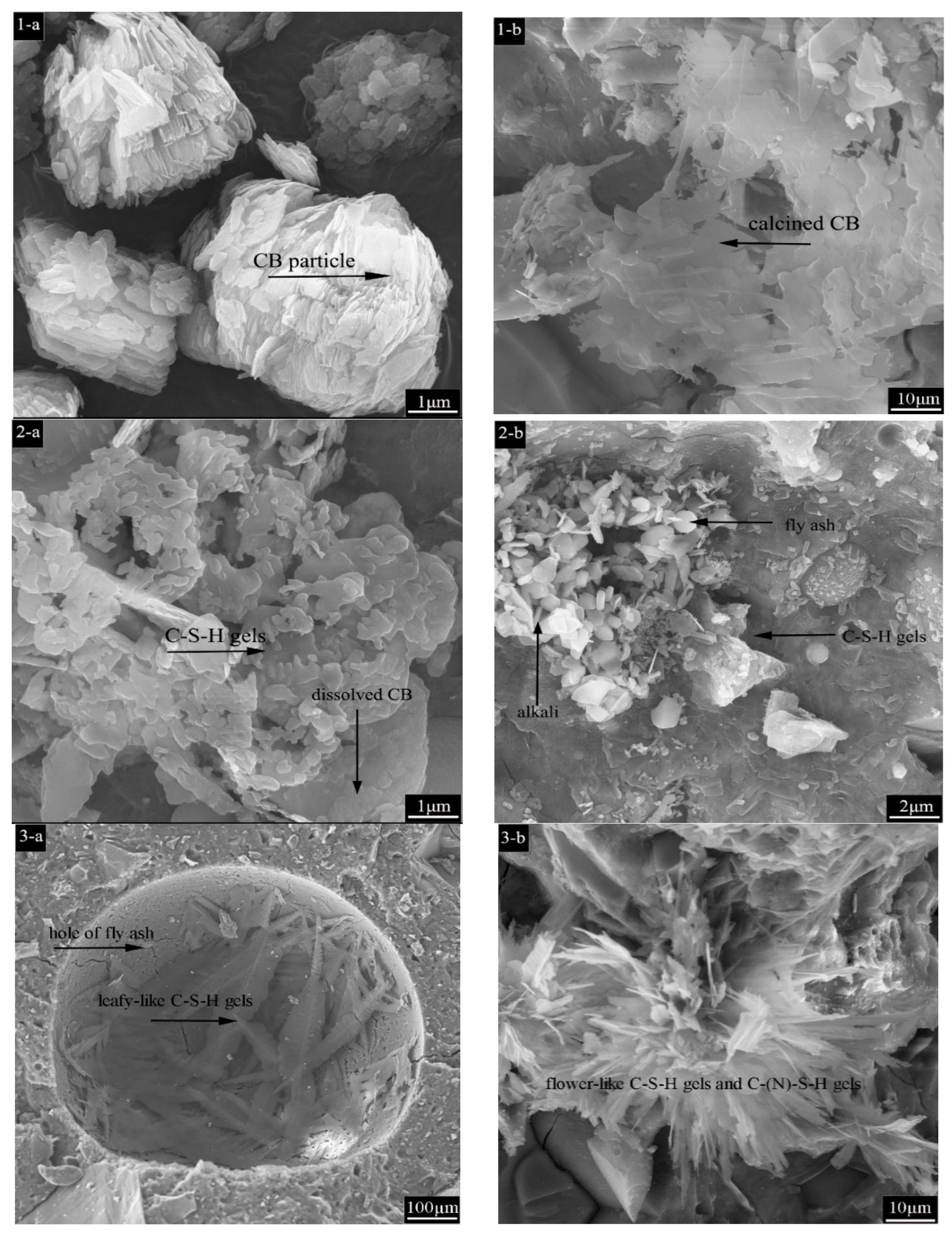
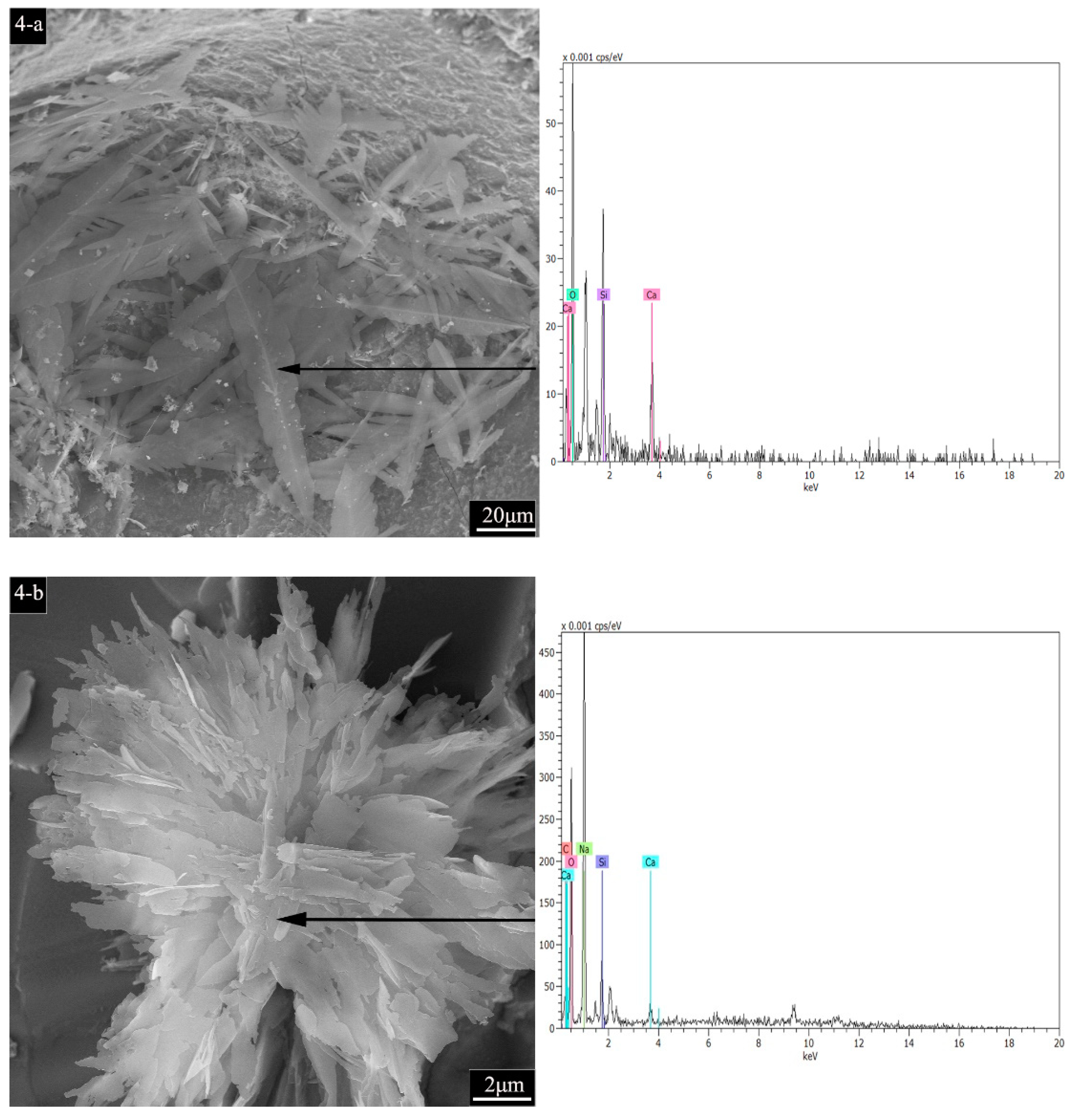
3.5.1. SEM Characterization
3.5.2. FTIR Characterization
4. Conclusions
- Calcination can change the pozzolanic activity of CB. In the temperature range of 150–560 °C, CB only removes water and does not change its properties. At 560–900 °C, CB underwent dehydroxylation and then recrystallization. The optimum activation temperature for CB is 750 °C. The recrystallization reaction at 900 °C is not conducive to improving the reactivity.
- Under room and low-temperature modification conditions, low purity CB is not an ideal SCM, and the presence of impurities leads to a decrease in strength. Only the CB modified at 750 °C fulfilled the Chinese standard, and its activity was still lower than that of the control group.
- The results of the alkali-activated tests differ, with low purity CB showing good compatibility with the AAC. At 750 °C, CB can replace parts of the slag without causing a loss of strength. The addition of CB increased the strength, and a difference in pozzolanic activity was observed at 150 °C. It is likely that low-temperature calcination enhances the water absorption and ion exchange capacities of CB, reduces the water content, increases the concentration of the alkali solution, and improves the rate and degree of hydration.
- At low temperatures, fly ash particles were involved in the hydration reaction, and leaf-like C-S-H gels were found in its hydration pores, which played a role in hydration filling. At high temperatures, intact fly ash particles, which did not participate in the hydration reaction and only acted as fillers, were observed, and flower-like C(N)-S-H gels coexisted inside. Due to the fact that CB absorbs water, the amplitude of the crystalline band decreases, whereas the degree of hydration increases. Meanwhile, dehydroxylation increased the Si/Al ratio, which shifted the position of the peaks.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharp, J.H. Surely we know all about cement—Don’t we? Adv. Appl. Ceram. 2007, 105, 162–174. [Google Scholar] [CrossRef]
- Jindal, B.B.; Sharma, R. The effect of nanomaterials on properties of geopolymers derived from industrial by-products: A state-of-the-art review. Constr. Build. Mater. 2020, 252, 119028. [Google Scholar] [CrossRef]
- Ambroise, J.; Murat, M.; Péra, J. Hydration reaction and hardening of calcined clays and related minerals V. Extension of the research and general conclusions. Cem. Concr. Res. 1985, 15, 261–268. [Google Scholar] [CrossRef]
- Tironi, A.; Trezza, M.A.; Scian, A.N.; Irassar, E.F. Assessment of pozzolanic activity of different calcined clays. Cem. Concr. Compos. 2013, 37, 319–327. [Google Scholar] [CrossRef]
- Zolfagharnasab, A.; Ramezanianpour, A.A.; Bahman-Zadeh, F. Investigating the potential of low-grade calcined clays to produce durable LC3 binders against chloride ions attack. Constr. Build. Mater. 2021, 303, 124541. [Google Scholar] [CrossRef]
- Zhou, S.; Tan, C.; Gao, Y.; Li, Y.; Guo, S. One-part alkali activated slag using Ca(OH)2 and Na2CO3 instead of NaOH as activator: More excellent compressive strength and microstructure. Mater. Res. Express. 2021, 8, 085501. [Google Scholar] [CrossRef]
- Siddique, S.; Gupta, V.; Chaudhary, S.; Park, S.; Jang, J.G. Influence of the Precursor, Molarity and Temperature on the Rheology and Structural Buildup of Alkali-Activated Materials. Materials 2021, 14, 3590. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Z.Q.; Wang, Y.Y.; Feng, J. Preparation and Properties of Alkali Activated Metakaolin-Based Geopolymer. Materials 2016, 9, 767. [Google Scholar] [CrossRef]
- Calvino, M.M.; Lisuzzo, L.; Cavallaro, G.; Lazzara, G.; Milioto, S. Halloysite based geopolymers filled with wax microparticles as sustainable building materials with enhanced thermo-mechanical performances. J. Environ. Chem. Eng. 2022, 10, 108594. [Google Scholar] [CrossRef]
- Davidovits, J. (Ed.) Geopolymer Chemistry and Sustainable Development: Proceedings of the World Congress Geopolymer 2005; Geopolymer Institute: Saint-Quentin, France, 2005. [Google Scholar]
- Phair, J.W.; Deventer, J. Effect of silicate activator pH on the leaching and material characteristics of waste-based inorganic polymers. Miner. Eng. 2001, 14, 289–304. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Deventer, J. The role of inorganic polymer technology in the development of ‘green concrete’. Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Maria Calvino, M.; Lisuzzo, L.; Cavallaro, G.; Lazzara, G.; Pipitone, C.; Giannici, F. Beeswax/halloysite microparticles embedded within a geopolymeric layer for the protective coating of steel. Mater. Lett. 2022, 330, 133257. [Google Scholar] [CrossRef]
- Liu, H.; Xie, B.; Qin, Y.L. Effect of Bentonite on the Pelleting Properties of Iron Concentrate. J. Chem. 2017, 2017, 7639326. [Google Scholar] [CrossRef]
- Fernandez, R.; Martirena, F.; Scrivener, K.L. The origin of the pozzolanic activity of calcined clay minerals: A comparison between kaolinite, illite and montmorillonite. Cem. Concr. Res. 2011, 41, 113–122. [Google Scholar] [CrossRef]
- Sen Gupta, S.; Bhattacharyya, K.G. Adsorption of heavy metals on kaolinite and montmorillonite: A review. Phys. Chem. Chem. Phys. 2012, 14, 6698–6723. [Google Scholar] [CrossRef]
- Taubald, H.; Bauer, A.; Schäfer, T.; Geckeis, H.; Kim, J.I. Experimental investigation of the effect of high-pH solutions on the Opalinus Shale and the Hammerschmiede Smectite. Clay Miner. 2000, 35, 515–524. [Google Scholar] [CrossRef]
- Sanchez, L.; Cuevas, J.; Ramirez, S.; Riuizdeleon, D.; Fernandez, R.; Vigildelavilla, R.; Leguey, S. Reaction kinetics of FEBEX bentonite in hyperalkaline conditions resembling the cement–bentonite interface. Appl. Clay. Sci. 2006, 33, 125–141. [Google Scholar] [CrossRef]
- Gates, W.P.; Bouazza, A. Bentonite transformations in strongly alkaline solutions. Geotext. Geomembr. 2010, 28, 219–225. [Google Scholar] [CrossRef]
- Ramírez, S.; Cuevas, J.; Vigil, R.; Leguey, S. Hydrothermal alteration of “La Serrata” bentonite (Almeria, Spain) by alkaline solutions. Appl. Clay. Sci. 2002, 21, 257–269. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Cherfa, N.; Zibouche, F.; Fernández-Jimenez, A.; Palomo, A. The role of aluminium in alkali-activated bentonites. Mater. Struct. 2014, 48, 585–597. [Google Scholar] [CrossRef]
- Huang, Y. Influence of Calcium Bentonite Addition on the Compressive Strength, Efflorescence Extent and Drying Shrinkage of Fly-Ash Based Geopolymer Mortar. Trans. Indian. Ceram. Soc. 2020, 79, 77–82. [Google Scholar] [CrossRef]
- Marsh, A.; Heath, A.; Patureau, P.; Evernden, M.; Walker, P. Alkali activation behaviour of un-calcined montmorillonite and illite clay minerals. Appl. Clay Sci. 2018, 166, 250–261. [Google Scholar] [CrossRef]
- Bayram, H.; Onal, M.; Yilmaz, H.; Sarikaya, Y. Thermal analysis of a white calcium bentonite. J Therm. Anal. Calorim. 2010, 101, 873–879. [Google Scholar] [CrossRef]
- Noyan, H.; Oenal, M.; Sarikaya, Y. The effect of heating on the surface area, porosity and surface acidity of a bentonite. Clays Clay Miner. 2006, 54, 375–381. [Google Scholar] [CrossRef]
- Mostafa, N.Y.; Mohsen, Q.; El-maghraby, A. Characterization of low-purity clays for geopolymer binder formulation. Int. J. Min. Met. Mater. 2014, 21, 609–619. [Google Scholar] [CrossRef]
- Oenal, M. Swelling and cation exchange capacity relationship for the samples obtained from a bentonite by acid activations and heat treatments. Appl. Clay Sci. 2007, 37, 74–80. [Google Scholar] [CrossRef]
- Ekosse, G.I.E.; Mulaba-Bafibiandi, A.F. Mineral thermochemistry of Bentonite and Kaolin related to their possible application in the ceramic industry. J. Appl. Sci. 2008, 8, 4145–4151. [Google Scholar] [CrossRef]
- Ferone, C.; Liguori, B.; Capasso, I.; Colangelo, F.; Cioffi, R.; Cappelletto, E.; Di Maggio, R. Thermally treated clay sediments as geopolymer source material. Appl. Clay Sci. 2015, 107, 195–204. [Google Scholar] [CrossRef]
- Castillo, R.; Fernández, R.; Antoni, M.; Scrivener, K.; Alujas, A.; Martirena, J.F. Activación de arcillas de bajo grado a altas temperaturas. Rev. Ing. Construcción 2010, 25, 329–352. [Google Scholar] [CrossRef]
- Elimbi, A.; Tchakoute, H.K.; Njopwouo, D. Effects of calcination temperature of kaolinite clays on the properties of geopolymer cements. Constr. Build. Mater. 2011, 25, 2805–2812. [Google Scholar] [CrossRef]
- Taylor-Lange, S.C.; Lamon, E.L.; Riding, K.A.; Juenger, M.C.G. Calcined kaolinite–bentonite clay blends as supplementary cementitious materials. Appl. Clay Sci. 2015, 108, 84–93. [Google Scholar] [CrossRef]
- Alujas, A.; Fernández, R.; Quintana, R.; Scrivener, K.L.; Martirena, F. Pozzolanic reactivity of low grade kaolinitic clays: Influence of calcination temperature and impact of calcination products on OPC hydration. Appl. Clay Sci. 2015, 108, 94–101. [Google Scholar] [CrossRef]
- Parolo, M.E.; Pettinari, G.R.; Musso, T.B.; Sánchez-Izquierdo, M.; Fernández, L. Characterization of organo-modified bentonite sorbents: The effect of modification conditions on adsorption performance. Appl. Surf. Sci. 2014, 320, 356–363. [Google Scholar] [CrossRef]
- Alizadeh, A.; Wang, M. Temperature effects on electrical double layer at solid-aqueous solution interface. Electrophoresis 2020, 41, 1067–1072. [Google Scholar] [CrossRef]
- Jastrzebska, A.M.; Kurtycz, P.; Olszyna, A.; Karwowska, E.; Miaskiewicz-Peska, E.; Zaleska-Radziwill, M.; Doskocz, N.; Basiak, D. The Impact of Zeta Potential and Physicochemical Properties of TiO2-Based Nanocomposites on Their Biological Activity. Int. J. Appl. Ceram. Technol. 2015, 12, 1157–1173. [Google Scholar] [CrossRef]
- Zuo, Q.; Gao, X.; Yang, J.; Zhang, P.; Chen, G.; Li, Y.; Shi, K.; Wu, W. Investigation on the thermal activation of montmorillonite and its application for the removal of U(VI) in aqueous solution. J. Taiwan. Inst. Chem. Eng. 2017, 80, 754–760. [Google Scholar] [CrossRef]
- Cantuaria, M.L.; Nascimento, E.S.; Neto, A.F.A.; Santos, O.A.A.d.; Vieira, M.G.A. Removal and Recovery of Silver by Dynamic Adsorption on Bentonite Clay Using a Fixed-Bed Column System. Adsorpt. Sci. Technol. 2015, 33, 91–103. [Google Scholar] [CrossRef]
- Shi, T.; Liu, Y.; Zhang, Y.; Lan, Y.; Zhao, Q.; Zhao, Y.; Wang, H. Calcined Attapulgite Clay as Supplementary Cementing Material: Thermal Treatment, Hydration Activity and Mechanical Properties. Int. J. Concr. Struct. Mater. 2022, 16, 1–10. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, P.; Chen, X.; Ge, X.; Wang, Y. Study on hydration characteristics of circulating fluidized bed combustion fly ash (CFBCA). Constr. Build. Mater. 2020, 251, 118993. [Google Scholar] [CrossRef]
- Peng, M.X.; Wang, Z.H.; Shen, S.H.; Xiao, Q.G.; Hu, L.L. Alkali fusion of bentonite to synthesize one-part geopolymeric cements cured at elevated temperature by comparison with two-part ones. Constr. Build. Mater. 2016, 130, 103–112. [Google Scholar] [CrossRef]
- Aydın, S.; Baradan, B. Mechanical and microstructural properties of heat cured alkali-activated slag mortars. Mater. Des. 2012, 35, 374–383. [Google Scholar] [CrossRef]
- Madejová, J. FTIR techniques in clay mineral studies. Vib. Spectrosc. 2003, 31, 1–10. [Google Scholar] [CrossRef]
- Tyagi, B.; Chudasama, C.; Jasra, R. Determination of structural modification in acid activated montmorillonite clay by FT-IR spectroscopy. Spectrochim. Acta. A. Mol. Biomol. 2006, 64, 273–278. [Google Scholar] [CrossRef]
- Gereli, G.; Seki, Y.; Kusoglu, I.; Yurdakoc, K. Equilibrium and kinetics for the sorption of promethazine hydrochloride onto K10 montmorillonite. J. Colloid. Interface. Sci. 2006, 299, 155–162. [Google Scholar] [CrossRef]
- Rakhimova, N.; Rakhimov, R.; Morozov, V.; Potapova, L.; Osin, Y. Marl as a supplementary material to alkali-activated blended cements. Eur. J. Environ. Civ. Eng. 2019, 25, 2491–2508. [Google Scholar] [CrossRef]




| Raw Materials | CaO | SiO2 | Al2O3 | SO3 | MgO | Fe2O3 | TiO2 | K2O | LOI |
|---|---|---|---|---|---|---|---|---|---|
| Cement | 62.50 | 20.44 | 4.67 | 2.86 | 2.53 | 3.25 | - | 0.53 | 1.98 |
| Slag | 39.3 | 34.53 | 12.73 | 0.4 | 8.27 | 1.93 | 0.9 | 0.8 | 0.3 |
| Fly ash | 4.8 | 56.2 | 22.5 | 1.02 | 1.56 | 5.09 | 1.8 | 0.9 | 3.8 |
| CB | 1.62 | 71.2 | 13 | - | 2.71 | 0.75 | 0.1 | 1.01 | 7.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Jiang, C.; Zhang, Q.; Li, S. Evaluation of Pozzolanic and Alkali-Activated Reactivity of Low-Purity Calcium Bentonite. Materials 2022, 15, 8015. https://doi.org/10.3390/ma15228015
Li W, Jiang C, Zhang Q, Li S. Evaluation of Pozzolanic and Alkali-Activated Reactivity of Low-Purity Calcium Bentonite. Materials. 2022; 15(22):8015. https://doi.org/10.3390/ma15228015
Chicago/Turabian StyleLi, Wanqiang, Chunmeng Jiang, Qin Zhang, and Shuangxi Li. 2022. "Evaluation of Pozzolanic and Alkali-Activated Reactivity of Low-Purity Calcium Bentonite" Materials 15, no. 22: 8015. https://doi.org/10.3390/ma15228015
APA StyleLi, W., Jiang, C., Zhang, Q., & Li, S. (2022). Evaluation of Pozzolanic and Alkali-Activated Reactivity of Low-Purity Calcium Bentonite. Materials, 15(22), 8015. https://doi.org/10.3390/ma15228015






