Engineering the Optical Properties of CsPbBr3 Nanoplatelets through Cd2+ Doping
Abstract
1. Introduction
2. Materials and Methods
3. Results
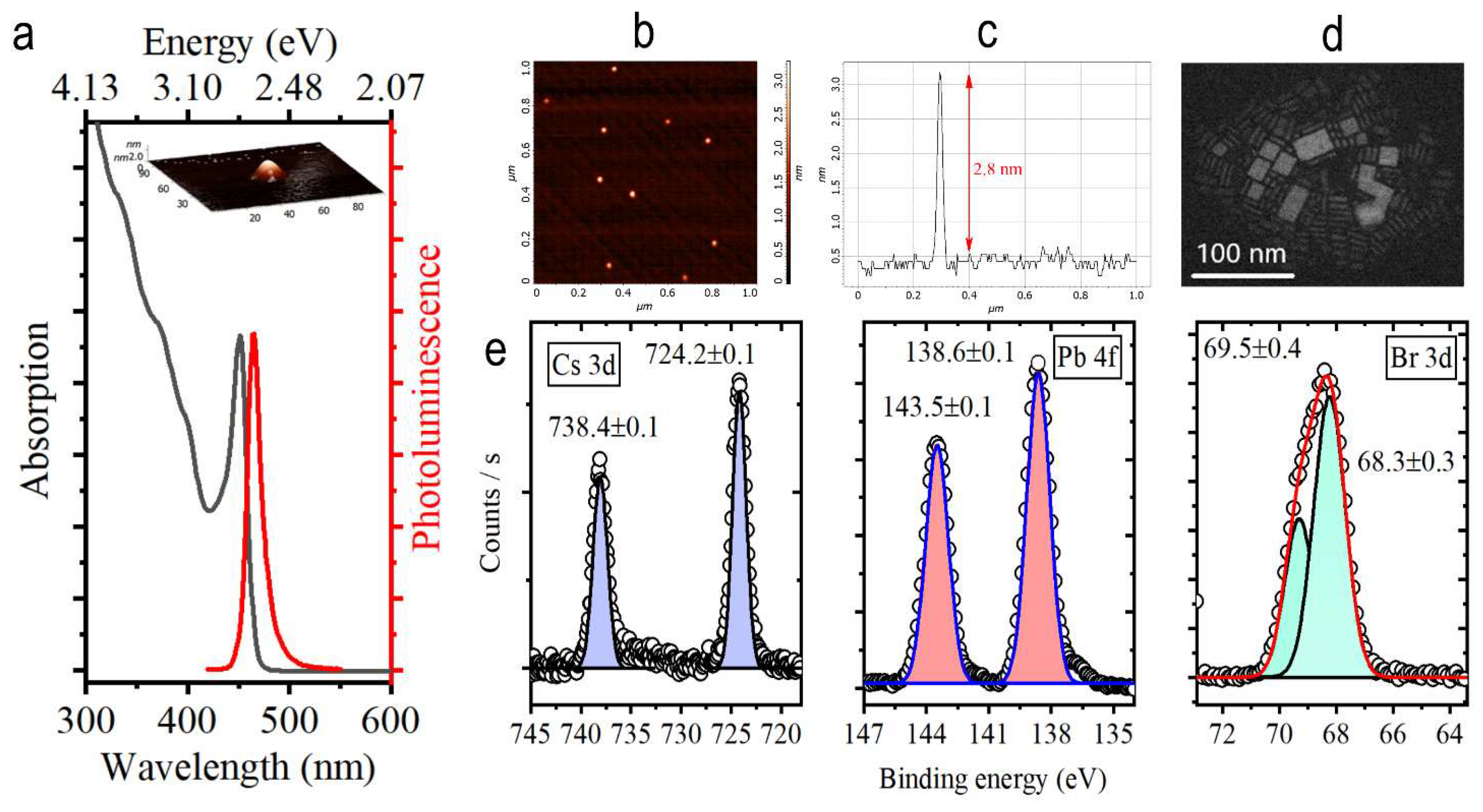
3.1. CsPbBr3 Nanoplatelets
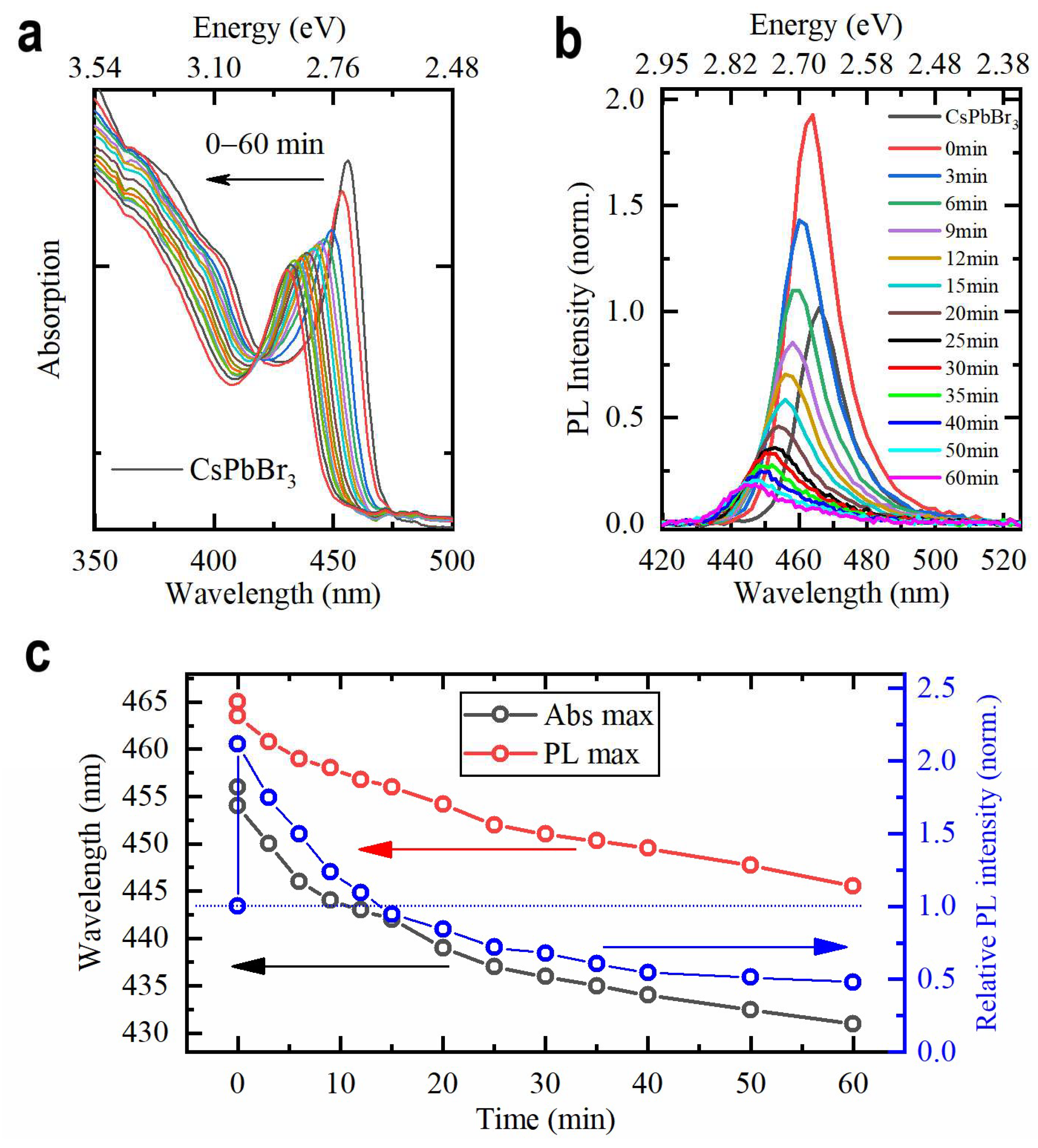
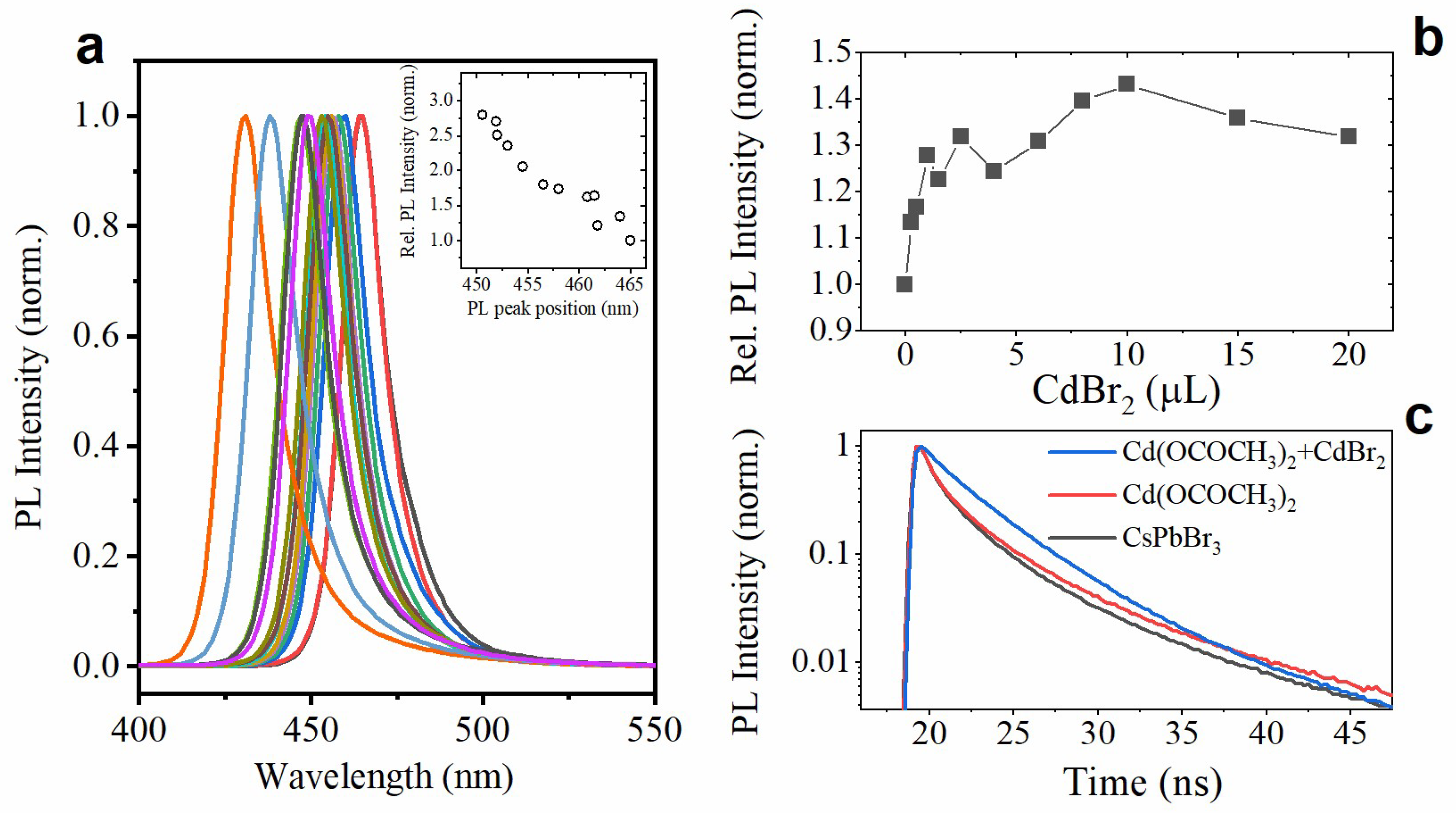
3.2. Doping with CdBr2
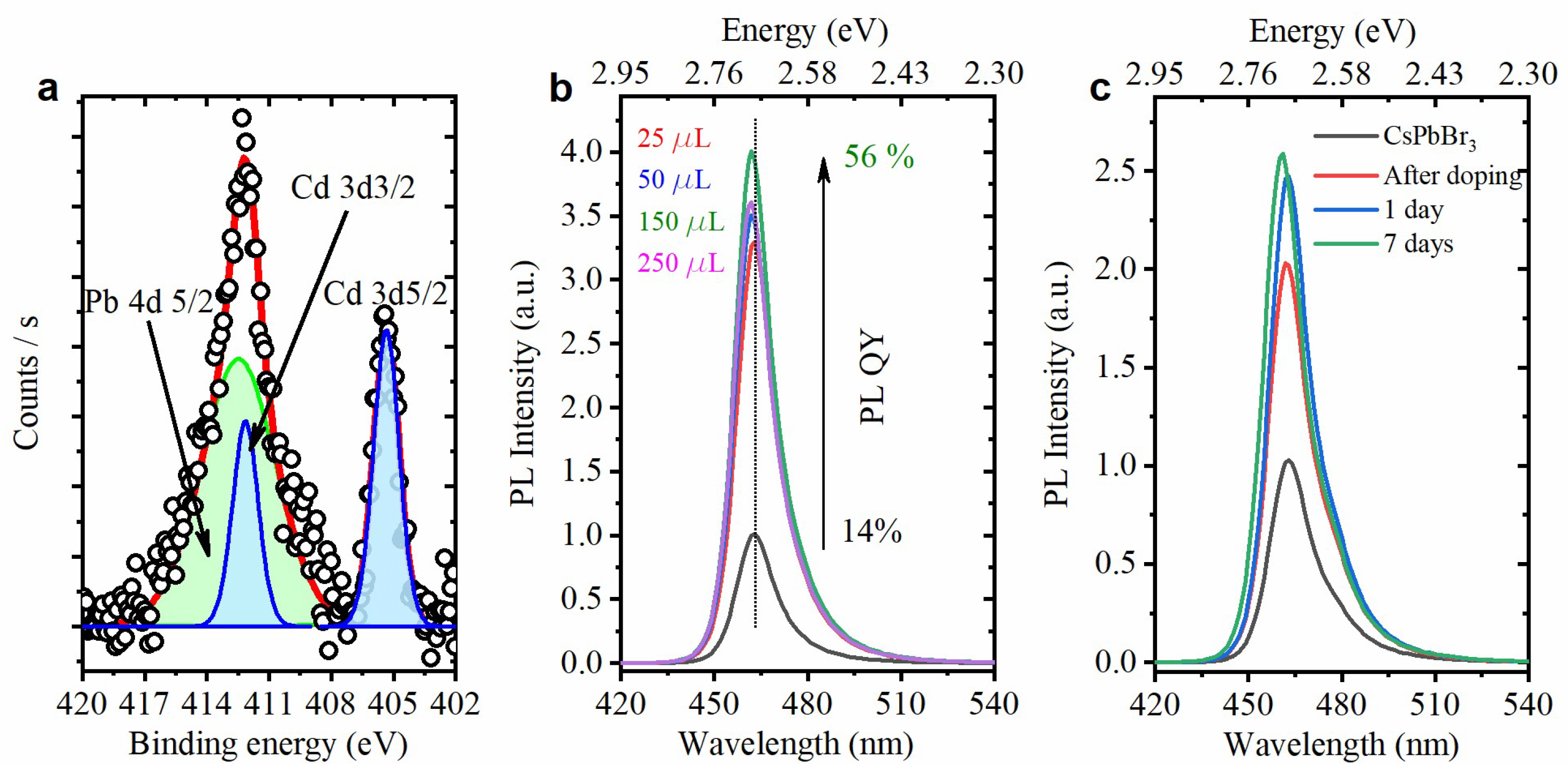
3.3. Doping with Cd(OCOCH3)2
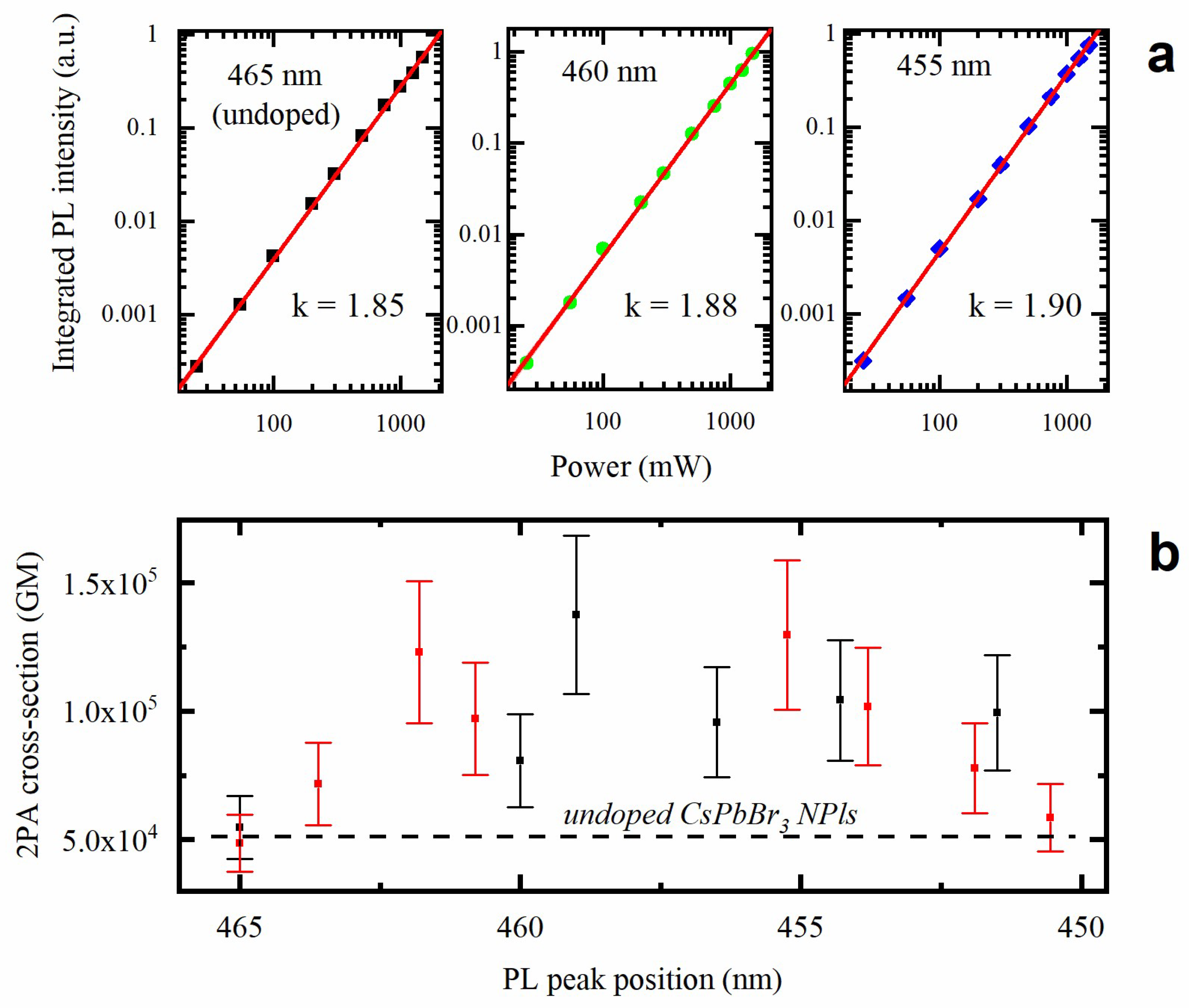
3.4. Two-Photon-Induced Photoluminescence
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weidman, M.C.; Goodman, A.J.; Tisdale, W.A. Colloidal Halide Perovskite Nanoplatelets: An Exciting New Class of Semiconductor Nanomaterials. Chem. Mater. 2017, 29, 5019–5030. [Google Scholar] [CrossRef]
- Otero-Martínez, C.; Ye, J.; Sung, J.; Pastoriza-Santos, I.; Pérez-Juste, J.; Xia, Z.; Rao, A.; Hoye, R.L.Z.; Polavarapu, L. Colloidal Metal-Halide Perovskite Nanoplatelets: Thickness-Controlled Synthesis, Properties, and Application in Light-Emitting Diodes. Adv. Mater. 2022, 34, e2107105. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P.; Arveson, S.M.; Tisdale, W.A. Colloidal Organohalide Perovskite Nanoplatelets Exhibiting Quantum Confinement. J. Phys. Chem. Lett. 2015, 6, 1911–1916. [Google Scholar] [CrossRef] [PubMed]
- Sichert, J.A.; Tong, Y.; Mutz, N.; Vollmer, M.; Fischer, S.; Milowska, K.Z.; García Cortadella, R.; Nickel, B.; Cardenas-Daw, C.; Stolarczyk, J.K.; et al. Quantum Size Effect in Organometal Halide Perovskite Nanoplatelets. Nano Lett. 2015, 15, 6521–6527. [Google Scholar] [CrossRef]
- Bekenstein, Y.; Koscher, B.A.; Eaton, S.W.; Yang, P.; Alivisatos, A.P. Highly Luminescent Colloidal Nanoplates of Perovskite Cesium Lead Halide and Their Oriented Assemblies. J. Am. Chem. Soc. 2015, 137, 16008–16011. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; Motti, S.G.; Srimath Kandada, A.R.; Mosconi, E.; D’Innocenzo, V.; Bertoni, G.; Marras, S.; Kamino, B.A.; Miranda, L.; De Angelis, F.; et al. Solution Synthesis Approach to Colloidal Cesium Lead Halide Perovskite Nanoplatelets with Monolayer-Level Thickness Control. J. Am. Chem. Soc. 2016, 138, 1010–1016. [Google Scholar] [CrossRef]
- Levchuk, I.; Osvet, A.; Tang, X.; Brandl, M.; Perea, J.D.; Hoegl, F.; Matt, G.J.; Hock, R.; Batentschuk, M.; Brabec, C.J. Brightly Luminescent and Color-Tunable Formamidinium Lead Halide Perovskite FAPbX3 (X = Cl, Br, I) Colloidal Nanocrystals. Nano Lett. 2017, 17, 2765–2770. [Google Scholar] [CrossRef]
- Zhai, W.; Lin, J.; Li, Q.; Zheng, K.; Huang, Y.; Yao, Y.; He, X.; Li, L.; Yu, C.; Liu, C.; et al. Solvothermal Synthesis of Ultrathin Cesium Lead Halide Perovskite Nanoplatelets with Tunable Lateral Sizes and Their Reversible Transformation into Cs4PbBr6 Nanocrystals. Chem. Mater. 2018, 30, 3714–3721. [Google Scholar] [CrossRef]
- Tong, Y.; Bladt, E.; Aygüler, M.F.; Manzi, A.; Milowska, K.Z.; Hintermayr, V.A.; Docampo, P.; Bals, S.; Urban, A.S.; Polavarapu, L.; et al. Highly Luminescent Cesium Lead Halide Perovskite Nanocrystals with Tunable Composition and Thickness by Ultrasonication. Angew. Chemie-Int. Ed. 2016, 55, 13887–13892. [Google Scholar] [CrossRef]
- Pan, Q.; Hu, H.; Zou, Y.; Chen, M.; Wu, L.; Yang, D.; Yuan, X.; Fan, J.; Sun, B.; Zhang, Q. Microwave-assisted synthesis of high-quality “all-inorganic” CsPbX3 (X = Cl, Br, I) perovskite nanocrystals and their application in light emitting diodes. J. Mater. Chem. C 2017, 5, 10947–10954. [Google Scholar] [CrossRef]
- Chen, S.; Shi, G.; Chen, S.; Shi, G.Q. Two-Dimensional Materials for Halide Perovskite-Based Optoelectronic Devices. Adv. Mater. 2017, 29, 1605448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Y.; Yang, Z.; Liu, S. Two dimensional metal halide perovskites: Promising candidates for light-emitting diodes. J. Energy Chem. 2019, 37, 97–110. [Google Scholar] [CrossRef]
- Zhou, F.; Ran, X.; Fan, D.; Lu, S.; Ji, W. Perovskites: Multiphoton Absorption and Applications. Adv. Opt. Mater. 2021, 9, 2100292. [Google Scholar] [CrossRef]
- Shen, W.; Chen, J.; Wu, J.; Li, X.; Zeng, H. Nonlinear Optics in Lead Halide Perovskites: Mechanisms and Applications. ACS Photonics 2021, 8, 113–124. [Google Scholar] [CrossRef]
- Yao, J.; Ge, J.; Wang, K.; Zhang, G.; Zhu, B.; Chen, C.; Zhang, Q.; Luo, Y.; Yu, S.; Yao, H. Few-Nanometer-Sized α—CsPbI 3 Quantum Dots Enabled by Strontium Substitution and Iodide Passivation for Efficient Red-Light Emitting Diodes. J. Am. Chem. Soc. 2019, 141, 2069–2079. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, Y.; Kershaw, S.V.; Li, T.; Wang, C.; Zhang, X.; Wang, W.; Li, D.; Wang, Y.; Lu, M.; et al. Zn-Alloyed CsPbI 3 Nanocrystals for Highly Efficient Perovskite Light-Emitting Devices. Nano Lett. 2019, 19, 1552–1559. [Google Scholar] [CrossRef]
- Zou, S.; Liu, Y.; Li, J.; Liu, C.; Feng, R.; Jiang, F.; Li, Y.; Song, J.; Zeng, H.; Hong, M.; et al. Stabilizing Cesium Lead Halide Perovskite Lattice through Mn(II) Substitution for Air-Stable Light-Emitting Diodes. J. Am. Chem. Soc. 2017, 139, 11443–11450. [Google Scholar] [CrossRef]
- Xu, K.; Lin, C.C.; Xie, X.; Meijerink, A. Efficient and Stable Luminescence from Mn2+ in Core and Core-Isocrystalline Shell CsPbCl3 Perovskite Nanocrystals. Chem. Mater. 2017, 29, 4265–4272. [Google Scholar] [CrossRef]
- Liu, F.; Ding, C.; Zhang, Y.; Ripolles, T.S.; Kamisaka, T.; Toyoda, T.; Hayase, S.; Minemoto, T.; Yoshino, K.; Dai, S.; et al. Colloidal Synthesis of Air-Stable Alloyed CsSn1-xPbxI3 Perovskite Nanocrystals for Use in Solar Cells. J. Am. Chem. Soc. 2017, 139, 16708–16719. [Google Scholar] [CrossRef]
- Mondal, N.; De, A.; Samanta, A. Achieving Near-Unity Photoluminescence Efficiency for Blue-Violet-Emitting Perovskite Nanocrystals. ACS Energy Lett. 2019, 4, 32–39. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, X.; Tu, D.; Gong, Z.; Li, R.; Yang, Y.; Zheng, W.; Xu, J.; Deng, S.; Chen, X. Engineering the Bandgap and Surface Structure of CsPbCl 3 Nanocrystals to Achieve Efficient Ultraviolet Luminescence. Angew. Chemie Int. Ed. 2021, 60, 9693–9698. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Lin, Q.; Li, H.; Wu, K.; Robel, I.; Pietryga, J.M.; Klimov, V.I. Mn2+-Doped Lead Halide Perovskite Nanocrystals with Dual-Color Emission Controlled by Halide Content. J. Am. Chem. Soc. 2016, 138, 14954–14961. [Google Scholar] [CrossRef] [PubMed]
- Cohen, T.A.; Milstein, T.J.; Kroupa, D.M.; Mackenzie, J.D.; Luscombe, C.K.; Gamelin, D.R. Quantum-cutting Yb3+-doped perovskite nanocrystals for monolithic bilayer luminescent solar concentrators. J. Mater. Chem. A 2019, 7, 9279–9288. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, D.; Pan, G.; Chen, X.; Li, D.; Xu, W.; Bai, X.; Song, H. Cerium and Ytterbium Codoped Halide Perovskite Quantum Dots: A Novel and Efficient Downconverter for Improving the Performance of Silicon Solar Cells. Adv. Mater. 2017, 29, 1704149. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.H.; Biesold-McGee, G.V.; Liu, Y.; Kang, Z.; Lin, Z. Doping and ion substitution in colloidal metal halide perovskite nanocrystals. Chem. Soc. Rev. 2020, 49, 4953–5007. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Hong, M. Cation-doping matters in caesium lead halide perovskite nanocrystals: From physicochemical fundamentals to optoelectronic applications. Nanoscale 2020, 12, 12228–12248. [Google Scholar] [CrossRef]
- Liu, M.; Grandhi, G.K.; Matta, S.; Mokurala, K.; Litvin, A.; Russo, S.; Vivo, P. Halide Perovskite Nanocrystal Emitters. Adv. Photonics Res. 2021, 2, 2000118. [Google Scholar] [CrossRef]
- Mir, W.J.; Jagadeeswararao, M.; Das, S.; Nag, A. Colloidal Mn-doped cesium lead halide perovskite nanoplatelets. ACS Energy Lett. 2017, 2, 537–543. [Google Scholar] [CrossRef]
- Li, Z.J.; Hofman, E.; Davis, A.H.; Khammang, A.; Wright, J.T.; Dzikovski, B.; Meulenberg, R.W.; Zheng, W. Complete Dopant Substitution by Spinodal Decomposition in Mn-Doped Two-Dimensional CsPbCl3 Nanoplatelets. Chem. Mater. 2018, 30, 6400–6409. [Google Scholar] [CrossRef]
- He, T.; Li, J.; Qiu, X.; Xiao, S.; Lin, X. Superior multiphoton absorption properties in nanoplatelets. Photonics Res. 2018, 6, 1021–1027. [Google Scholar] [CrossRef]
- Wu, L.; Wang, Y.; Kurashvili, M.; Dey, A.; Cao, M.; Döblinger, M.; Zhang, Q.; Feldmann, J.; Huang, H.; Debnath, T. Interfacial Manganese-Doping in CsPbBr 3 Nanoplatelets by Employing a Molecular Shuttle. Angew. Chemie Int. Ed. 2022, 61, e202115852. [Google Scholar]
- Cao, Q.; Ilyas, A.; Zhang, S.; Ju, Z.; Sun, F.; Liu, T.; Yang, Y.; Lu, Y.; Liu, X.; Deng, R. Lanthanide-doping enables kinetically controlled growth of deep-blue two-monolayer halide perovskite nanoplatelets. Nanoscale 2021, 13, 11552–11560. [Google Scholar] [CrossRef]
- Wang, H.; Ye, F.; Sun, J.; Wang, Z.; Zhang, C.; Qian, J.; Zhang, X.; Choy, W.C.H.; Sun, X.W.; Wang, K.; et al. Efficient CsPbBr 3 Nanoplatelet-Based Blue Light-Emitting Diodes Enabled by Engineered Surface Ligands. ACS Energy Lett. 2022, 7, 1137–1145. [Google Scholar] [CrossRef]
- Liu, H.; Worku, M.; Mondal, A.; Shonde, T.B.; Chaaban, M.; Ben-Akacha, A.; Lee, S.; Gonzalez, F.; Olasupo, O.; Lin, X.; et al. Efficient and Stable Blue Light Emitting Diodes Based on CsPbBr 3 Nanoplatelets with Surface Passivation by a Multifunctional Organic Sulfate. Adv. Energy Mater. 2022, 2201605. [Google Scholar] [CrossRef]
- Van der Stam, W.; Geuchies, J.J.; Altantzis, T.; Van Den Bos, K.H.W.; Meeldijk, J.D.; Van Aert, S.; Bals, S.; Vanmaekelbergh, D.; De Mello Donega, C. Highly Emissive Divalent-Ion-Doped Colloidal CsPb1-xMxBr3 Perovskite Nanocrystals through Cation Exchange. J. Am. Chem. Soc. 2017, 139, 4087–4097. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Zhao, Y.; Shi, W.; Yang, P. Postsynthetic Surface-Treatment of CsPbX3(X = Cl, Br, or I) Nanocrystals via CdX2Precursor Solution toward High Photoluminescence Quantum Yield. Langmuir 2021, 37, 1183–1193. [Google Scholar] [CrossRef]
- Skurlov, I.D.; Yin, W.; Ismagilov, A.O.; Tcypkin, A.N.; Hua, H.; Wang, H.; Zhang, X.; Litvin, A.P.; Zheng, W. Improved one-and multiple-photon excited photoluminescence from cd2+-doped cspbbr3 perovskite ncs. Nanomaterials 2022, 12, 151. [Google Scholar] [CrossRef]
- Zhao, Y.; Shen, C.; Ding, L.; Liu, J.; Xiang, W.; Liang, X. Novel B-site Cd2+ doped CsPbBr3 quantum dot glass toward strong fluorescence and high stability for wLED. Opt. Mater. 2020, 107, 110046. [Google Scholar] [CrossRef]
- Yang, D.; Zou, Y.; Li, P.; Liu, Q.; Wu, L.; Hu, H.; Xu, Y.; Sun, B.; Zhang, Q.; Lee, S. Nano Energy Large-scale synthesis of ultrathin cesium lead bromide perovskite nanoplates with precisely tunable dimensions and their application in blue light- emitting diodes. Nano Energy 2018, 47, 235–242. [Google Scholar] [CrossRef]
- Borri, C.; Calisi, N.; Galvanetto, E.; Falsini, N.; Biccari, F.; Vinattieri, A.; Cucinotta, G.; Caporali, S. First Proof-of-Principle of Inorganic Lead Halide Perovskites Deposition by Magnetron-Sputtering. Nanomaterials 2019, 10, 60. [Google Scholar] [CrossRef]
- Jaffe, A.; Lin, Y.; Karunadasa, H.I. Halide Perovskites under Pressure: Accessing New Properties through Lattice Compression. ACS Energy Lett. 2017, 2, 1549–1555. [Google Scholar] [CrossRef]
- Melnikov, A.S.; Serdobintsev, P.Y.; Vedyaykin, A.D.; Khodorkovskii, M.A. Two-photon absorption cross section for Coumarins 102, 153 and 307. J. Phys. Conf. Ser. 2017, 917, 062029. [Google Scholar] [CrossRef]
- Ahmed, G.H.; Liu, Y.; Bravić, I.; Ng, X.; Heckelmann, I.; Narayanan, P.; Fernández, M.S.; Monserrat, B.; Congreve, D.N.; Feldmann, S. Luminescence Enhancement Due to Symmetry Breaking in Doped Halide Perovskite Nanocrystals. J. Am. Chem. Soc. 2022, 144, 15862–15870. [Google Scholar] [CrossRef]
| Doping Type | Doping Rate | PL Peak Position Tuning | Stability | Passivation |
|---|---|---|---|---|
| CdBr2, diluted | High | Yes | Low | Moderate |
| CdBr2, concentrated | Low | No | High | High |
| CdBr2, ligands | Low | No | High | High |
| Cd(OCOCH3)2 | Moderate | Yes | Moderate | Moderate |
| Double-step | Moderate | Yes | High | High |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skurlov, I.D.; Sokolova, A.V.; Tatarinov, D.A.; Parfenov, P.S.; Kurshanov, D.A.; Ismagilov, A.O.; Koroleva, A.V.; Danilov, D.V.; Zhizhin, E.V.; Mikushev, S.V.; et al. Engineering the Optical Properties of CsPbBr3 Nanoplatelets through Cd2+ Doping. Materials 2022, 15, 7676. https://doi.org/10.3390/ma15217676
Skurlov ID, Sokolova AV, Tatarinov DA, Parfenov PS, Kurshanov DA, Ismagilov AO, Koroleva AV, Danilov DV, Zhizhin EV, Mikushev SV, et al. Engineering the Optical Properties of CsPbBr3 Nanoplatelets through Cd2+ Doping. Materials. 2022; 15(21):7676. https://doi.org/10.3390/ma15217676
Chicago/Turabian StyleSkurlov, Ivan D., Anastasiia V. Sokolova, Danila A. Tatarinov, Peter S. Parfenov, Danil A. Kurshanov, Azat O. Ismagilov, Aleksandra V. Koroleva, Denis V. Danilov, Evgeniy V. Zhizhin, Sergey V. Mikushev, and et al. 2022. "Engineering the Optical Properties of CsPbBr3 Nanoplatelets through Cd2+ Doping" Materials 15, no. 21: 7676. https://doi.org/10.3390/ma15217676
APA StyleSkurlov, I. D., Sokolova, A. V., Tatarinov, D. A., Parfenov, P. S., Kurshanov, D. A., Ismagilov, A. O., Koroleva, A. V., Danilov, D. V., Zhizhin, E. V., Mikushev, S. V., Tcypkin, A. N., Fedorov, A. V., & Litvin, A. P. (2022). Engineering the Optical Properties of CsPbBr3 Nanoplatelets through Cd2+ Doping. Materials, 15(21), 7676. https://doi.org/10.3390/ma15217676








