The First Examples of [3+2] Cycloadditions with the Participation of (E)-3,3,3-Tribromo-1-Nitroprop-1-Ene
Abstract
1. Introduction
2. Results and Discussion
3. Methods and Procedures
3.1. Experimental
3.1.1. Analytical Techniques
3.1.2. Materials
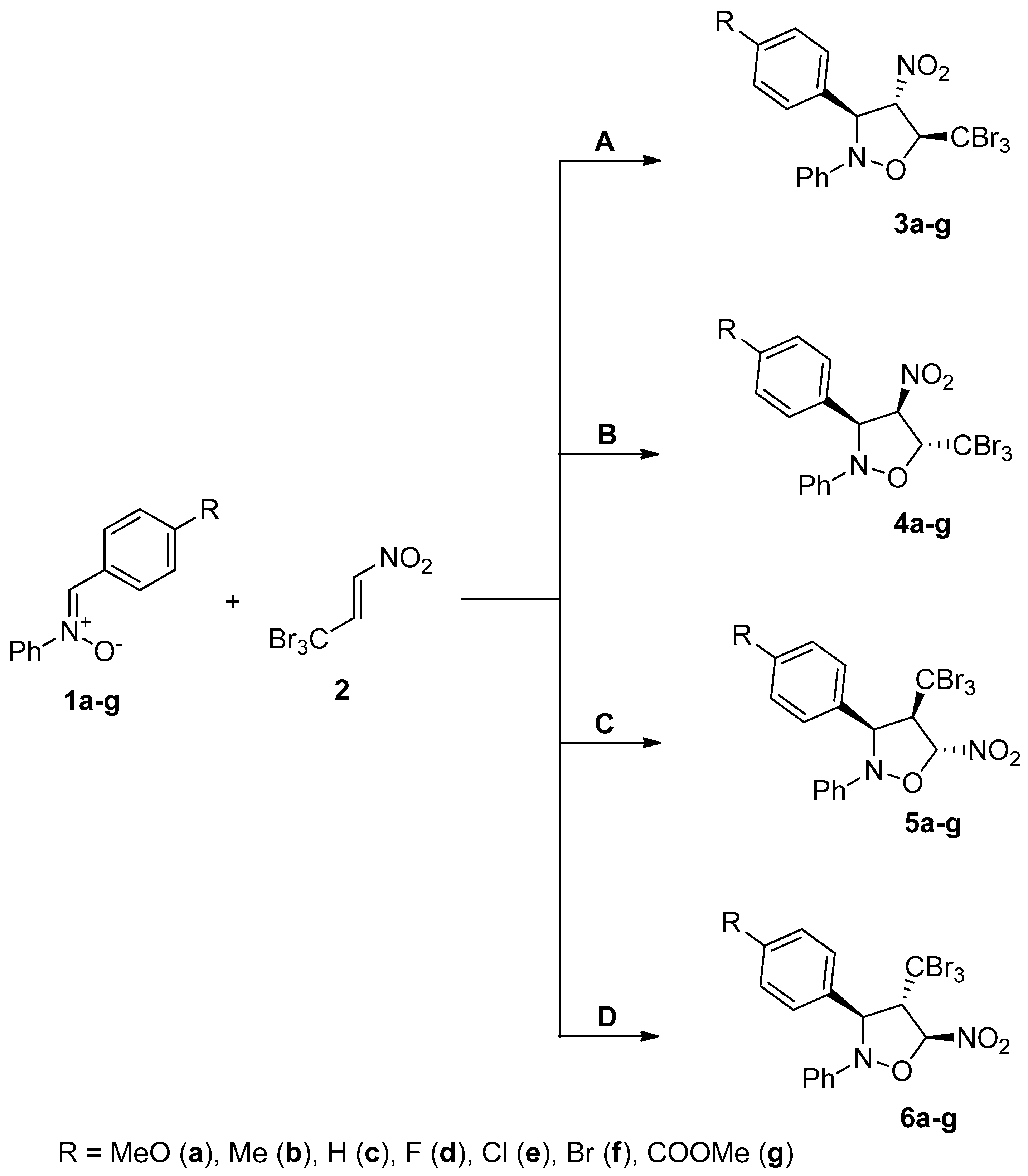
3.1.3. Cycloaddition between Z-C-aryl-N-Phenylnitrones (1a–g) and TBMN (2) General Procedure
3.2. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ito, S.; Koyasu, K.; Takano, S.; Tsukuda, T. Critical Role of CF3 Groups in the Electronic Stabilization of [PdAu24(C≡CC6H3(CF3)2)18]2− as Revealed by Gas-Phase Anion Photoelectron Spectroscopy. J. Phys. Chem. Lett. 2021, 12, 10417–10421. [Google Scholar] [CrossRef] [PubMed]
- Jagodzinska, M.; Huguenot, F.; Candiani, G.; Zanda, M. Assessing the Bioisosterism of the Trifluoromethyl Group with a Protease Probe. ChemMedChem 2009, 4, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Komarov, I.V.; Afonin, S.; Ulrich, A.S. 19F-Labeled Amino Acids for NMR Structure Analysis of Membrane-Bound Peptides. In Fluorine in Life Sciences: Pharmaceuticals, Medicinal Diagnostics, and Agrochemicals; Elsevier: Amsterdam, The Netherlands, 2019; pp. 349–395. [Google Scholar]
- Siadati, S.A. The Effect of Position Replacement of Functional Groups on the Stepwise character of 1,3-Dipolar Reaction of a Nitrile Oxide and an Alkene. Helv. Chim. Acta 2016, 99, 273–280. [Google Scholar] [CrossRef]
- Wójtowicz-Rajchel, H.; Koroniak, H. Synthesis of 5-fluorovinyl derivatives of pyrimidines via Suzuki–Miyaura coupling and their 1,3-dipolar cycloaddition reactions with nitrones. J. Fluor. Chem. 2012, 135, 225–230. [Google Scholar] [CrossRef]
- Jakowiecki, J.; Loska, R.; Makosza, M. Synthesis of αTrifluoromethyl-β-Lactams and Esters of β-Amino Acids via 1,3-Dipolar Cycloaddition of Nitrones to Fluoroalkenes. J. Org. Chem. 2008, 73, 5436–5441. [Google Scholar] [CrossRef]
- Łapczuk-Krygier, A.; Kącka-Zych, A.; Kula, K. Recent progress in the field of cycloaddition reactions involving conjugated nitroalkenes. Curr. Chem. Lett 2019, 8, 13–38. [Google Scholar] [CrossRef]
- Kula, K.; Kącka-Zych, A.; Łapczuk-Krygier, A.; Wzorek, Z.; Nowak, A.; Jasiński, R. Experimental and theoretical mechanistic study on the thermal decomposition of 3,3-diphenyl-4-(trichloromethyl)-5-nitropyrazoline. Molecules 2021, 26, 1364. [Google Scholar] [CrossRef]
- Kula, K.; Dobosz, J.; Jasiński, R.; Kącka-Zych, A.; Łapczuk-Krygier, A.; Mirosław, B.; Demchuk, O.M. [3+2] Cycloaddition of diaryldiazomethanes with (E)-3,3,3-trichloro-1-nitroprop-1-ene: Experimental, theoretical and structural study. J. Mol. Struct. 2020, 1203, 127473. [Google Scholar] [CrossRef]
- Zawadzińska, K.; Rios-Gutierrez, M.; Kula, K.; Woliński, P.; Mirosław, B.; Krawczyk, T.; Jasiński, R. The participation of 3,3,3-trichloro-1-nitroprop-1-ene in the [3+2] cycloaddition reaction with selected nitrile N-oxides in the light of the experimental and MEDT quantum chemical study. Molecules 2021, 26, 6774. [Google Scholar] [CrossRef]
- Fryzlewicz, A.; Łapczuk-Krygier, A.; Kula, K.; Demchuk, O.M.; Dresler, E.; Jasiński, R. Regio- and stereoselective synthesis of nitrofunctionalized 1,2-oxazolidine analogs of nicotine. Chem. Heterocycl. Compd. 2020, 56, 120–122. [Google Scholar] [CrossRef]
- Jasiński, R.; Zmigrodzka, M.; Dresler, E.; Kula, K. A full regio- and stereoselective synthesis of 4-nitroisoxazolidines via stepwise [3+2] cycloaddition reactions between (Z)-C-(9-anthryl)-N-arylnitrones and (E)-3,3,3-trichloro-1-nitroprop-1-ene: Comprehensive experimental and theoretical study. J. Heterocycl. Chem. 2017, 54, 3314–3320. [Google Scholar] [CrossRef]
- Jasiński, R.; Mróz, K.; Kącka, A. Experimental and theoretical DFT study on synthesis of sterically crowded 2,3,3,(4)5-tetrasubstituted-4-nitroisoxazolidines via 1,3-dipolar cycloaddition reactions between ketonitrones and conjugated nitroalkenes. J. Heterocycl. Chem. 2016, 53, 1424–1429. [Google Scholar] [CrossRef]
- Jasiński, R.; Mróz, K. Kinetic aspects of [3+2] cycloaddition reactions between (E)-3,3,3-trichloro-1-nitroprop-1-ene and ketonitrones. React Kinet. Mech. Catal. 2015, 116, 35–41. [Google Scholar] [CrossRef]
- Kącka-Zych, A.; Jasiński, R. Molecular mechanism of Hetero Diels-Alder reactions between (E)-1,1,1-trifluoro-3-nitrobut-2-enes and enamine systems in the light of Molecular Electron Density Theory. J. Mol. Graph. Model. 2020, 101, 107714. [Google Scholar] [CrossRef]
- Jasiński, R. B-Trifluoromethylated nitroethenes in Diels-Alder reaction with cyclopentadiene: A DFT computational study. J. Fluor. Chem. 2018, 206, 1–7. [Google Scholar] [CrossRef]
- Anisimova, N.A.; Slobodchikova, E.K.; Ivanova, M.E.; Rybalova, T.V. Reaction of 3,3,3-Tribromo- and 1,3,3,3-Tetrabromo-1-nitroprop-1-enes with Aliphatic Dienes. Russ. J. Gen. Chem. 2020, 90, 1388–1397. [Google Scholar] [CrossRef]
- Anisimova, N.A.; Slobodchikova, E.K.; Kuzhaeva, A.A.; Rybalova, T.V.; Stukan, E.V.; Berestovitskaya, V.M. 3,3,3-Tribromo-1-nitropropene: Synthesis and structure. Russ. J. Gen. Chem. 2014, 84, 834–838. [Google Scholar] [CrossRef]
- Ríos-Gutiérrez, M.; Domingo, L.R. Unravelling the Mysteries of the [3+2] Cycloaddition Reactions. Eur. J. Org. Chem. 2019, 2019, 267–282. [Google Scholar] [CrossRef]
- Feuer, H.; Savides, C.; Rao, C.N.R. The infrared spectra of the salts of nitro compound. Characteristic frequencies of the carbonitronate group. Spectrochim. Acta 1963, 19, 431–434. [Google Scholar] [CrossRef]
- Park, C.; Menini, C.; Valverde, J.L.; Keane, M.A. Carbon-chlorine and carbon-bromine bond cleavage in the catalytic hydrodehalogenation of halogenated aromatics. J. Catal. 2002, 211, 451–463. [Google Scholar] [CrossRef]
- Jasiński, R.; Ziółkowska, M.; Demchuk, O.M.; Maziarka, A. Regio- and stereoselectivity of polar [2+3] cycloaddition reactions between (Z)-C-(3,4,5-trimethoxyphenyl)-N-methylnitrone and selected (E)-2-substituted nitroethenes. Cent. Eur. J. Chem. 2014, 12, 586–593. [Google Scholar] [CrossRef]
- Domingo, L.R. Molecular Electron Density Theory: A Modern View of Reactivity in Organic Chemistry. Molecules 2016, 21, 1319. [Google Scholar] [CrossRef] [PubMed]
- Jasiński, R.; Kula, K.; Kącka, A.; Mirosław, B. Unexpected course of reaction between (E)-2-aryl-1-cyano-1-nitroethenes and diazafluorene: Why is there no 1,3-dipolar cycloaddition? Mon. Chem. 2017, 148, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Jasiński, R. One-step versus two-step mechanism of Diels-Alder reaction of 1-chloro-1-nitroethene with cyclopentadiene and furan. J. Mol. Graph. Model. 2017, 75, 55–61. [Google Scholar] [CrossRef]
- Mirosław, B.; Babyuk, D.; Łapczuk-Krygier, A.; Kącka-Zych, A.; Demchuk, O.M.; Jasiński, R. Regiospecific formation of the nitromethyl-substituted 3-phenyl-4,5-dihydroisoxazole via [3+2] cycloaddition. Mon. Chem. 2018, 149, 1877–1884. [Google Scholar] [CrossRef]
- Jasinski, R. Competition between one-step and two-step mechanism in polar [3+2] cycloadditions of (Z)-C-(3,4,5-trimethoxyphenyl)-N-methyl-nitrone with (Z)-2-EWG-1-bromo-1-nitroethenes. Comp. Theor. Chem. 2018, 1125, 77–85. [Google Scholar] [CrossRef]
- Kącka-Zych, A.; Jasiński, R. Understanding the molecular mechanism of the stereoselective conversion of N-trialkylsilyloxy nitronates into bicyclic isoxazoline derivatives. New J. Chem. 2021, 45, 9491–9500. [Google Scholar] [CrossRef]
- Fryźlewicz, A.; Kącka-Zych, A.; Demchuk, O.M.; Mirosław, B.; Woliński, P.; Jasiński, R. Green synthesis of nitrocyclopropane-type precursors of inhibitors for the maturation of fruits and vegetables via domino reactions of diazoalkanes with 2-nitroprop-1-ene. J. Clean. Prod. 2021, 292, 126079. [Google Scholar] [CrossRef]
- Woliński, P.; Kącka-Zych, A.; Mirosław, B.; Wielgus, E.; Olszewska, A.; Jasiński, R. Green, One-pot Synthesis of 1,2-oxazine-type Herbicides Via Non-catalyzed Hetero Diels-alder Reactions Comprising (2E)-3-aryl-2-nitroprop-2-enenitriles. J. Clean. Prod. 2022, 356, 131878. [Google Scholar] [CrossRef]
- Padwa, A.; Bullock, W.H.; Kline, D.N.; Perumattam, J. Heterocyclic synthesis via the reaction of nitrones and hydroxylamines with substituted allenes. J. Org. Chem. 1989, 54, 2862–2869. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Fox, D.J.; et al. (Eds.) Gaussian 16; Revision, A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef]
- Domingo, L.R. A new C–C bond formation model based on the quantum chemical topology of electron density. RSC Adv. 2014, 4, 32415–32428. [Google Scholar] [CrossRef]
- Jasiński, R. A stepwise, zwitterionic mechanism for the 1,3-dipolar cycloaddition between (Z)-C4-methoxyphenyl-N-phenylnitrone and gem-chloronitroethene catalysed by 1-butyl-3-methylimidazolium ionic liquid cations. Tetrahedron Lett. 2015, 56, 532–535. [Google Scholar] [CrossRef]
- Perez, P.; Domingo, L.R.; Aurell, M.J.; Contreras, R. Quantitative characterization of the global electrophilicity pattern of some reagents involved in 1,3-dipolar cycloaddition reactions. Tetrahedron 2003, 59, 3117–3125. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpaly, L.; Liu, S. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Domingo, L.R.; Chamorro, E.; Perez, P. Understanding the Reactivity of Captodative Ethylenes in Polar Cycloaddition Reactions. A Theoretical Study. J. Org. Chem. 2008, 73, 4615–4624. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Perez, P.; Saez, J.A. Understanding the local reactivity in polar organic reactions through electrophilic and nucleophilic Parr functions. RSC Adv. 2013, 3, 1486–1494. [Google Scholar] [CrossRef]
| R | ω [eV] | N [eV] | P-C | P-O | NC [eV] | NO [eV] | P+α | P+β | ωα [eV] | ωβ [eV] | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1a | OMe | 1.48 | 3.98 | 0.04 | 0.41 | 0.16 | 1.62 | ||||
| 1b | Me | 1.60 | 3.77 | 0.10 | 0.44 | 0.38 | 1.64 | ||||
| 1c | H | 1.67 | 3.64 | 0.14 | 0.44 | 0.51 | 1.61 | ||||
| 1d | F | 1.71 | 3.60 | 0.12 | 0.43 | 0.44 | 1.56 | ||||
| 1e | Cl | 1.87 | 3.48 | 0.12 | 0.42 | 0.41 | 1.47 | ||||
| 1f | Br | 1.88 | 3.49 | 0.11 | 0.41 | 0.37 | 1.45 | ||||
| 1g | COOMe | 2.11 | 3.39 | 0.15 | 0.43 | 0.50 | 1.47 | ||||
| 2 | - | 3.23 | 1.09 | 0.13 | 0.22 | 0.41 | 0.71 |
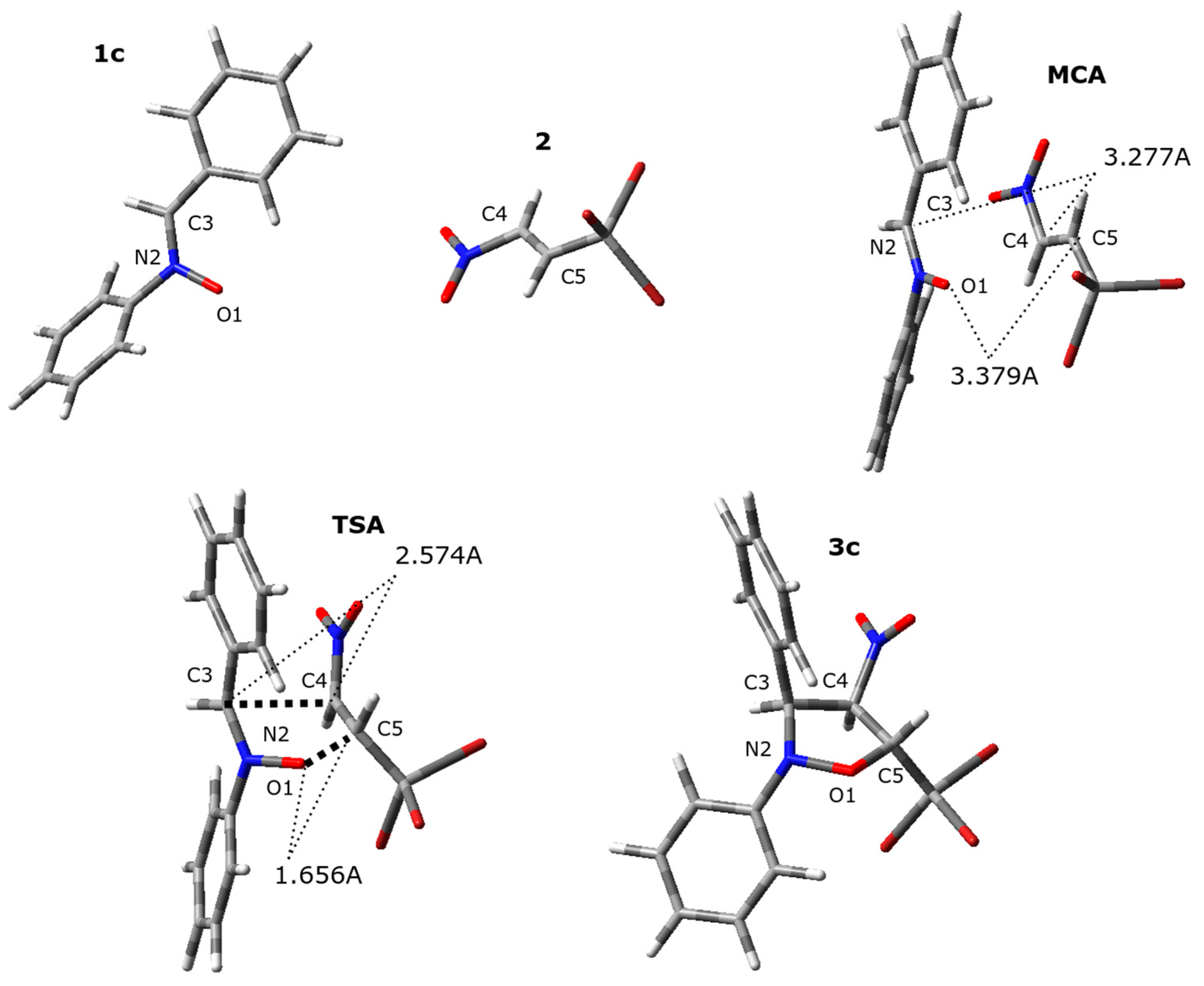
| Structure | Interatomic Distances [Å] | lC3-C4 | lC5-O1 | Δl | GEDT [e] | ||||
|---|---|---|---|---|---|---|---|---|---|
| O1-N2 | N2-C3 | C3-C4 | C4-C5 | C5-O1 | |||||
| 1c | 1.276 | 1.307 | |||||||
| 2 | 1.322 | ... | |||||||
| MCA | 1.276 | 1.307 | 3.277 | 1.323 | 3.379 | 0.00 | |||
| TSA | 1.332 | 1.315 | 2.574 | 1.424 | 1.656 | 0.353 | 0.822 | 0.47 | 0.66 |
| 3c | 1.407 | 1.474 | 1.563 | 1.530 | 1.405 | ||||
| Transition | ΔH | ΔG | ΔS |
|---|---|---|---|
| 1c+2→LMA | −9.0 | 3.8 | −43.2 |
| 1c+2→TSA | 6.0 | 22.0 | −53.5 |
| 1c+2→3c | −27.5 | −11.6 | −53.5 |
| 1c+2→LMB | −8.3 | 4.0 | −41.1 |
| 1c+2→TSB | 4.9 | 20.2 | −51.5 |
| 1c+2→4c | −31.7 | −15.8 | −53.1 |
| 1c+2→LMC | −8.5 | 3.9 | −41.6 |
| 1c+2→TSC | 8.5 | 24.0 | −51.9 |
| 1c+2→5c | −29.6 | −13.2 | −55.1 |
| 1c+2→LMD | −10.3 | 3.6 | −46.6 |
| 1c+2→TSD | 12.8 | 28.8 | −53.9 |
| 1c+2→6c | −26.4 | −10.2 | −54.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zawadzińska, K.; Gadocha, Z.; Pabian, K.; Wróblewska, A.; Wielgus, E.; Jasiński, R. The First Examples of [3+2] Cycloadditions with the Participation of (E)-3,3,3-Tribromo-1-Nitroprop-1-Ene. Materials 2022, 15, 7584. https://doi.org/10.3390/ma15217584
Zawadzińska K, Gadocha Z, Pabian K, Wróblewska A, Wielgus E, Jasiński R. The First Examples of [3+2] Cycloadditions with the Participation of (E)-3,3,3-Tribromo-1-Nitroprop-1-Ene. Materials. 2022; 15(21):7584. https://doi.org/10.3390/ma15217584
Chicago/Turabian StyleZawadzińska, Karolina, Zuzanna Gadocha, Kamila Pabian, Aneta Wróblewska, Ewelina Wielgus, and Radomir Jasiński. 2022. "The First Examples of [3+2] Cycloadditions with the Participation of (E)-3,3,3-Tribromo-1-Nitroprop-1-Ene" Materials 15, no. 21: 7584. https://doi.org/10.3390/ma15217584
APA StyleZawadzińska, K., Gadocha, Z., Pabian, K., Wróblewska, A., Wielgus, E., & Jasiński, R. (2022). The First Examples of [3+2] Cycloadditions with the Participation of (E)-3,3,3-Tribromo-1-Nitroprop-1-Ene. Materials, 15(21), 7584. https://doi.org/10.3390/ma15217584








