Selected Properties of Two Alternative Plant Fibers: Canola and Sweet Clover Fibers
Abstract
1. Introduction
2. Materials and Methods
2.1. Types of Plant Fibers
2.2. Fiber Characterization
2.2.1. Chemical Compositions
2.2.2. Microstructural Characterization
2.2.3. Thermal Characterization
2.2.4. Contact Angle Measurement
2.2.5. Mechanical Characterization
3. Results and Discussions
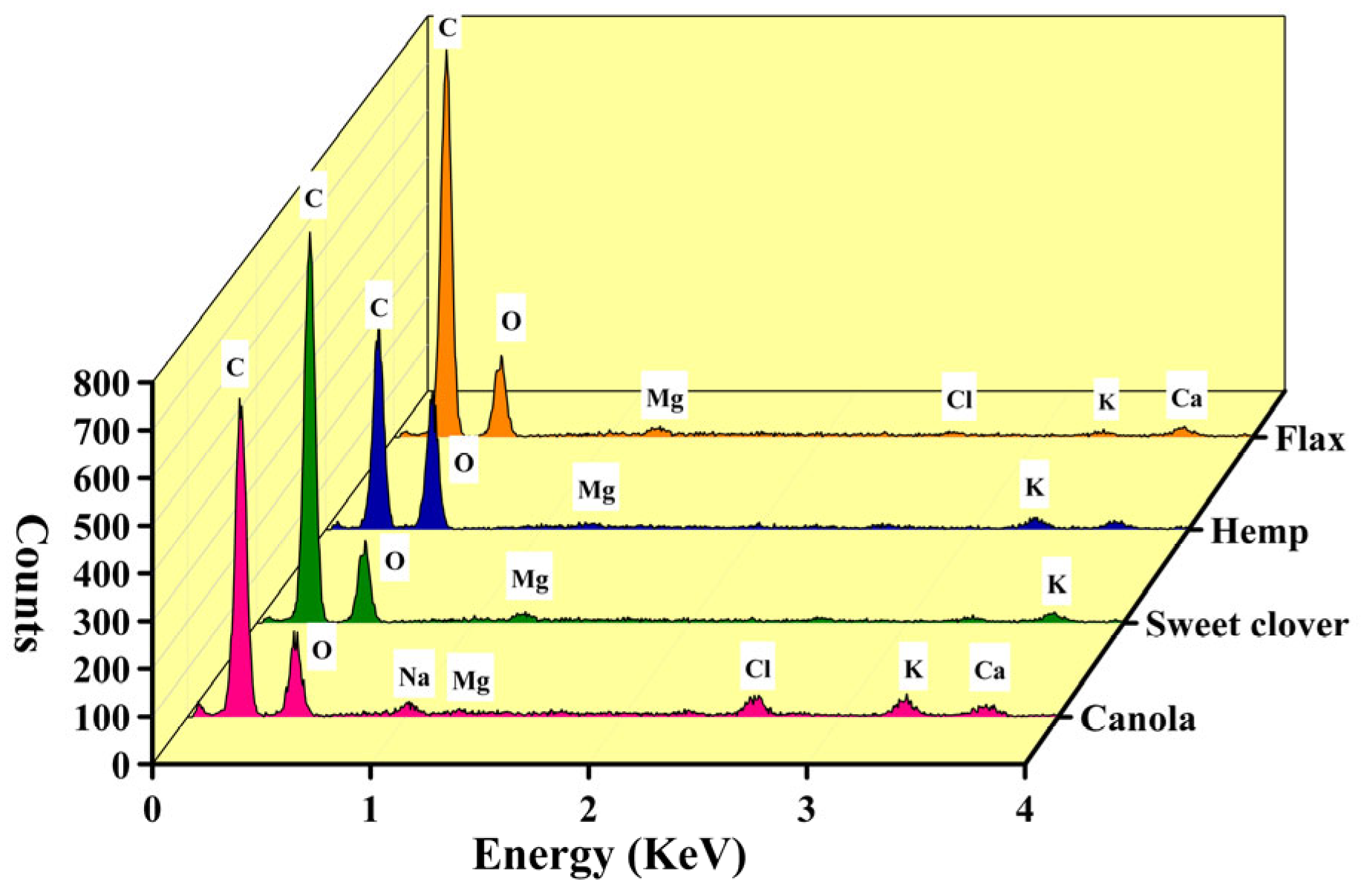
3.1. Chemical Compositions
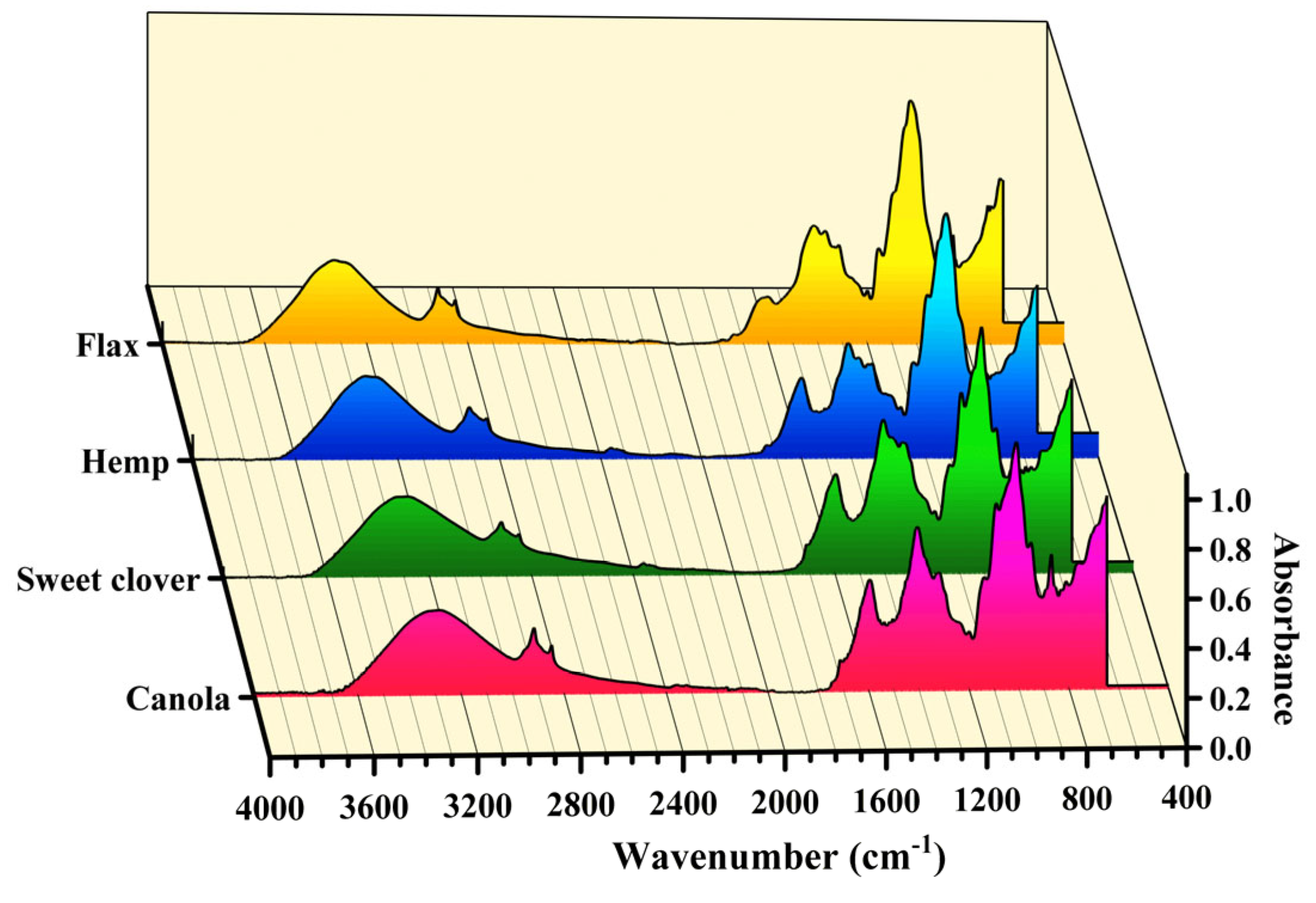
3.2. Microstructure Analysis
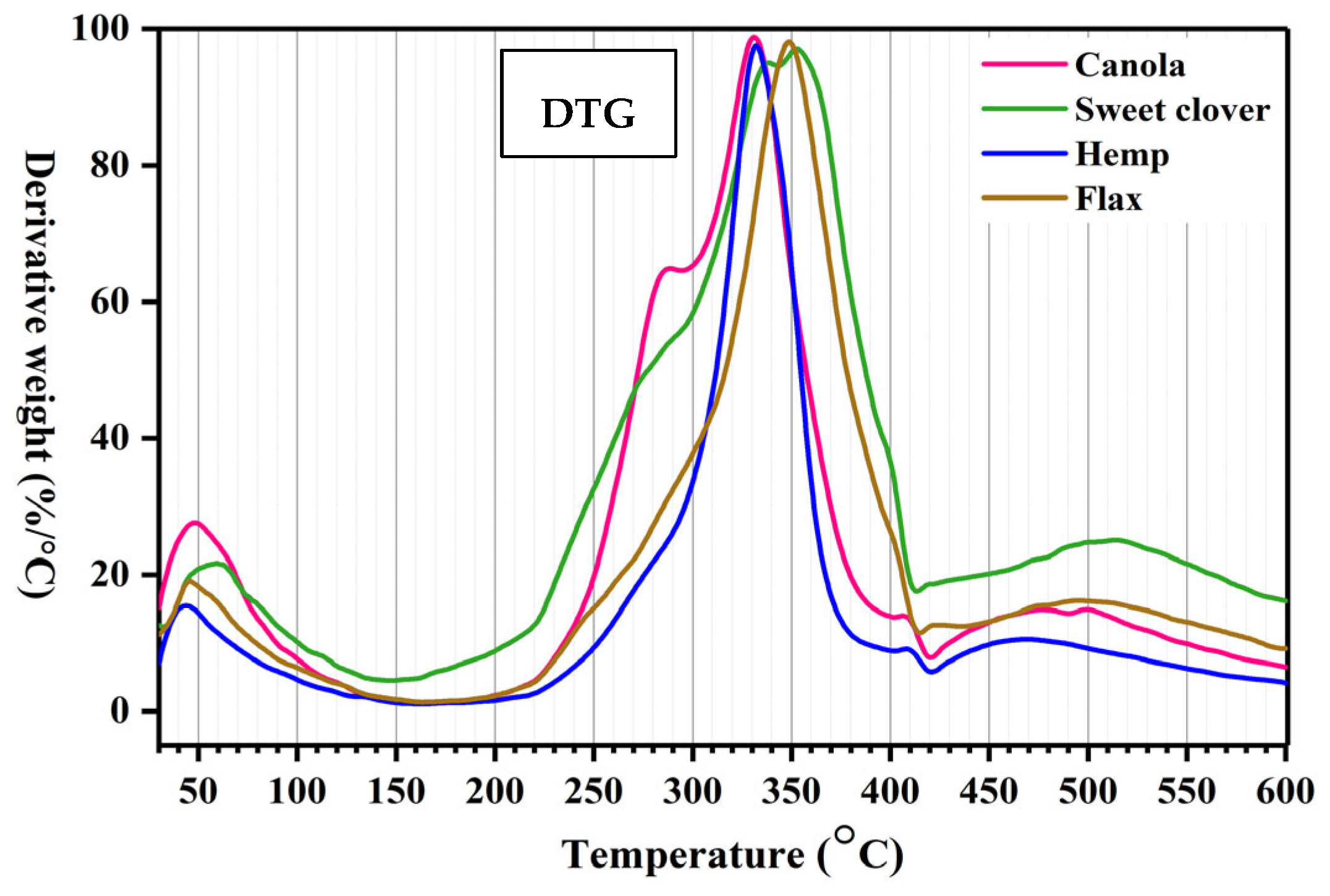
3.3. Thermal Analysis
3.4. Contact Angle Analysis
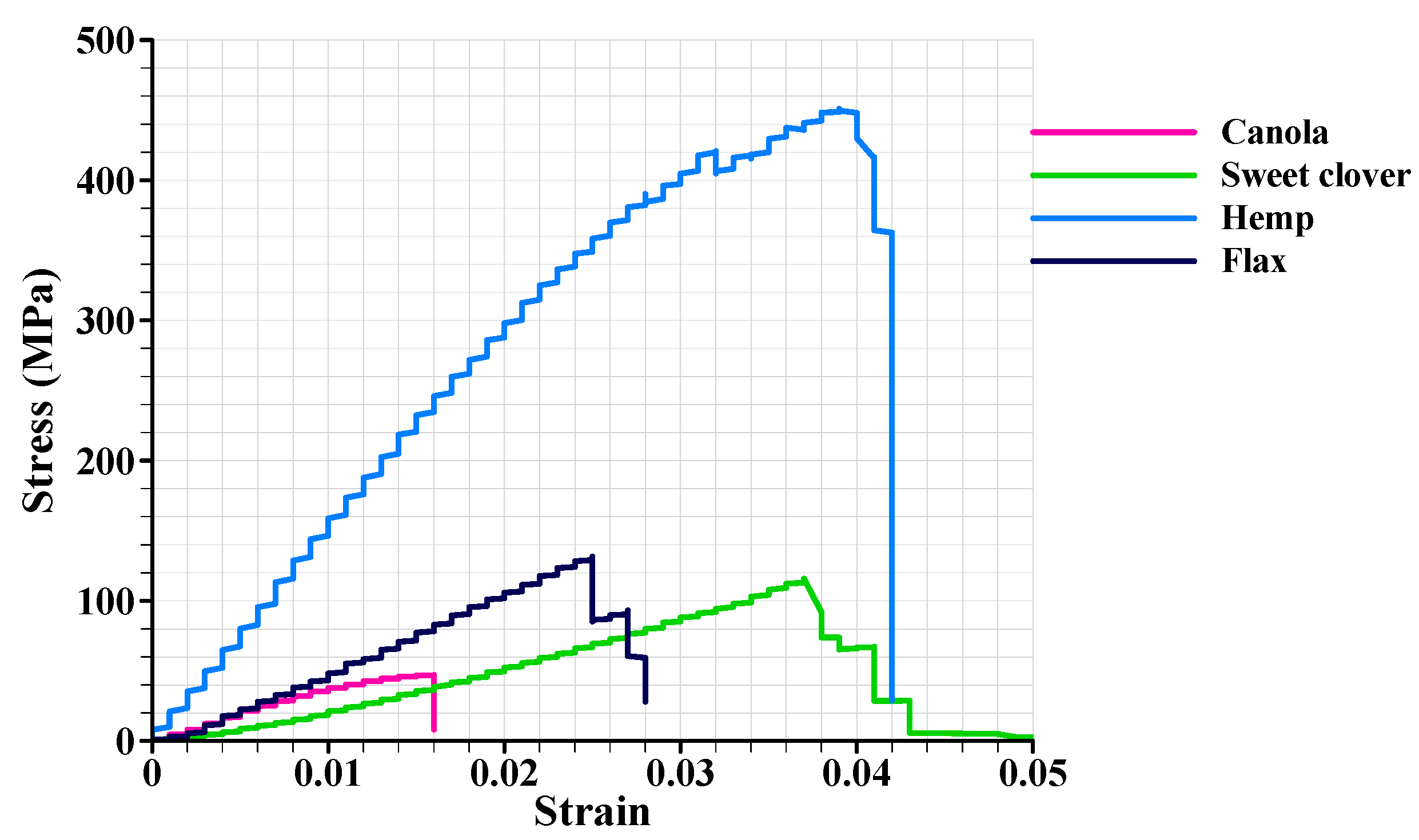
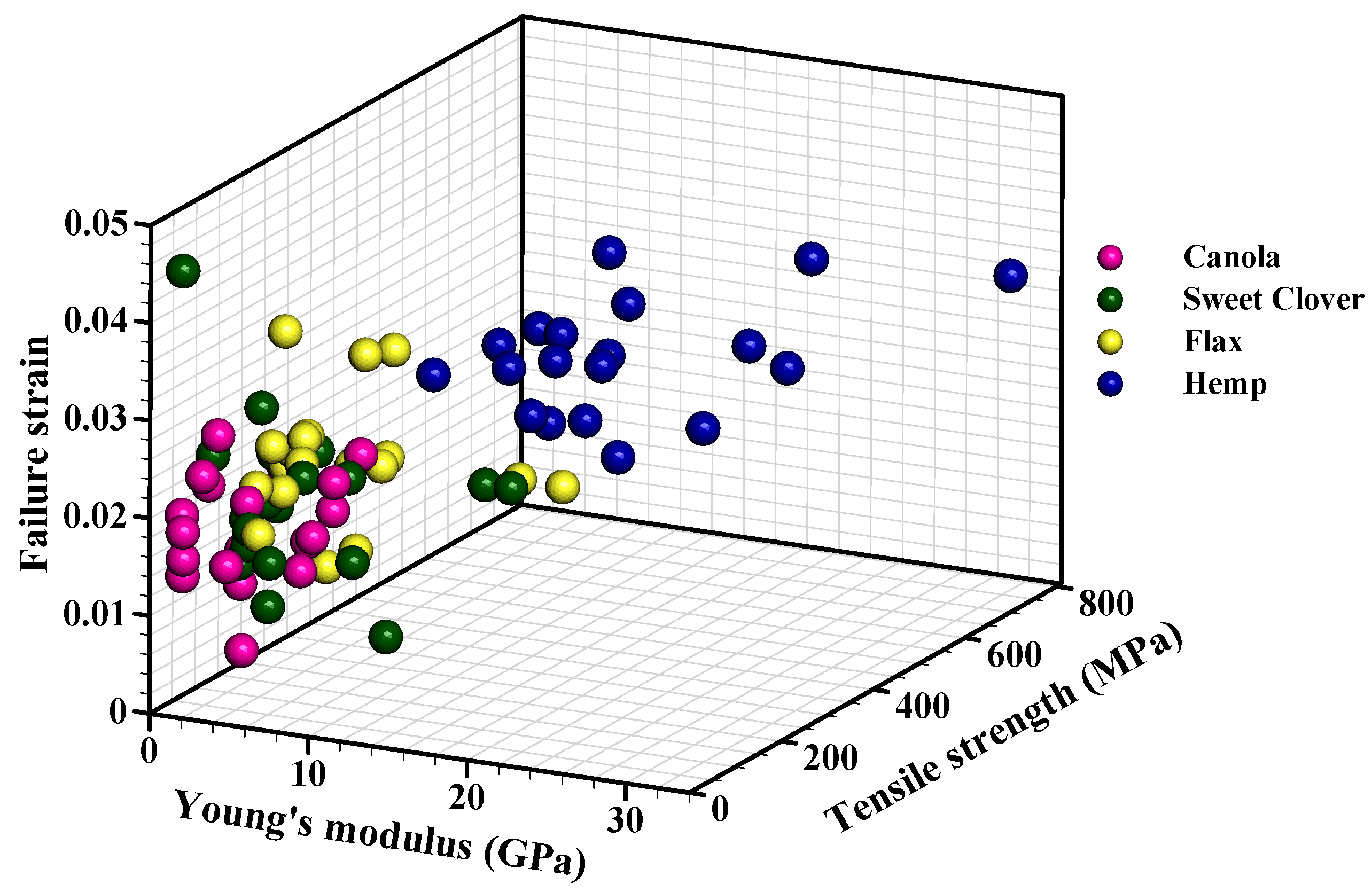
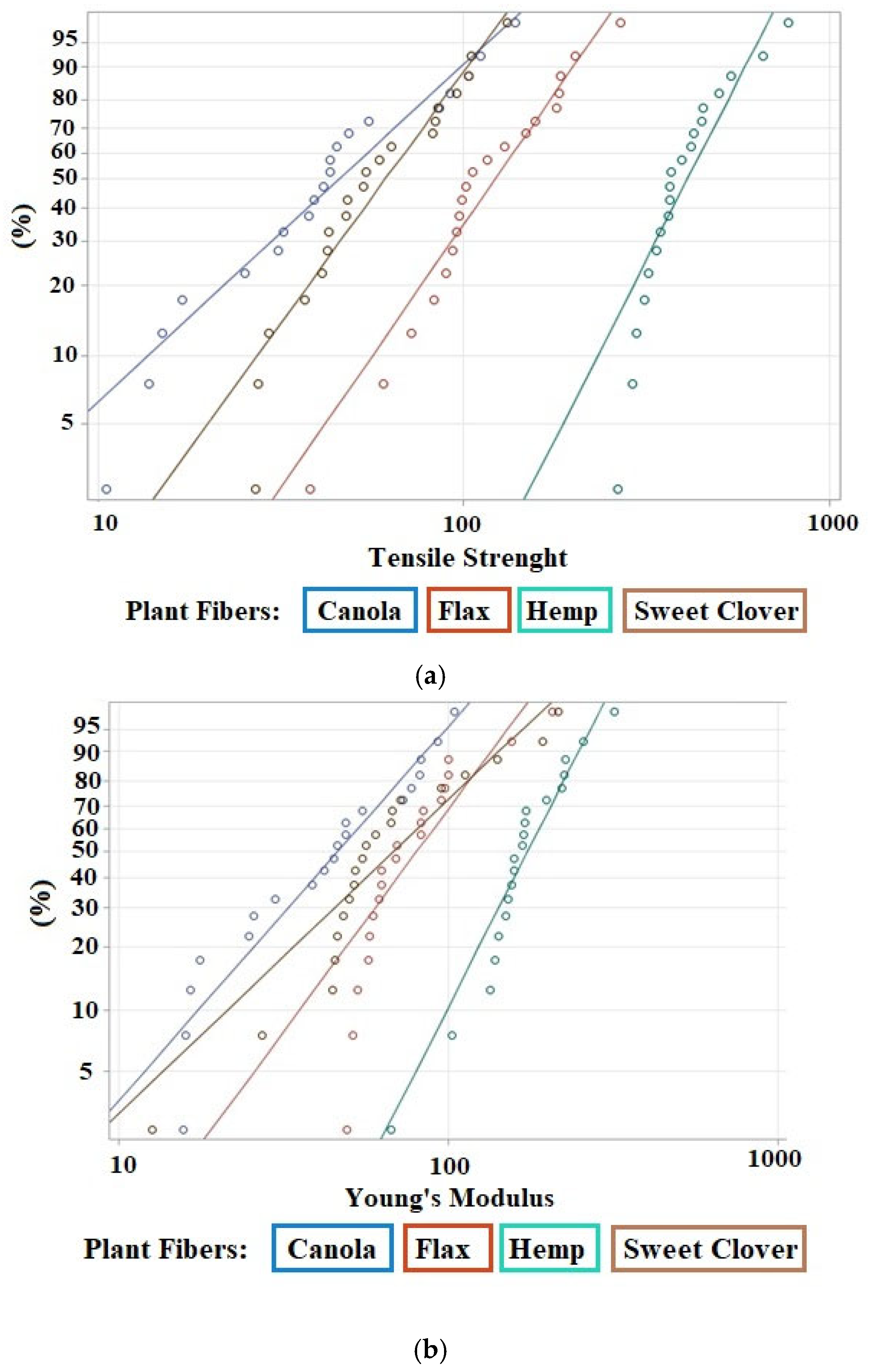
3.5. Mechanical Analysis
4. Conclusions
- The alternative fibers had the same microstructure as the conventional fibers. The main chemical elements, chemical bonds, and crystallinity index of the alternative fibers were highly similar among these fibers. For example, the percentage of cellulose as the main chemical component for all the fibers was in the range of 44 to 60. Besides, the main compositional elements of all the fibers were carbon, oxygen, magnesium, and potassium. The CI index of the alternative fibers was around 62–71% which was higher than for other new fibers introduced by other researchers.
- When heated, the behaviors of all the fibers were similar. The thermal stability of the alternative fibers (220 °C) was close to the conventional fibers (230 °C). This thermal stability is high enough for fibers to be used in the development of commercial bioproducts.
- The contact angles of the alternative fibers were less than 90° showing high surface energy and high wettability which leads to a high IFSS.
- Canola and sweet clover fibers were associated with a low Young’s modulus (5.57 and 8.52 GPa, respectively), and acceptable tensile strength (57.45 and 71.26 MPa, respectively), while hemp and flax fibers were associated with high stiffness (19.36 and 9.39 GPa, respectively), and high strength (456.26 MPa and 141.23 MPa, respectively).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Xu, X.; Cheng, F.; Ramakrishna, S. Study Progress on Inorganic Fibers from Industry Solid Wastes and the Key Factors Determining Their Characteristics. Materials 2022, 15, 7256. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Khairandish, M.I.; Razahi, M.M.; Kumar, R.; Chohan, J.S.; Tiwary, A.; Sharma, S.; Li, C.; Ilyas, R.A.; Asyraf, M.R.M.; et al. Preference Index of Sustainable Natural Fibers in Stone Matrix Asphalt Mixture Using Waste Marble. Materials 2022, 15, 2729. [Google Scholar] [CrossRef] [PubMed]
- Abotbina, W.; Sapuan, S.M.; Ilyas, R.A.; Sultan, M.T.H.; Alkbir, M.F.M.; Sulaiman, S.; Harussani, M.M.; Bayraktar, E. Recent Developments in Cassava (Manihot Esculenta) Based Biocomposites and Their Potential Industrial Applications: A Comprehensive Review. Materials 2022, 15, 6992. [Google Scholar] [CrossRef] [PubMed]
- Prasanthi, P.; Kondapalli, S.B.; Morampudi, N.K.S.R.; Vallabhaneni, V.V.M.; Saxena, K.K.; Mohammed, K.A.; Linul, E.; Prakash, C.; Buddhi, D. Elastic Properties of Jute Fiber Reinforced Polymer Composites with Different Hierarchical Structures. Materials 2022, 15, 7032. [Google Scholar] [CrossRef] [PubMed]
- Sadrmanesh, V.; Chen, Y. Bast Fibres: Structure, Processing, Properties, and Applications. Int. Mater. Rev. 2019, 64, 381–406. [Google Scholar] [CrossRef]
- Szatkowski, P.; Szatkowska, M.; Gralewski, J.; Czechowski, L.; Kedziora, S. Biocomposites with Epoxy Resin Matrix Modified with Ingredients of Natural Origin. Materials 2022, 15, 7167. [Google Scholar] [CrossRef]
- Da Silva Santos, R.; de Souza, A.A.; De Paoli, M.-A.; de Souza, C.M.L. Cardanol–Formaldehyde Thermoset Composites Reinforced with Buriti Fibers: Preparation and Characterization. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1123–1129. [Google Scholar] [CrossRef]
- De Rosa, I.M.; Kenny, J.M.; Puglia, D.; Santulli, C.; Sarasini, F. Morphological, Thermal and Mechanical Characterization of Okra (Abelmoschus Esculentus) Fibres as Potential Reinforcement in Polymer Composites. Compos. Sci. Technol. 2010, 70, 116–122. [Google Scholar] [CrossRef]
- Fiore, V.; Valenza, A.; Di Bella, G. Artichoke (Cynara cardunculus L.) Fibres as Potential Reinforcement of Composite Structures. Compos. Sci. Technol. 2011, 71, 1138–1144. [Google Scholar] [CrossRef]
- Seki, Y.; Sarikanat, M.; Sever, K.; Durmuşkahya, C. Extraction and Properties of Ferula Communis (Chakshir) Fibers as Novel Reinforcement for Composites Materials. Compos. Part B Eng. 2013, 44, 517–523. [Google Scholar] [CrossRef]
- Obi Reddy, K.; Uma Maheswari, C.; Shukla, M.; Song, J.I.; Varada Rajulu, A. Tensile and Structural Characterization of Alkali Treated Borassus Fruit Fine Fibers. Compos. Part B Eng. 2013, 44, 433–438. [Google Scholar] [CrossRef]
- Kılınç, A.Ç.; Köktaş, S.; Seki, Y.; Atagür, M.; Dalmış, R.; Erdoğan, Ü.H.; Göktaş, A.A.; Seydibeyoğlu, M.Ö. Extraction and Investigation of Lightweight and Porous Natural Fiber from Conium Maculatum as a Potential Reinforcement for Composite Materials in Transportation. Compos. Part B Eng. 2018, 140, 1–8. [Google Scholar] [CrossRef]
- Senthamaraikannan, P.; Kathiresan, M. Characterization of Raw and Alkali Treated New Natural Cellulosic Fiber from Coccinia Grandis.L. Carbohydr. Polym. 2018, 186, 332–343. [Google Scholar] [CrossRef]
- Ding, L.; Han, X.; Cao, L.; Chen, Y.; Ling, Z.; Han, J.; He, S.; Jiang, S. Characterization of Natural Fiber from Manau Rattan (Calamus Manan) as a Potential Reinforcement for Polymer-Based Composites. J. Bioresour. Bioprod. 2022, 7, 190–200. [Google Scholar] [CrossRef]
- Statistics Canada. Table 001-0010—Estimated Areas, Yield, Production and Average Farm Price of Principal Field Crops, in Metric Units, Annual; CANSIM: Ottawa, ON, Canada, 2017. [Google Scholar]
- Rigal, M.; Rigal, L.; Vilarem, G.; Vandenbossche, V. Sweet Clovers, a Source of Fibers Adapted for Growth on Wet and Saline Soils. J. Nat. Fibers 2016, 13, 410–422. [Google Scholar] [CrossRef]
- Yun, K.K.; Hossain, M.S.; Han, S.; Seunghak, C. Rheological, Mechanical Properties, and Statistical Significance Analysis of Shotcrete with Various Natural Fibers and Mixing Ratios. Case Stud. Constr. Mater. 2022, 16, e00833. [Google Scholar] [CrossRef]
- Thakur, S. Cleaning Flax Fibre; Extracting and Identifying Antimicrobials and Measuring Water Absorption of Plant Stems. Master’s Thesis, University of Manitoba, Winnipeg, MB, Canada, 2015. [Google Scholar]
- Garside, P.; Wyeth, P. Identification of Cellulosic Fibres by FTIR Spectroscopy—Thread and Single Fibre Analysis by Attenuated Total Reflectance. Stud. Conserv. 2003, 48, 269–275. [Google Scholar] [CrossRef]
- ASTM D3822/D3822M-14; Standard Test Method for Tensile Properties of Single Textile Fibers. ASTM International: West Conshohocken, PA, USA, 2015; pp. 1–10. [CrossRef]
- Sadrmanesh, V.; Chen, Y. Simulation of Tensile Behavior of Plant Fibers Using the Discrete Element Method (DEM). Compos. Part A Appl. Sci. Manuf. 2018, 114, 196–203. [Google Scholar] [CrossRef]
- Sadek, M.A.; Guzman, L.; Chen, Y.; Laguë, C.; Landry, H. Simulation of Tensile Tests of Hemp Fibre Using Discrete Element Method. Agric. Eng. Int. CIGR J. 2014, 16, 126–135. [Google Scholar]
- Kiaei, M.; Mahdavi, S.; Kialashaki, A.; Nemati, M.; Samariha, A.; Saghafi, A. Chemical Composition and Morphological Properties of Canola Plant and Its Potential Application in Pulp and Paper Industry. Cellul. Chem. Technol. 2014, 48, 105–110. [Google Scholar]
- Manimaran, P.; Senthamaraikannan, P.; Sanjay, M.R.; Marichelvam, M.K.; Jawaid, M. Study on Characterization of Furcraea Foetida New Natural Fiber as Composite Reinforcement for Lightweight Applications. Carbohydr. Polym. 2018, 181, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Olsson, A.-M.; Salmén, L. The Association of Water to Cellulose and Hemicellulose in Paper Examined by FTIR Spectroscopy. Carbohydr. Res. 2004, 339, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Konczewicz, W.; Zimniewska, M.; Valera, M.A. The Selection of a Retting Method for the Extraction of Bast Fibers as Response to Challenges in Composite Reinforcement. Text. Res. J. 2018, 88, 2104–2119. [Google Scholar] [CrossRef]
- Sgriccia, N.; Hawley, M.C.; Misra, M. Characterization of Natural Fiber Surfaces and Natural Fiber Composites. Compos. Part A Appl. Sci. Manuf. 2008, 39, 1632–1637. [Google Scholar] [CrossRef]
- Le Troedec, M.; Sedan, D.; Peyratout, C.; Bonnet, J.P.; Smith, A.; Guinebretiere, R.; Gloaguen, V.; Krausz, P. Influence of Various Chemical Treatments on the Composition and Structure of Hemp Fibres. Compos. Part A Appl. Sci. Manuf. 2008, 39, 514–522. [Google Scholar] [CrossRef]
- Fiore, V.; Scalici, T.; Nicoletti, F.; Vitale, G.; Prestipino, M.; Valenza, A. A New Eco-Friendly Chemical Treatment of Natural Fibres: Effect of Sodium Bicarbonate on Properties of Sisal Fibre and Its Epoxy Composites. Compos. Part B Eng. 2016, 85, 150–160. [Google Scholar] [CrossRef]
- Li, Y.; Pickering, K.L. Hemp Fibre Reinforced Composites Using Chelator and Enzyme Treatments. Compos. Sci. Technol. 2008, 68, 3293–3298. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystalline of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Sathishkumar, T.P.; Navaneethakrishnan, P.; Shankar, S.; Rajasekar, R. Characterization of New Cellulose Sansevieria Ehrenbergii Fibers for Polymer Composites. Compos. Interfaces 2013, 20, 575–593. [Google Scholar] [CrossRef]
- Sreenivasan, V.S.; Somasundaram, S.; Ravindran, D.; Manikandan, V.; Narayanasamy, R. Microstructural, Physico-Chemical and Mechanical Characterisation of Sansevieria Cylindrica Fibres-An Exploratory Investigation. Mater. Des. 2010, 32, 453–461. [Google Scholar] [CrossRef]
- Sarikanat, M.; Seki, Y.; Sever, K.; Durmuşkahya, C. Determination of Properties of Althaea officinalis L. (Marshmallow) Fibres as a Potential Plant Fibre in Polymeric Composite Materials. Compos. Part B Eng. 2014, 57, 180–186. [Google Scholar] [CrossRef]
- Efendy, M.G.A.; Pickering, K.L. Comparison of Harakeke with Hemp Fibre as a Potential Reinforcement in Composites. Compos. PART A 2014, 67, 259–267. [Google Scholar] [CrossRef]
- Sathishkumar, T.P.; Navaneethakrishnan, P.; Shankar, S.; Rajasekar, R.; Rajini, N. Characterization of Natural Fiber and Composites—A Review. J. Reinf. Plast. Compos. 2013, 32, 1457–1476. [Google Scholar] [CrossRef]
- Fiore, V.; Scalici, T.; Valenza, A. Characterization of a New Natural Fiber from Arundo Donax L. as Potential Reinforcement of Polymer Composites. Carbohydr. Polym. 2014, 106, 77–83. [Google Scholar] [CrossRef]
- Xue, Y.; Du, Y.; Elder, S.; Wang, K.; Zhang, J. Temperature and Loading Rate Effects on Tensile Properties of Kenaf Bast Fiber Bundles and Composites. Compos. Part B Eng. 2009, 40, 189–196. [Google Scholar] [CrossRef]
- Liu, D.; Han, G.; Huang, J.; Zhang, Y. Composition and Structure Study of Natural Nelumbo Nucifera Fiber. Carbohydr. Polym. 2009, 75, 39–43. [Google Scholar] [CrossRef]
- Andersons, J.; Sparnins, E.; Joffe, R.; Wallstrom, L. Strength Distribution of Elementary Flax Fibres. Compos. Sci. Technol. 2005, 65, 693–702. [Google Scholar] [CrossRef]
- Orue, A.; Jauregi, A.; Unsuain, U.; Labidi, J.; Eceiza, A.; Arbelaiz, A. The Effect of Alkaline and Silane Treatments on Mechanical Properties and Breakage of Sisal Fibers and Poly(Lactic Acid)/Sisal Fiber Composites. Compos. Part A 2016, 84, 186–195. [Google Scholar] [CrossRef]
- Weibull, W. A Statistical Theory of the Strength of Materials; Generalstabens Litografiska Anstalts Förlag: Stockholm, Sweden, 1939. [Google Scholar]
- Komuraiah, A.; Kumar, N.S.; Prasad, B.D. Chemical Composition of Natural Fibers and Its Influence on Their Mechanical Properties. Mech. Compos. Mater. 2014, 50, 359–376. [Google Scholar] [CrossRef]



| Fiber | Cellulose (%) | Hemicellulose (%) | Lignin (%) |
|---|---|---|---|
| Canola | 44 | 6 | 19.21 |
| Sweet clover | 53 | 16.77 | 13.6 |
| Flax | 60 | 7 | 27 |
| Hemp | 60.2 | 14.8 | 11.2 |
| Fiber | Advancing Angle (°) | Receding Angle (°) |
|---|---|---|
| Canola | 79.17 | 29.36 |
| Sweet clover | 71.63 | 52.13 |
| Flax | 81.42 | 64.96 |
| Hemp | 77.36 | 59.89 |
| Property | Parameter | Canola | Sweet Clover | Flax | Hemp |
|---|---|---|---|---|---|
| Tensile strength, MPa | Shape parameter | 1.57 | 2.34 | 2.43 | 3.32 |
| Scale parameter | 57.45 | 71.26 | 141.23 | 456.26 | |
| Mean | 51.61 | 63.14 | 125.24 | 409.40 | |
| Standard deviation | 33.64 | 28.71 | 54.79 | 135.85 | |
| Young’s modulus, GPa | Shape parameter | 1.93 | 1.62 | 2.30 | 3.34 |
| Scale parameter | 5.57 | 8.52 | 9.39 | 19.36 | |
| Mean | 4.94 | 7.63 | 8.32 | 17.38 | |
| Standard deviation | 2.66 | 4.82 | 3.83 | 5.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadrmanesh, V.; Chen, Y. Selected Properties of Two Alternative Plant Fibers: Canola and Sweet Clover Fibers. Materials 2022, 15, 7877. https://doi.org/10.3390/ma15227877
Sadrmanesh V, Chen Y. Selected Properties of Two Alternative Plant Fibers: Canola and Sweet Clover Fibers. Materials. 2022; 15(22):7877. https://doi.org/10.3390/ma15227877
Chicago/Turabian StyleSadrmanesh, Vahid, and Ying Chen. 2022. "Selected Properties of Two Alternative Plant Fibers: Canola and Sweet Clover Fibers" Materials 15, no. 22: 7877. https://doi.org/10.3390/ma15227877
APA StyleSadrmanesh, V., & Chen, Y. (2022). Selected Properties of Two Alternative Plant Fibers: Canola and Sweet Clover Fibers. Materials, 15(22), 7877. https://doi.org/10.3390/ma15227877





