Study on the Densification of Osmium by Experiment and First Principle Calculations
Abstract
1. Introduction
2. Experiment and Calculation
3. Results
3.1. Morphological Characterization
3.2. MSC of Nanosized Osmium
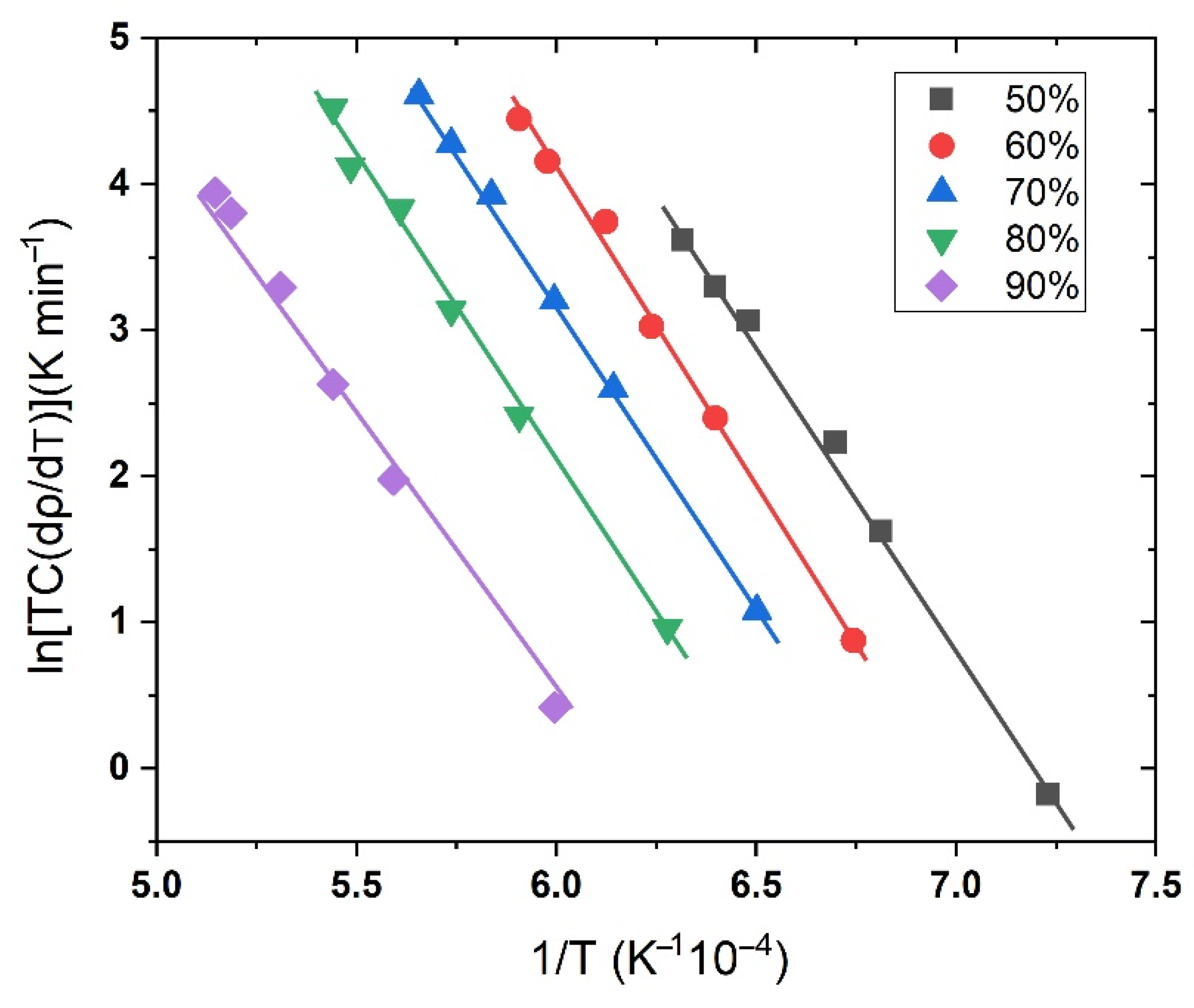
3.2.1. Theoretical Background: Master Sintering Curve Analysis
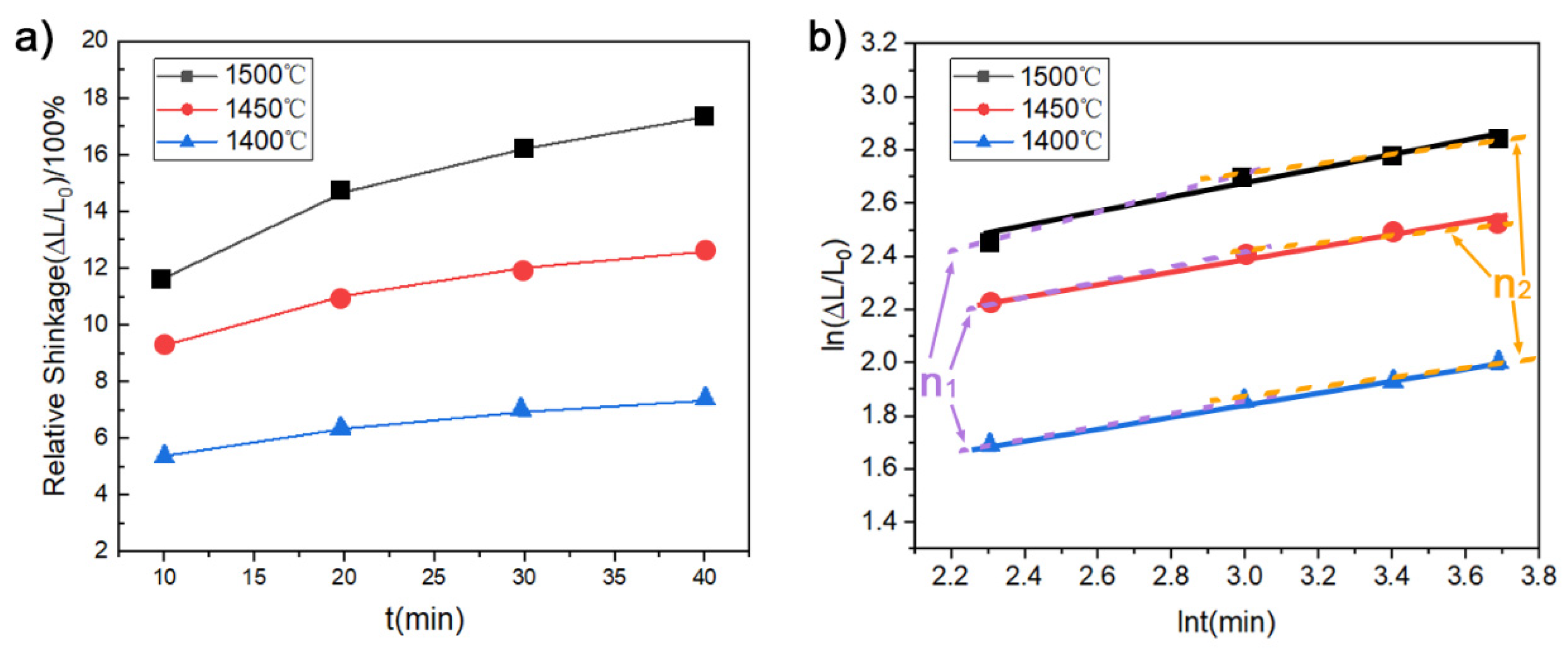
3.2.2. MSC Results of Nanosized Osmium
3.2.3. Densification Mechanism of Osmium
3.3. First Principle Study on Densification of Osmium Nanocrystals
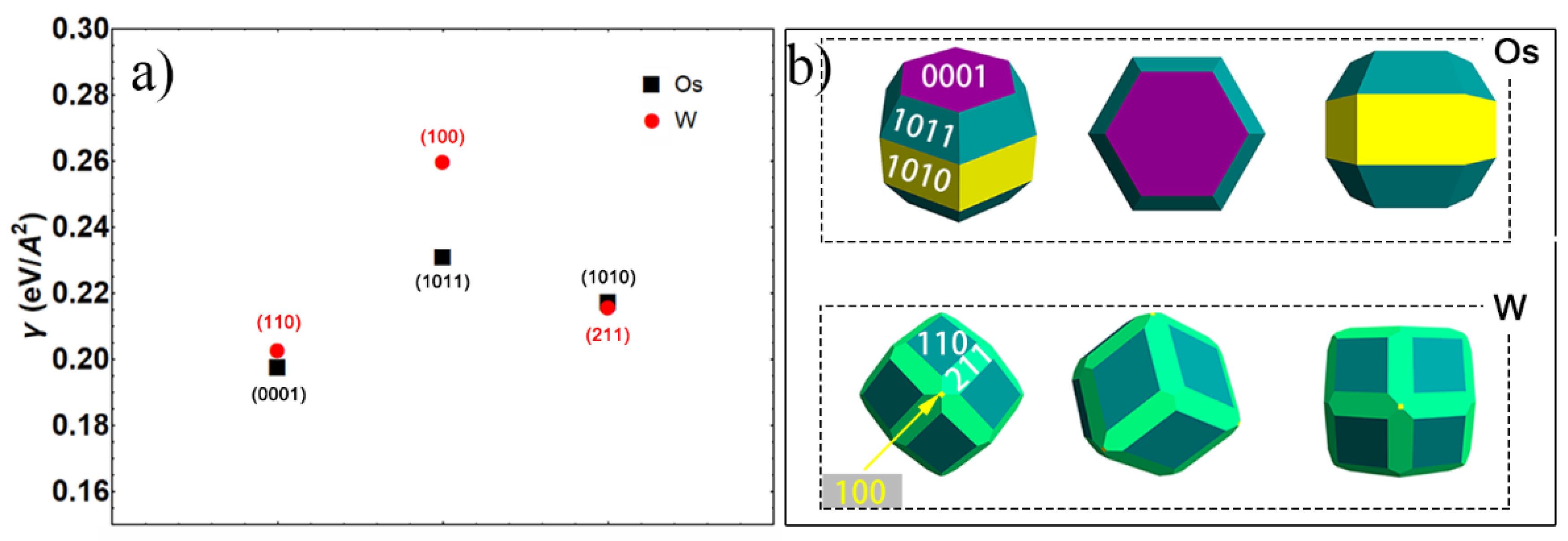
3.3.1. Thermodynamic Analysis of Densification Depending on Surface Energy
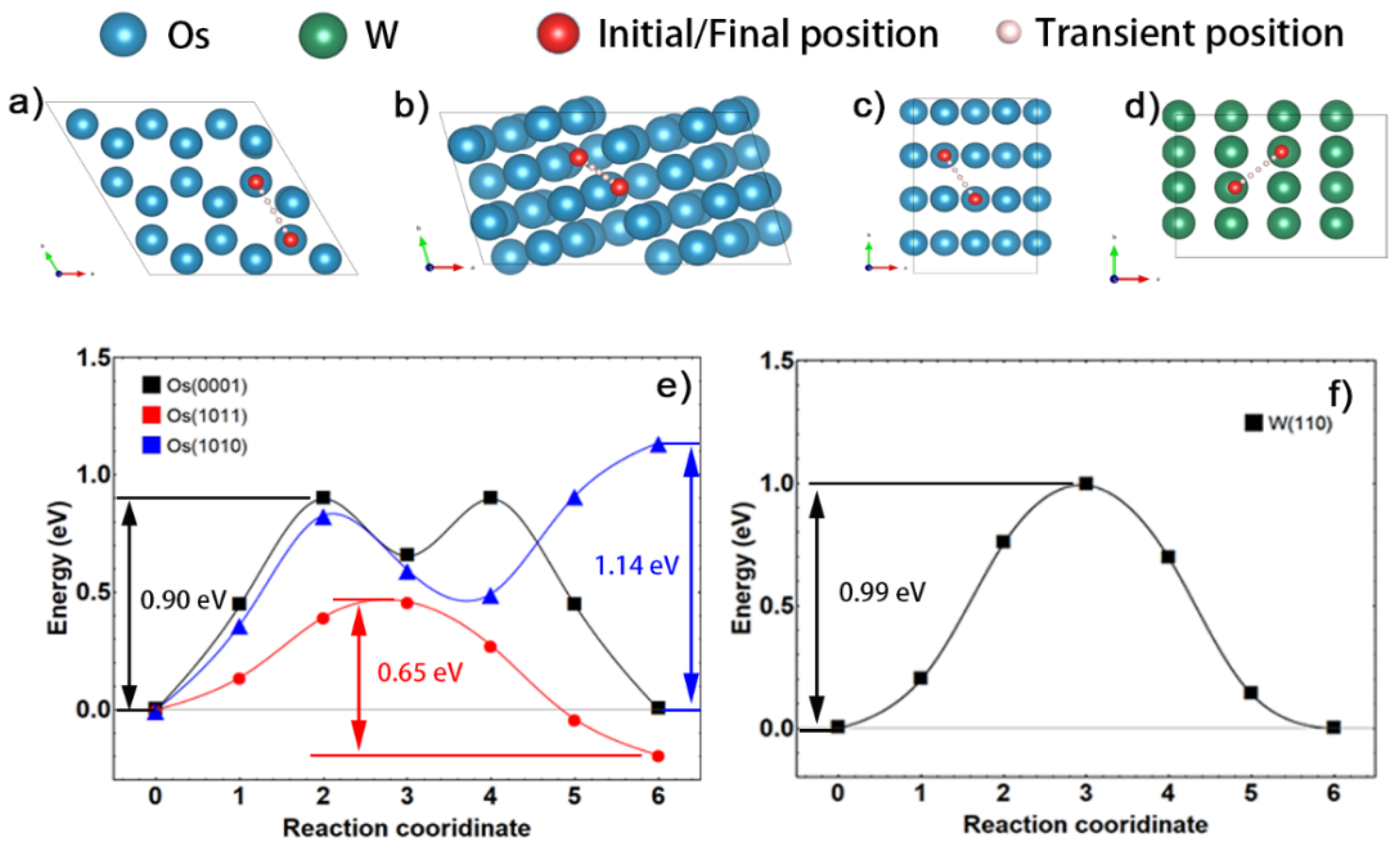
3.3.2. Kinetic Analysis of Densification by Vacancy Migration Model through Multiple Paths
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubrovinsky, L.; Dubrovinskaia, N.; Bykova, E.; Bykov, M.; Prakapenka, V.; Prescher, C.; Glazyrin, K.; Liermann, H.-P.; Hanfland, M.; Ekholm, M.; et al. The most incompressible metal osmium at static pressures above 750 gigapascals. Nature 2015, 525, 226–229. [Google Scholar] [CrossRef]
- Young, D.A. Phase Diagrams of the Elements; Univ of California Press: Berkeley, CA, USA; Los Angeles, CA, USA, 1991. [Google Scholar]
- Armentrout, M.M.; Kavner, A. Incompressibility of osmium metal at ultrahigh pressures and temperatures. J. Appl. Phys. 2010, 107, 093528. [Google Scholar] [CrossRef]
- Ma, J.; Xia, Y.; Xie, Y.; Lv, H.; Zhang, C. Preparation Process and Quality Characterization of High Density Fine Grain Osmium Target for Coated Cathode. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2022; Volume 2194, p. 012029. [Google Scholar]
- Forleo, A.; Capone, S.; Epifani, M.; Siciliano, P.; Rella, R. Role of osmium in the electrical transport mechanism of polycrystalline tin oxide thin films. Appl. Phys. Phys. Lett. 2004, 84, 744–746. [Google Scholar] [CrossRef]
- Kapustin, V.I.; Li, I.P.; Moskalenko, S.O.; Shumanov, A.V.; Zalialiev, R.R.; Kozhevnikova, N.E. Physical Mechanism of Operation of Osmium-Coated Cathodes of Microwave Devices. Inorg. Mater. Appl. Res. 2021, 12, 1175–1184. [Google Scholar] [CrossRef]
- Hämäläinen, J.; Sajavaara, T.; Puukilainen, E.; Ritala, M.; Leskelä, M. Atomic layer deposition of osmium. Chem. Mater. 2012, 24, 55–60. [Google Scholar] [CrossRef]
- Hämäläinen, J.; Ritala, M.; Leskelä, M. Atomic layer deposition of noble metals and their oxides. Chem. Mater. 2014, 26, 786–801. [Google Scholar] [CrossRef]
- Chi, Y.; Yu, H.-L.; Ching, W.-L.; Liu, C.-S.; Chen, Y.-L.; Chou, T.-Y.; Peng, S.-M.; Lee, G.-H. Deposition of osmium thin films using pyrazolate complexes as CVD source reagents. J. Mater. Chem. 2002, 12, 1363–1369. [Google Scholar] [CrossRef][Green Version]
- Wall, R.N.; Jacobson, D.L.; Bosch, D.R. The high-temperature electron emission and vaporization of tungsten-osmium alloys. Metall. Trans. A 1993, 24, 951–958. [Google Scholar] [CrossRef]
- Akahori, H.; Handa, M.; Yoshida, H.; Kozuka, Y. Osmium-metal coating device using hollowcathode plasma CVD method. Microscopy 2000, 49, 735–744. [Google Scholar] [CrossRef]
- Molefe, T.; Forbes, R.P.; Coville, N.J. Osmium@ Hollow carbon spheres as Fischer–Tropsch synthesis catalysts. Catal. Lett. 2021, 151, 875–887. [Google Scholar] [CrossRef]
- Josell, D.; Witt, C.; Moffat, T.P. Osmium barriers for direct copper electrodeposition in damascene processing. Electrochem. Solid-State Lett. 2005, 9, C41. [Google Scholar] [CrossRef][Green Version]
- Mitu, A.; Dumitru, M.; Șuvăilă, R.; Oprea, A.; Gheorghe, I.; Mereuță, P.; Brajnicov, S.; Burducea, I.; Florea, N.M.; Mărginean, N.; et al. Refractory osmium targets for accelerator based nuclear activation experiments prepared by Pulsed Laser Deposition technique. Vacuum 2019, 161, 162–167. [Google Scholar] [CrossRef]
- Reuter, W.; Gursky, J.C.; Shera, E.B.; Johnson, M.W. Thick osmium targets. Nucl. Instrum. Methods Phys. Res. 1984, 220, 288–292. [Google Scholar] [CrossRef]
- Fremont, G.; Ngono-Ravache, Y.; Schmitt, C.; Stodel, C. Preparation of osmium targets with carbon backing. AIP Conf. Proc. 2018, 1962, 030002. [Google Scholar]
- Gómez, S.Y.; Hotza, D. Predicting powder densification during sintering. J. Eur. Ceram. Soc. 2018, 38, 1736–1741. [Google Scholar] [CrossRef]
- Ferreira, A.F.; Ferreira, I.; Almeida, R.P.; Castro, J.A.; Sales, R.C.; Júnior, Z.A. Microstructural evolution and microsegregation in directional solidification of hypoeutectic Al− Cu alloy: A comparison between experimental data and numerical results obtained via phase-field model. Trans. Nonferrous Met. Soc. China 2021, 31, 1853–1867. [Google Scholar] [CrossRef]
- Yi, S.; Li, Y.Y.; Zhou, Z.Y.; Zheng, Z.X.; Chen, P.Q. Improved model and 3D simulation of densification process for iron powder. Trans. Nonferrous Met. Soc. China 2010, 20, 1470–1475. [Google Scholar]
- Cao, P.; Liu, S.; Wu, Y.; Xia, Y.; Wu, Z.; Zhou, Z.; Liu, Y.; Sun, A. Master sintering curve of binary mutually immiscible molybdenum-copper powders. J. Alloy. Compd. 2020, 834, 155194. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, K.; Zhou, H. Master sintering curve of W-Mo-Cu alloy prepared by electric field sintering. J. Manuf. Sci. Eng. 2022, 144, 1–14. [Google Scholar] [CrossRef]
- Dong, J.; Pouchly, V.; Biesuz, M.; Tyrpekl, V.; Vilémová, M.; Kermani, M.; Reece, M.; Hu, C.; Grasso, S. Thermally-insulated ultra-fast high temperature sintering (UHS) of zirconia: A master sintering curve analysis. Scr. Mater. 2021, 203, 114076. [Google Scholar] [CrossRef]
- Frueh, T.; Ozer, I.; Poterala, S.; Lee, H.; Kupp, E.; Compson, C.; Atria, J.; Messing, G. A critique of master sintering curve analysis. J. Eur. Ceram. Soc. 2018, 38, 1030–1037. [Google Scholar] [CrossRef]
- Talimian, A.; Wondraczek, L.; Galusek, D. Densification of glass powder studied by master sintering curve and master kinetic curve methods. Int. J. Appl. Glass Sci. 2021, 12, 541–550. [Google Scholar] [CrossRef]
- Du, J. A differentiating approach to the master sintering curve analysis. Ceram. Int. 2020, 46, 6945–6950. [Google Scholar] [CrossRef]
- Baruah, B.; Anand, R.; Behera, S.K. Master sintering curve and activation energy of sintering of ZrO2-doped Al2O3. Ceram. Int. 2021, 47, 7253–7257. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Perdew, burke, and ernzerhof reply. Phys. Rev. Lett. 1998, 80, 891. [Google Scholar] [CrossRef]
- Harl, J.; Schimka, L.; Kresse, G. Assessing the quality of the random phase approximation for lattice constants and atomization energies of solids. Phys. Rev. B 2010, 81, 115126. [Google Scholar] [CrossRef]
- Shang, S.L.; Hector, J.L.G.; Wang, Y.; Liu, Z.K. Anomalous energy pathway of vacancy migration and self-diffusion in hcp Ti. Phys. Rev. B 2011, 83, 224104. [Google Scholar] [CrossRef]
- Zou, N.; Lu, H.; Lu, X. Impurity diffusion coefficients in BCC Nb from first-principles calculations. J. Alloy. Compd. 2019, 803, 684–688. [Google Scholar] [CrossRef]
- Ren, Z.; Xue, Z.; Zhang, X.; Qin, J.; Ma, M.; Liu, R. Atomic diffusion mediated by vacancy defects in L12-Zr3Al: A first-principles study. J. Alloy. Compd. 2020, 821, 153223. [Google Scholar] [CrossRef]
- Hirata, K.; Iikubo, S.; Koyama, M.; Tsuzaki, K.; Ohtani, H. First-principles study on hydrogen diffusivity in BCC, FCC, and HCP iron. Metall. Mater. Trans. A 2018, 49, 5015–5022. [Google Scholar] [CrossRef]
- Meyers, M.A.; Olevsky, E.A. Sintering Theory and Practice. J. Mater. Eng. Perform. 1997, 6, 278. [Google Scholar]
- Su, H.; Johnson, D.L. Master sintering curve: A practical approach to sintering. J. Am. Ceram. Soc. 1996, 79, 3211–3217. [Google Scholar] [CrossRef]
- Pouchly, V.; Maca, K. Master sintering curve: A practical approach to its construction. Sci. Sinter. 2010, 42, 25–32. [Google Scholar] [CrossRef]
- Blaine, D.C.; Gurosik, J.D.; Park, S.J.; German, R.M.; Heaney, D.F. Master sintering curve concepts as applied to the sintering of molybdenum. Metall. Mater. Trans. A 2006, 37, 715–720. [Google Scholar] [CrossRef]
- Kiani, S.; Pan, J.; Yeomans, J.A. A new scheme of finding the master sintering curve. J. Am. Ceram. Soc. 2006, 89, 3393–3396. [Google Scholar] [CrossRef]
- Blaine, D.C.; Park, S.; German, R.M. Linearization of master sintering curve. J. Am. Ceram. Soc. 2009, 92, 1403–1409. [Google Scholar] [CrossRef]
- Duan, B.-H.; Zhang, Z.; Wang, D.-Z.; Zhou, T. Microwave sintering of Mo nanopowder and its densification behavior. Trans. Nonferrous Met. Soc. China 2019, 29, 1705–1713. [Google Scholar] [CrossRef]
- Deng, S.; Li, J.; Li, R.; Zhao, H.; Yuan, T.; Li, L.; Zhang, Y. The effect of particle size on the densification kinetics of tungsten powder during spark plasma sintering. Int. J. Refract. Met. Hard Mater. 2020, 93, 105358. [Google Scholar] [CrossRef]
- Srivastav, A.K.; Sankaranarayana, M.; Murty, B.S. Initial-stage sintering kinetics of nanocrystalline tungsten. Metall. Mater. Trans. A 2011, 42, 3863–3866. [Google Scholar] [CrossRef]
- Li, B.; Sun, Z.; Hou, G.; Ding, F.; Hu, P.; Yuan, F. The sintering behavior of quasi-spherical tungsten nanopowders. Int. J. Refract. Met. Hard Mater. 2016, 56, 44–50. [Google Scholar] [CrossRef]
- Raj, R. Analysis of the sintering pressure. J. Am. Ceram. Soc. 1987, 70, C-210. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Punkkinen, M.; Schönecker, S.; Nabi, Z.; Kádas, K.; Zólyomi, V.; Koo, Y.; Hu, Q.-M.; Ahuja, R.; Johansson, B.; et al. The surface energy and stress of metals. Surf. Sci. 2018, 674, 51–68. [Google Scholar] [CrossRef]
- Zhang, J.-M.; Wang, D.-D.; Xu, K.-W. Calculation of the surface energy of hcp metals by using the modified embedded atom method. Appl. Surf. Sci. 2006, 253, 2018–2024. [Google Scholar] [CrossRef]
- Dobrushin, R.L.; Kotecký, R.; Shlosman, S. Wulff Construction: A Global Shape from Local Interaction; American Mathematical Society: Providence, RI, USA, 1992; Volume 104. [Google Scholar]
- Tran, R.; Xu, Z.; Radhakrishnan, B.; Winston, D.; Sun, W.; Persson, K.A.; Ong, S.P. Surface energies of elemental crystals. Sci. Data 2016, 3, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zucker, R.V.; Chatain, D.; Dahmen, U.; Hagège, S.; Carter, W.C. New software tools for the calculation and display of isolated and attached interfacial-energy minimizing particle shapes. J. Mater. Sci. 2012, 47, 8290–8302. [Google Scholar] [CrossRef]
- Vasilos, T.; Smith, J.T. Diffusion mechanism for tungsten sintering kinetics. J. Appl. Phys. 1964, 35, 215–217. [Google Scholar] [CrossRef]
- Chen, L.C. Dilatometric analysis of sintering of tungsten and tungsten with ceria and hafnia dispersions. Int. J. Refract. Met. Hard Mater. 1993, 12, 41–51. [Google Scholar] [CrossRef]
- Somfai, E.; van Gastel, R.; van Albada, S.B.; van Saarloos, W.; Frenken, J.W.M. Vacancy diffusion in the Cu (001) surface II: Random walk theory. Surf. Sci. 2002, 521, 26–33. [Google Scholar] [CrossRef][Green Version]
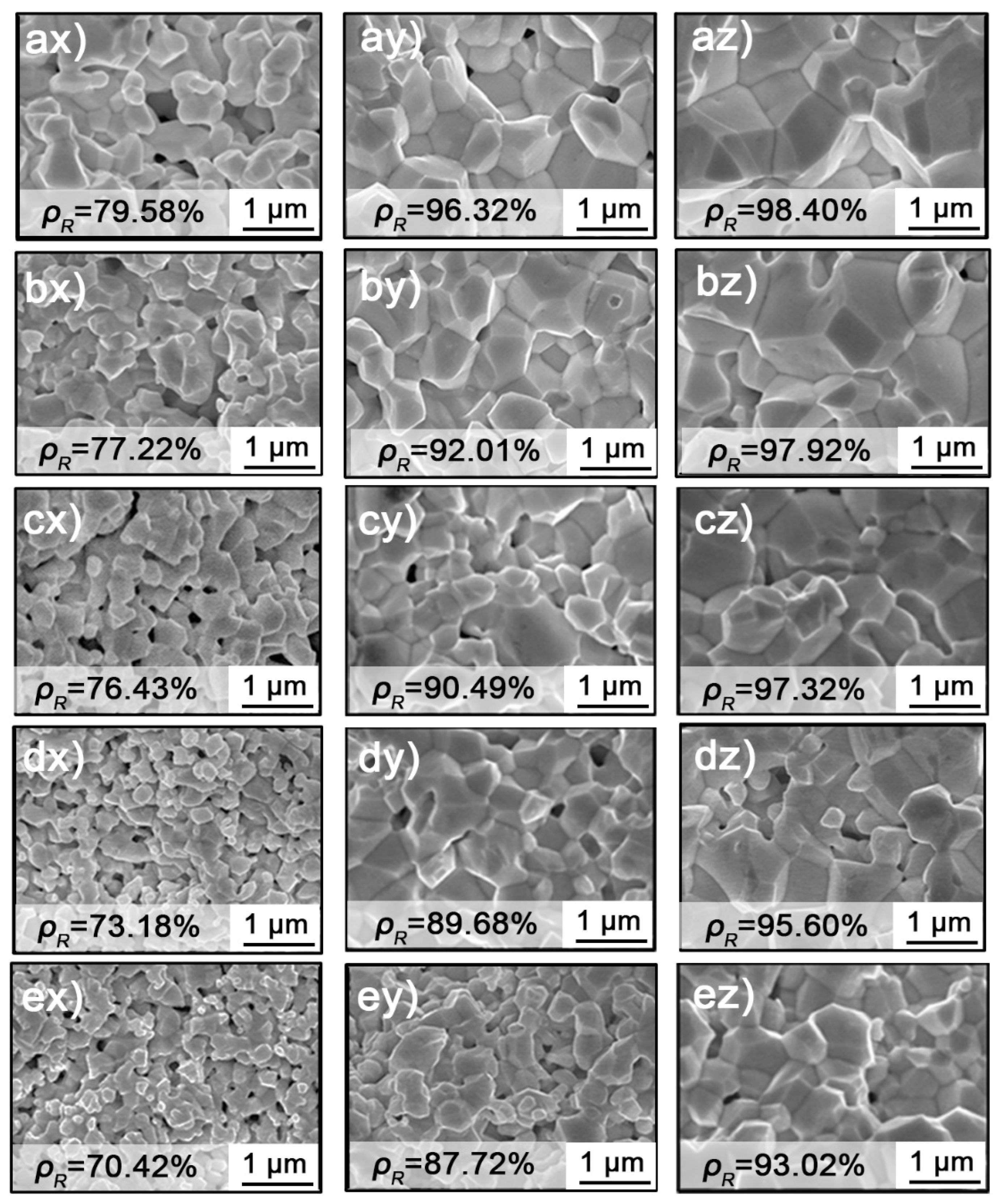
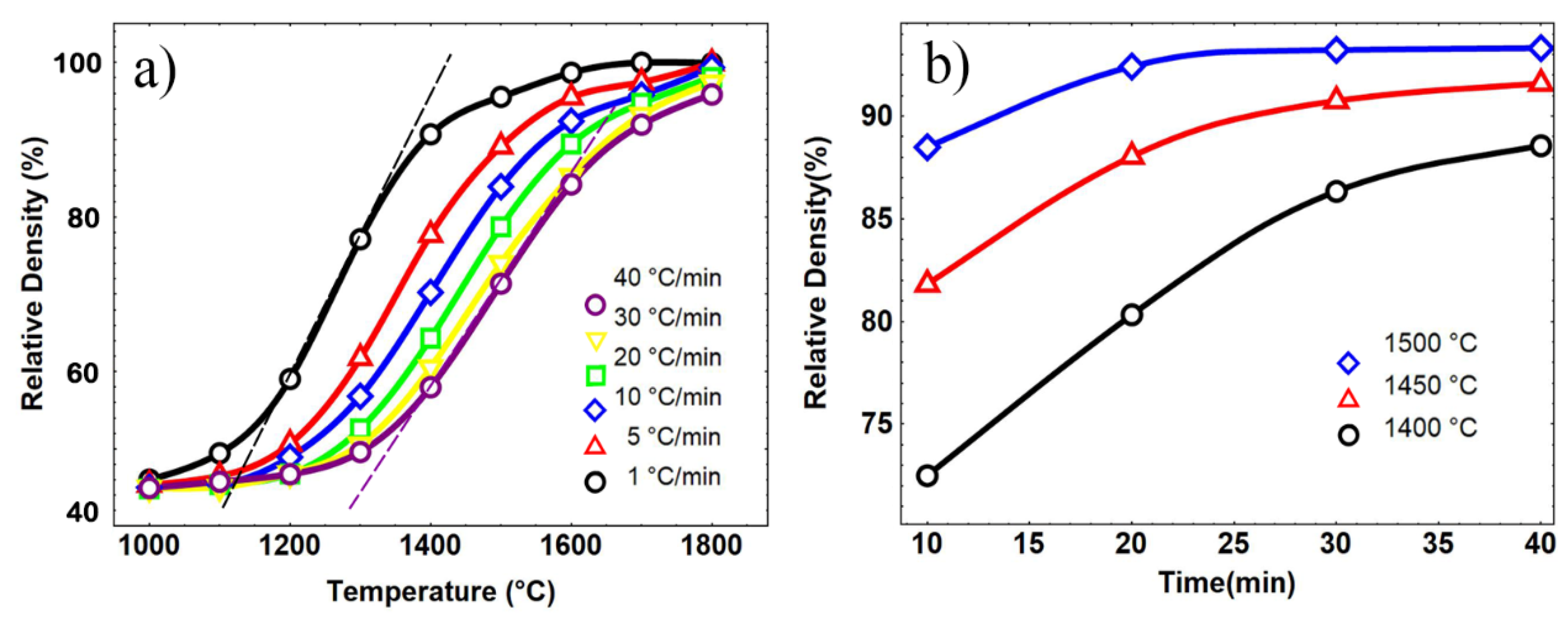
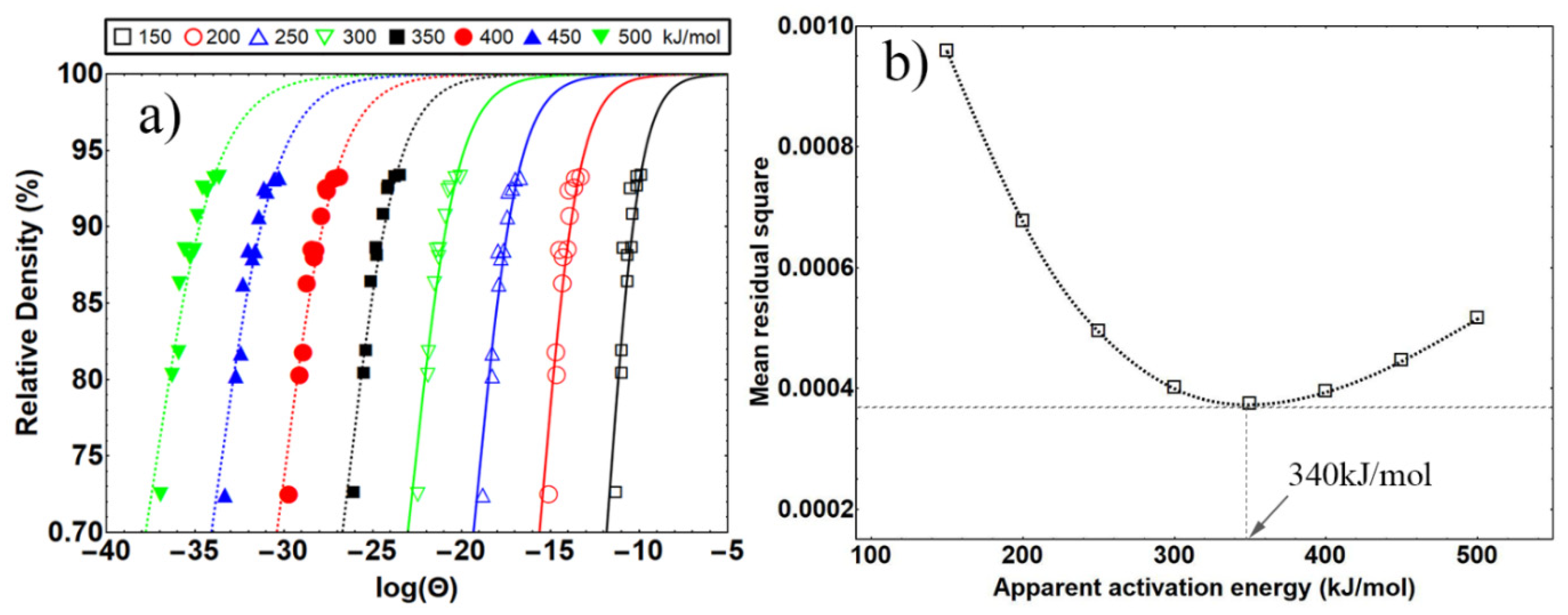
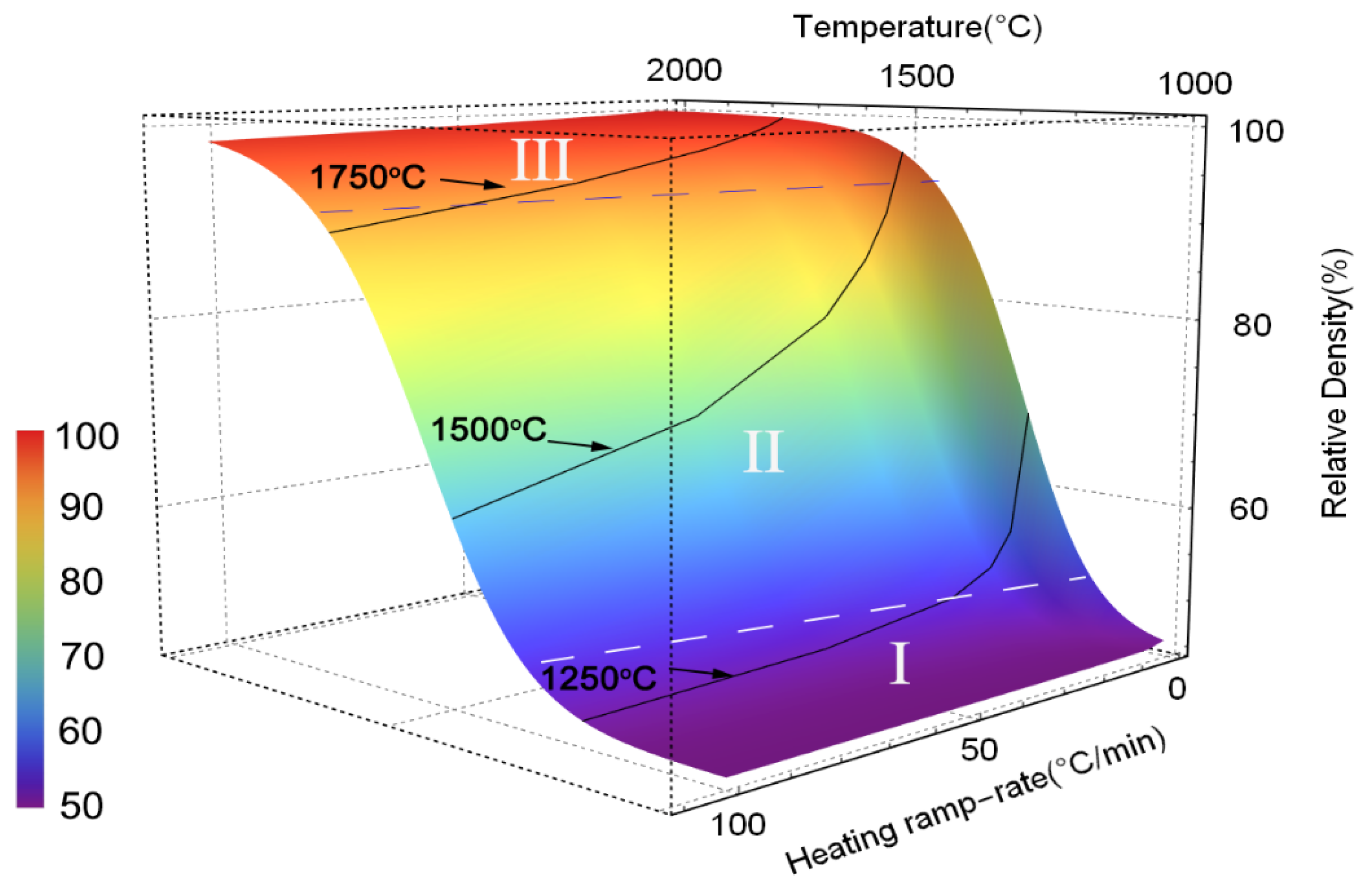
| Heating Rate °C/min | Termination Temperature °C | Relative Density (%) | Predicted Density (%) |
|---|---|---|---|
| 1 | 1100 | 51.43 | 48.65 |
| 1 | 1500 | 95.55 | 96.09 |
| 1 | 1700 | 97.97 | 98.31 |
| 5 | 1100 | 50.49 | 46.31 |
| 5 | 1500 | 89.12 | 89.29 |
| 5 | 1700 | 96.45 | 97.38 |
| 10 | 1100 | 49.38 | 45.80 |
| 10 | 1500 | 83.93 | 84.37 |
| 10 | 1700 | 95.80 | 96.60 |
| 20 | 1100 | 48.38 | 45.49 |
| 20 | 1500 | 78.73 | 78.29 |
| 20 | 1700 | 94.73 | 94.63 |
| 40 | 1100 | 47.75 | 45.30 |
| 40 | 1500 | 71.38 | 71.55 |
| 40 | 1700 | 91.65 | 91.70 |
| T/°C | 1400 | 1450 | 1500 |
|---|---|---|---|
| n | 3.49 | 4.24 | 4.48 |
| n1 | 2.94 | 3.92 | 4.08 |
| n2 | 4.31 | 4.76 | 4.83 |
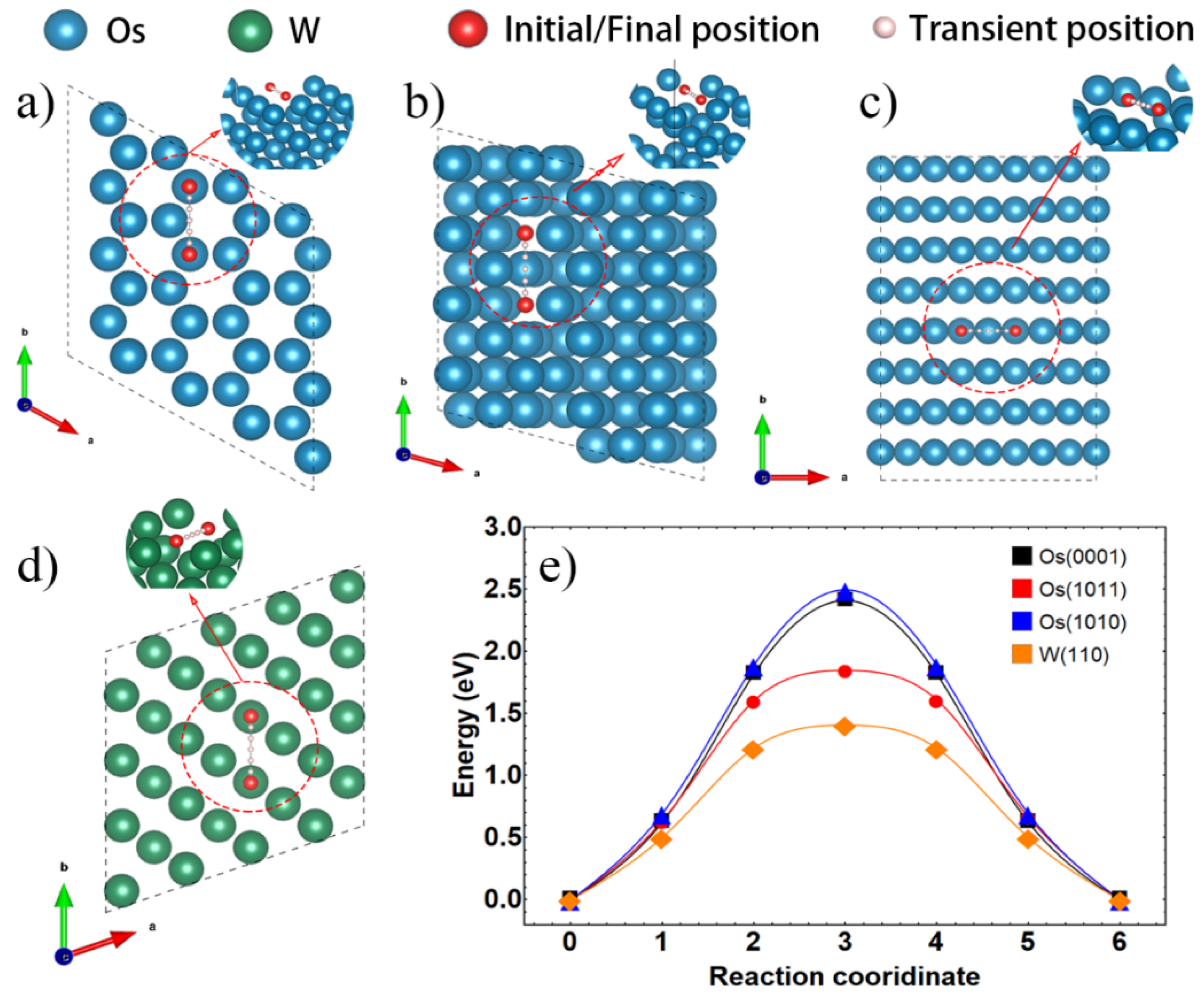
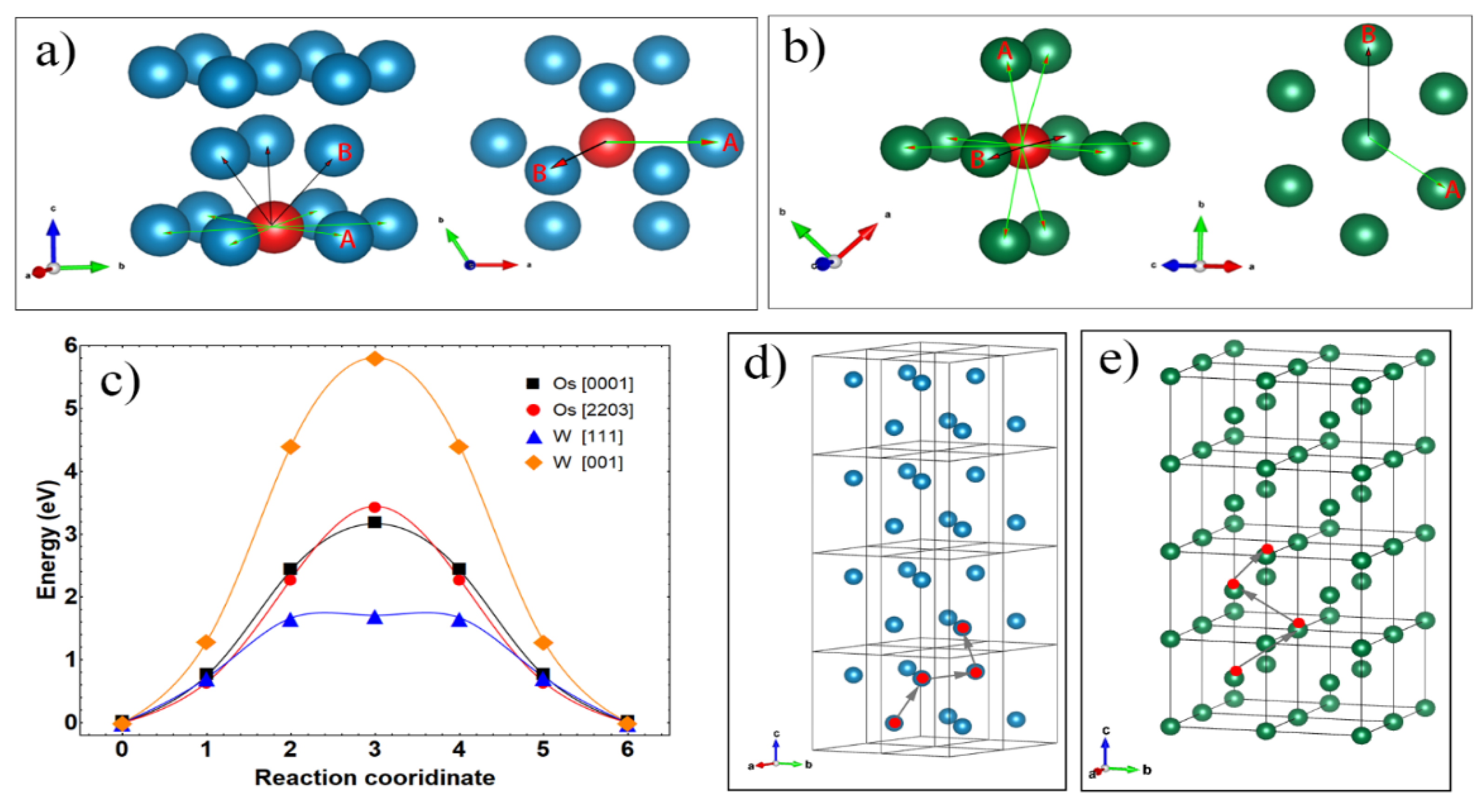
| Surface Atom | Surface Vacancy | Inside Vacancy | ||||
|---|---|---|---|---|---|---|
| Plane | Energy Barrier (eV) | Plane | Energy Barrier (eV) | Direction | Energy Barrier (eV) | |
| W | (110) | 0.99 | (110) | 1.41 | <001> | 5.95 |
| <111> | 1.70 | |||||
| Os | (0001) | 0.90 | (0001) | 2.41 | <0001> | 3.08 |
| (1010) | 1.14 | (1010) | 2.50 | <2203> | 3.21 | |
| (1011) | 0.65 | (1011) | 1.85 | / | / | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Sun, J.; Liu, W.; Hu, P.; Zhang, R.; Liu, H.; Gao, J.; Wang, J. Study on the Densification of Osmium by Experiment and First Principle Calculations. Materials 2022, 15, 8011. https://doi.org/10.3390/ma15228011
Yang Y, Sun J, Liu W, Hu P, Zhang R, Liu H, Gao J, Wang J. Study on the Densification of Osmium by Experiment and First Principle Calculations. Materials. 2022; 15(22):8011. https://doi.org/10.3390/ma15228011
Chicago/Turabian StyleYang, Yunfei, Junhao Sun, Wei Liu, Peng Hu, Ruimin Zhang, Hexiong Liu, Junyan Gao, and Jinshu Wang. 2022. "Study on the Densification of Osmium by Experiment and First Principle Calculations" Materials 15, no. 22: 8011. https://doi.org/10.3390/ma15228011
APA StyleYang, Y., Sun, J., Liu, W., Hu, P., Zhang, R., Liu, H., Gao, J., & Wang, J. (2022). Study on the Densification of Osmium by Experiment and First Principle Calculations. Materials, 15(22), 8011. https://doi.org/10.3390/ma15228011






