Physical Properties and Biodegradability Evaluation of Vulcanized Epoxidized Natural Rubber/Thermoplastic Potato Starch Blends
Abstract
1. Introduction
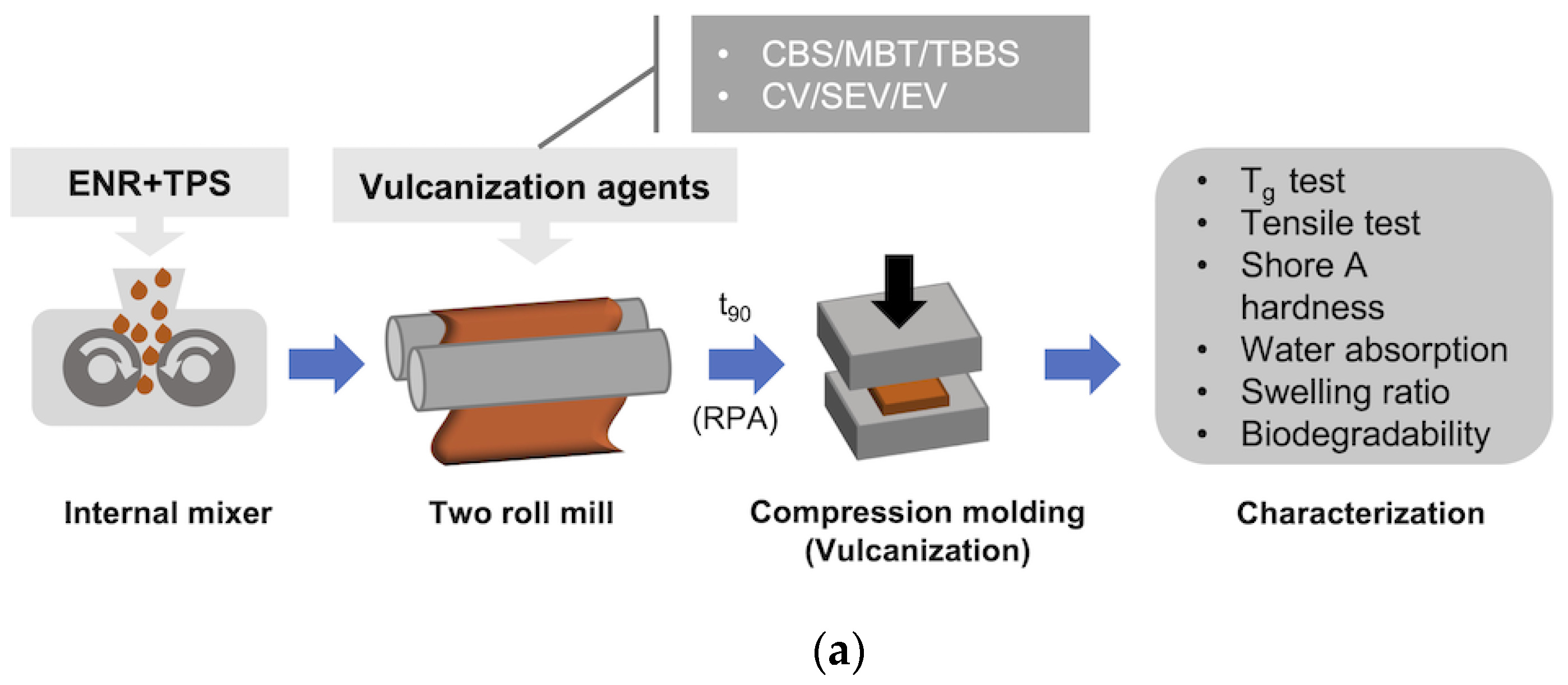
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Vulcanization Characteristics
2.2.2. Glass Transition Temperature
2.2.3. Tensile Properties
2.2.4. Hardness
2.2.5. Water Absorption
2.2.6. Swelling Ratio
2.2.7. Biodegradability by Composting Test
3. Results and Discussion
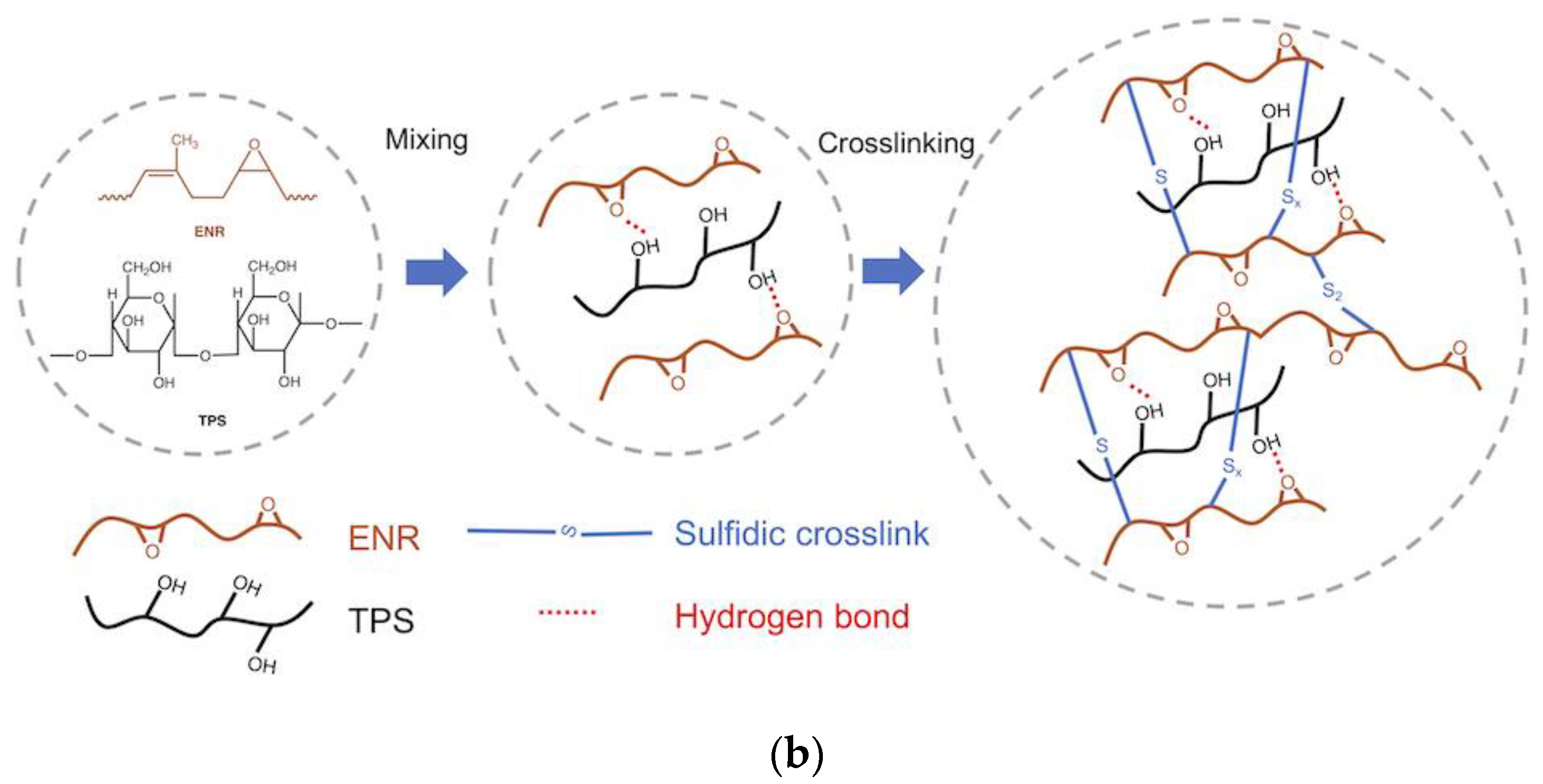
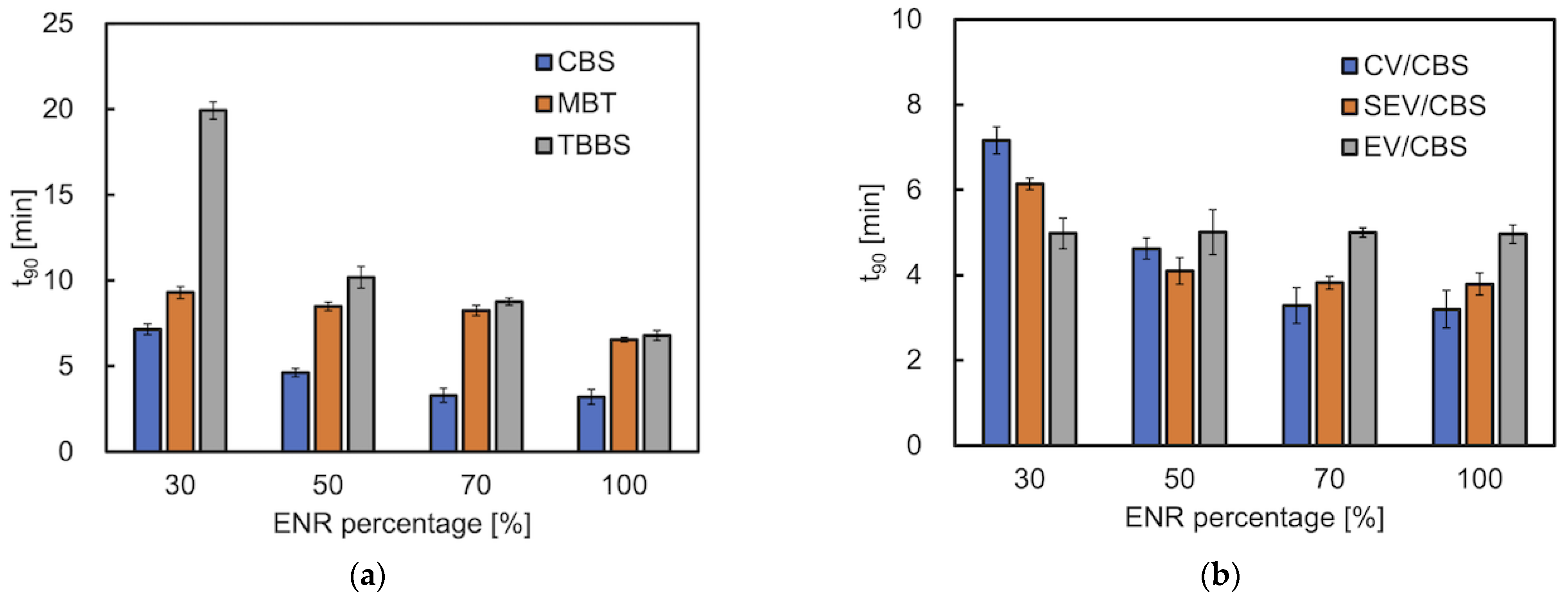
3.1. Curing Behavior
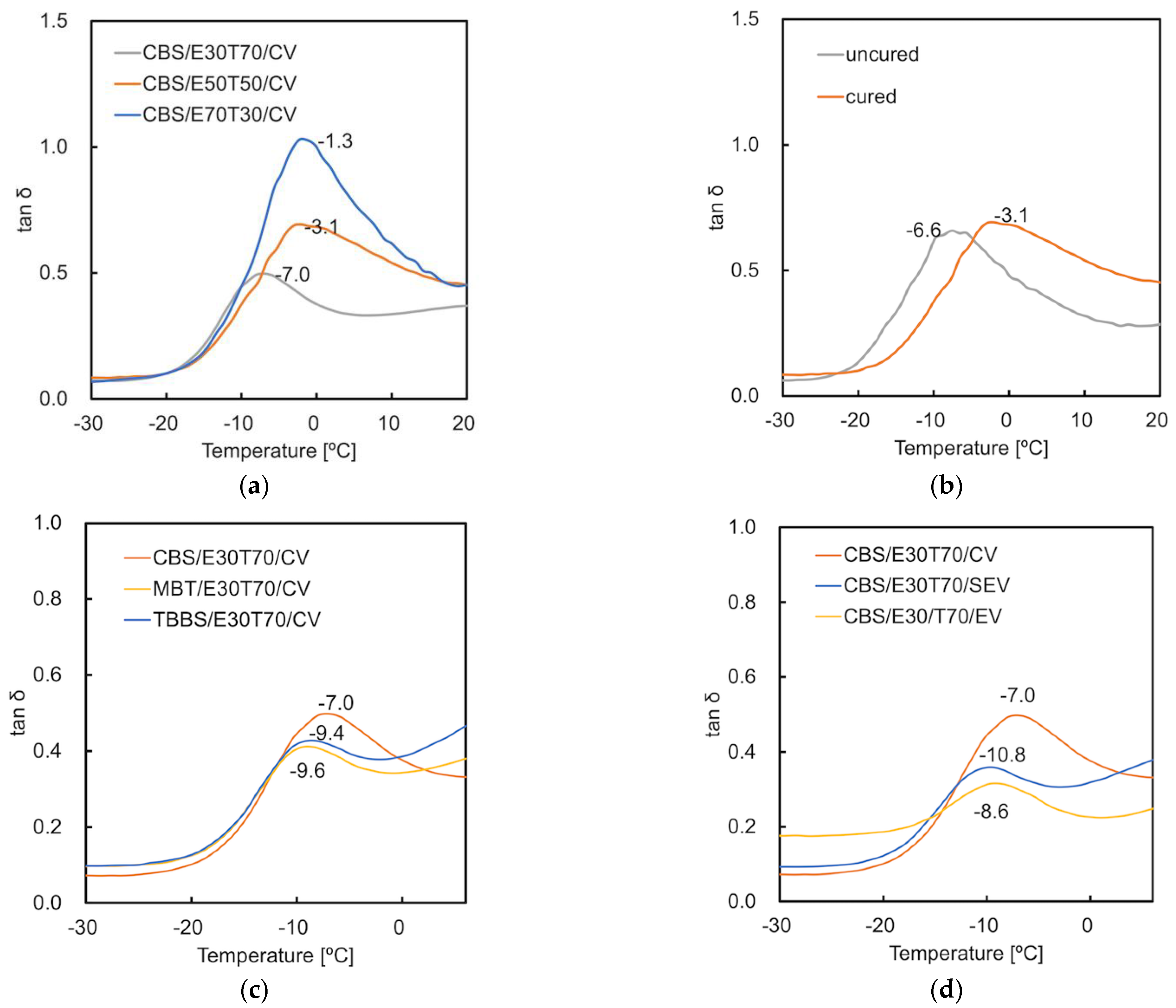
3.2. Glass Transition Temperature (Tg)
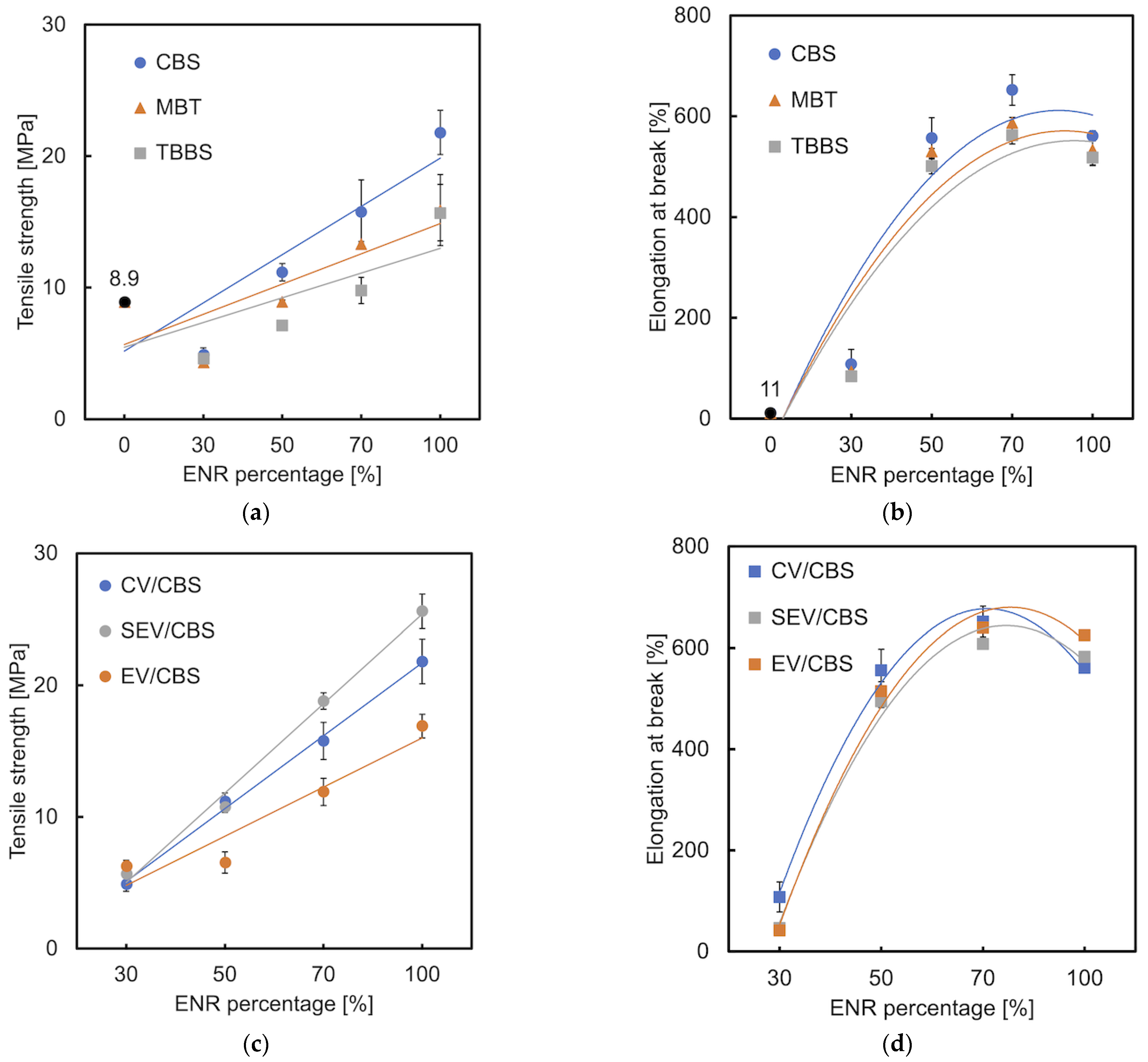
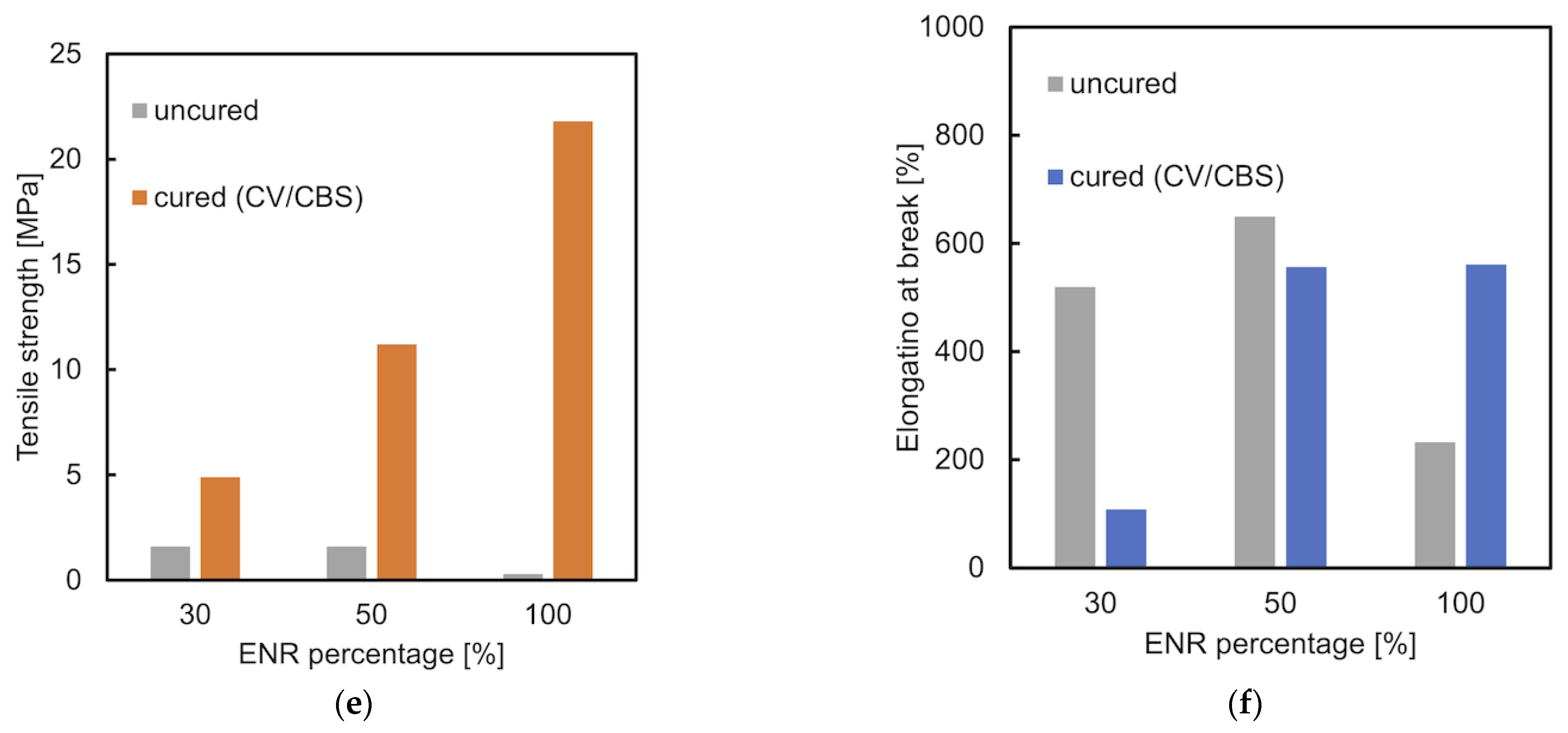
3.3. Tensile Properties
3.4. Shore A Hardness
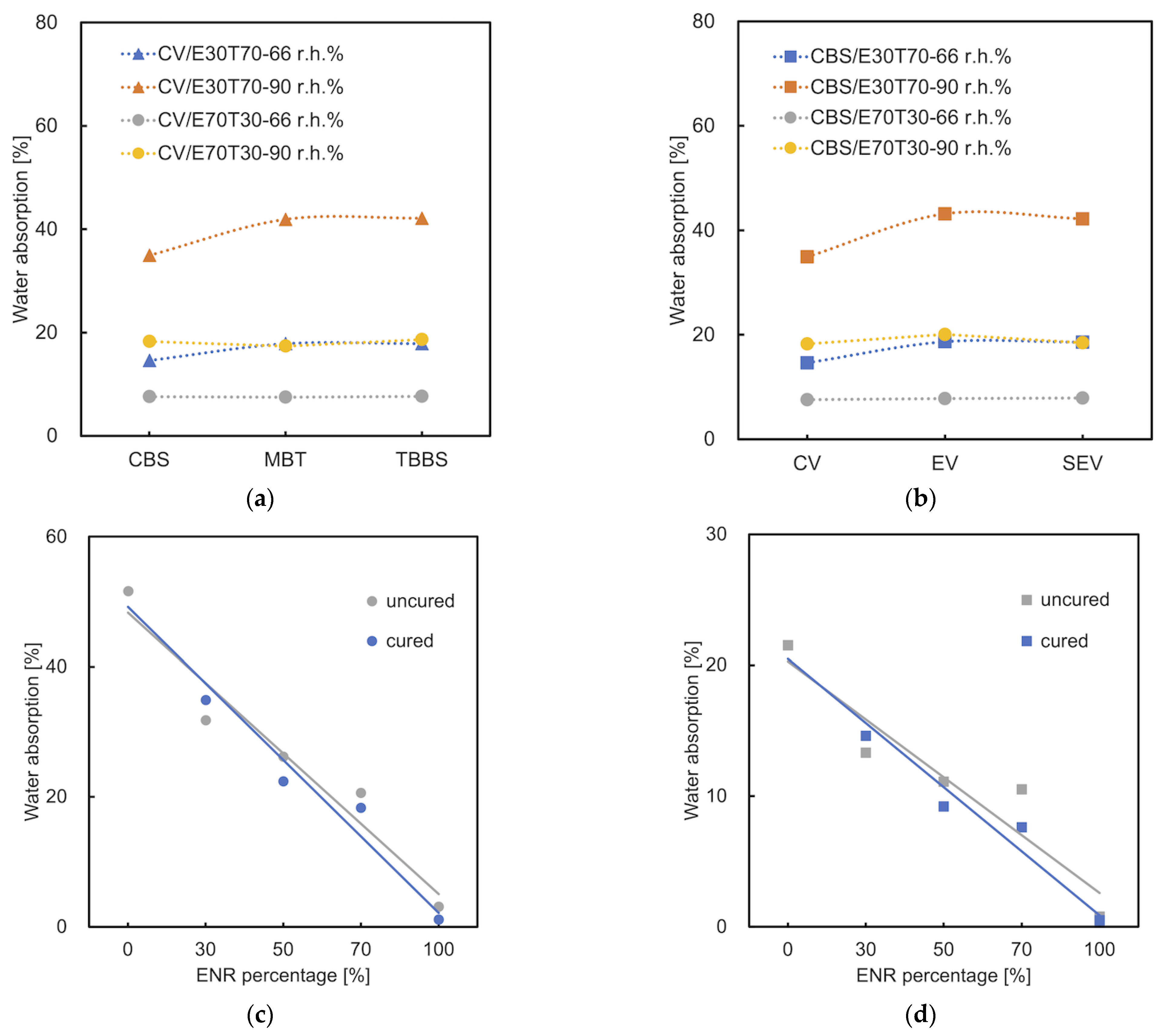
3.5. Water Absorption
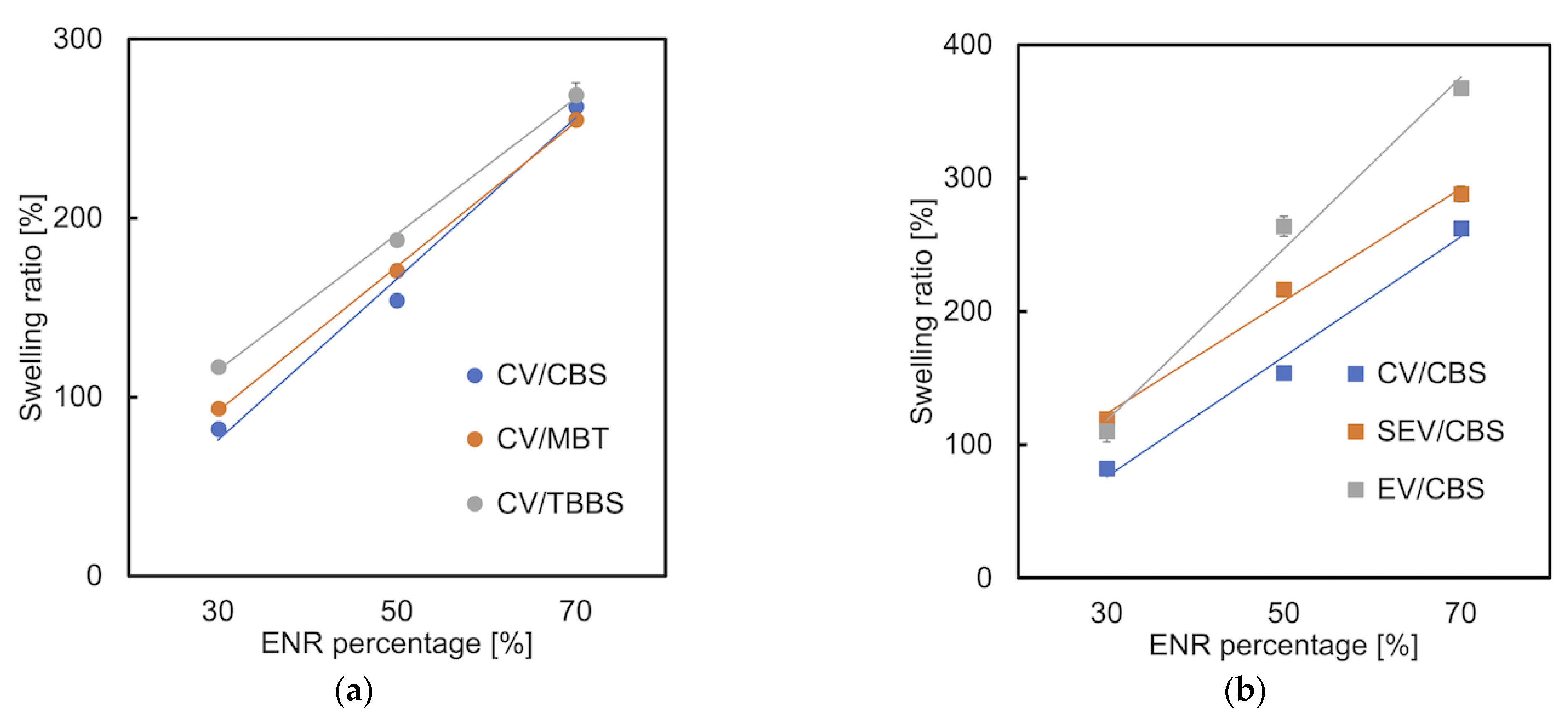
3.6. Swelling Ratio
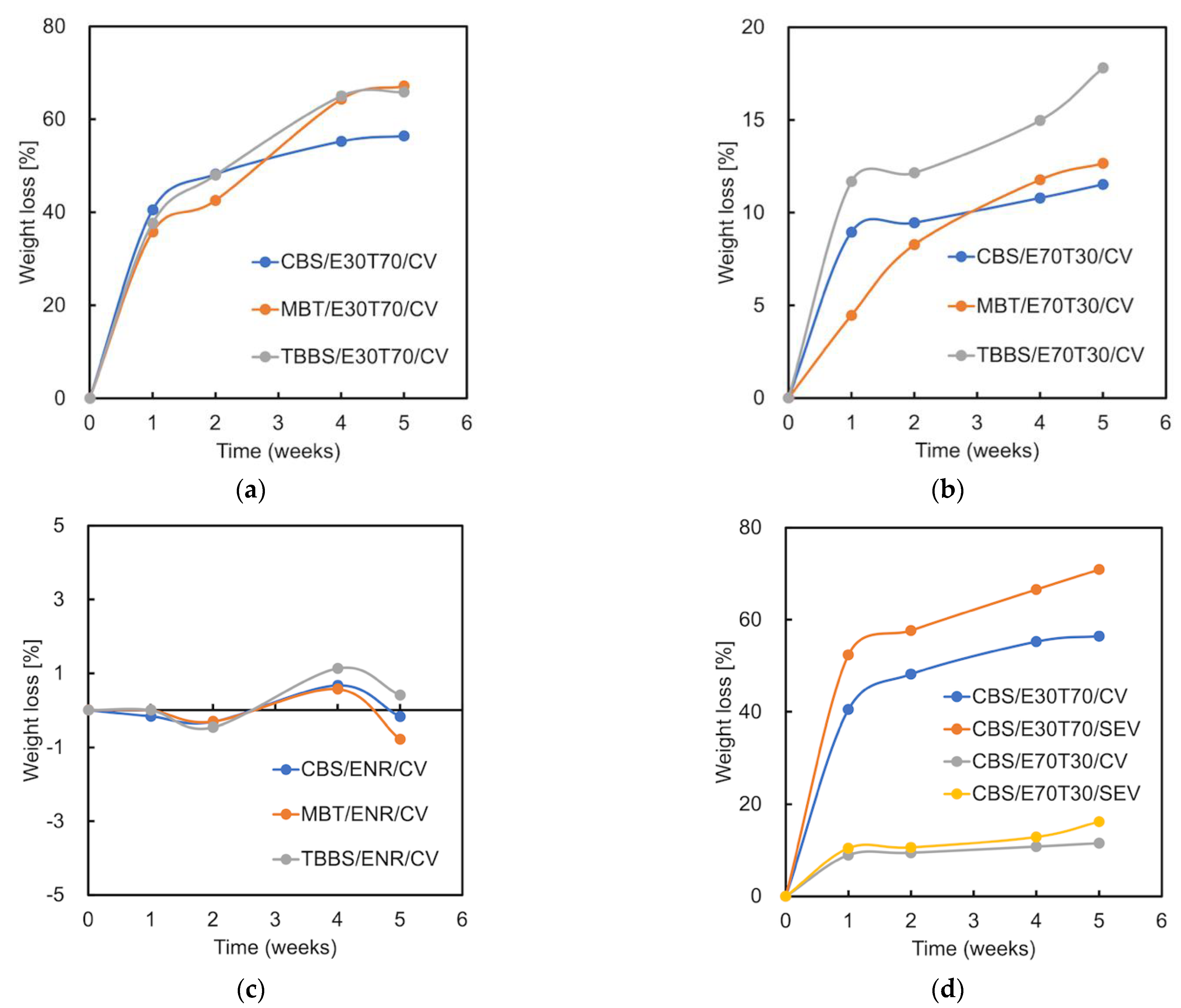
3.7. Composting Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaseem, M.; Hamad, K.; Deri, F. Rheological and mechanical properties of polypropylene/thermoplastic starch blend. Polym. Bull. 2012, 68, 1079–1091. [Google Scholar] [CrossRef]
- Nafchi, M.; Moradpour, M.; Saeidi, M.; Alias, A.K. Thermoplastic starches: Properties, challenges, and prospects. Starch-Stärke 2013, 65, 61–72. [Google Scholar] [CrossRef]
- Cai, Z.; Čadek, D.; Šmejkalová, P.; Kadeřábková, A.; Nová, M.; Kuta, A. The modification of properties of thermoplastic starch materials: Combining potato starch with natural rubber and epoxidized natural rubber. Mater. Today Commun. 2021, 26, 101912. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ahmad, I.; Abdullah, I.; Thomas, R.; Dufresne, A.; Thomas, S.; Hassan, A. Functionalized liquid natural rubber and liquid epoxidized natural rubber: A promising green toughening agent for polyester. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Kruželák, J.; Sýkora, R.; Hudec, I. Vulcanization of rubber compounds with peroxide curing systems. Rubber Chem. Technol. 2017, 90, 60–88. [Google Scholar] [CrossRef]
- Hofmann, W. Rubber Technology Handbook; Hanser Publishers: Cincinnati, OH, USA, 1989. [Google Scholar]
- Nagdi, K. Rubber as an Engineering Material: Guideline for Users; Hanser Verlag: Munich, Germany, 1993. [Google Scholar]
- Blow, C.M. Rubber Technology and Manufacture; CRC Press: Boca Raton, FL, USA, 1971. [Google Scholar]
- Mark, J.E.; Erman, B.; Roland, M. The Science and Technology of Rubber; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Cheremisinoff, N.P. Condensed Encyclopedia of Polymer Engineering Terms; Butterworth-Heinemann: Oxford, UK, 2001. [Google Scholar]
- Coran, A.Y. Vulcanization, Science and Technology of Rubber; Elsevier: Amsterdam, The Netherlands, 1994; pp. 339–385. [Google Scholar]
- Nakason, C.; Wannavilai, P.; Kaesaman, A. Effect of vulcanization system on properties of thermoplastic vulcanizates based on epoxidized natural rubber/polypropylene blends. Polym. Test. 2006, 25, 34–41. [Google Scholar] [CrossRef]
- Sadequl; Ishiaku, U.; Ismail, H.; Poh, B. The effect of accelerator/sulphur ratio on the scorch time of epoxidized natural rubber. Eur. Polym. J. 1998, 34, 51–57. [Google Scholar] [CrossRef]
- Poh, B.T.; Chen, M.; Ding, B. Cure characteristics of unaccelerated sulfur vulcanization of epoxidized natural rubber. J. Appl. Polym. Sci. 1996, 60, 1569–1574. [Google Scholar] [CrossRef]
- Larpkasemsek, A.; Raksaksri, L.; Chuayjuljit, S.; Chaiwutthinan, P.; Boonmahitthisud, A. Effects of sulfur vulcanization system on cure characteristics, physical properties and thermal aging of epoxidized natural rubber. J. Met. Mater. Miner. 2019, 29, 49–57. [Google Scholar]
- Poh, B.T.; Khok, G. Tensile property of epoxidized natural rubber/natural rubber blends. Polym.-Plast. Technol. Eng. 2000, 39, 151–161. [Google Scholar] [CrossRef]
- Chuayjuljit, S.; Nutchapong, T.; Saravari, O.; Boonmahitthisud, A. Preparation and characterization of epoxidized natural rubber and epoxidized natural rubber/carboxylated styrene butadiene rubber blends. J. Met. Mater. Miner. 2015, 25, 27–34. [Google Scholar]
- MWahab, K.A.; Ismail, H.; Othman, N. Effects of dynamic vulcanization on the physical, mechanical, and morphological properties of high-density polyethylene/(natural rubber)/(thermoplastic tapioca starch) blends. J. Vinyl Addit. Technol. 2012, 18, 192–197. [Google Scholar]
- Formela, K.; Wąsowicz, D.; Formela, M.; Hejna, A.; Haponiuk, J. Curing characteristics, mechanical and thermal properties of reclaimed ground tire rubber cured with various vulcanizing systems. Iran. Polym. J. 2015, 24, 289–297. [Google Scholar] [CrossRef]
- Riyajan, S.-A. Physical Properties of Cured Self-green Epoxidized Natural Rubber with Amino Acid. J. Polym. Environ. 2022, 30, 2304–2313. [Google Scholar] [CrossRef]
- Kahar, A.W.M.; Low, K.Y.; Ismail, H. Study of Thermoplastic Starch Incorporation on Polylactic Acid/Natural Rubber Blends via Dynamic Vulcanization Approach, Biofiller-Reinforced Biodegradable Polymer Composites; CRC Press: Boca Raton, FL, USA, 2020; pp. 91–117. [Google Scholar]
- ASTM D 5289; Standard Test Method for Rubber Property—Vulcanization Using Rotorless Cure Meters. ASTM: West Conshohocken, PA, USA, 2019.
- ISO 37; Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties. ISO: Geneva, Switzerland, 2017.
- ISO 48; Rubber, Vulcanized or Thermoplastic—Determination of Hardness—Part 2: Hardness between 10 IRHD and 100 IRHD. ISO: Geneva, Switzerland, 2018.
- ASTM E 104; Standard Practice for Maintaining Constant Relative Humidity by Means of Aqueous Solutions. ASTM: West Conshohocken, PA, USA, 2020.
- ISO 1817; Resilient floor coverings—Specification for Homogeneous and Heterogeneous Smooth Rubber Floor Coverings. ISO: Geneva, Switzerland, 2020.
- Joseph, R.; George, K.; Francis, D.J. Studies on the cure characteristics and vulcanizate properties of 50/50 NR/SBR blend. J. Appl. Polym. Sci. 1988, 35, 1003–1017. [Google Scholar] [CrossRef]
- Ahmed, K.; Nizami, S.S.; Raza, N.Z.; Mahmood, K. Mechanical, swelling, and thermal aging properties of marble sludge-natural rubber composites. Int. J. Ind. Chem. 2012, 3, 1–12. [Google Scholar] [CrossRef]
- ASTM D5338; Biodegradation Test—Composting. ASTM: West Conshohocken, PA, USA, 2021.
- Khimi, S.R.; Pickering, K.L. A new method to predict optimum cure time of rubber compound using dynamic mechanical analysis. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Pacáková, J.V.V. PLASTICS, Encyclopedia of Analytical Science, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 8. [Google Scholar]
- Mathew, V.S.; George, S.C.; Parameswaranpillai, J.; Thomas, S. Epoxidized natural rubber/epoxy blends: Phase morphology and thermomechanical properties. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Mansilla, M.; Silva, L.; Salgueiro, W.; Marzocca, A.; Somoza, A. A study about the structure of vulcanized natural rubber/styrene butadiene rubber blends and the glass transition behavior. J. Appl. Polym. Sci. 2012, 125, 992–999. [Google Scholar] [CrossRef]
- Hernández, L.G.; Díaz, A.R.; Valentín, J.L.; Marcos-Fernández, Á.; Posadas, P. Conventional and efficient crosslinking of natural rubber: Effect of heterogeneities on the physical properties. Elastomer Kunstst. 2005, 58, 638–643. [Google Scholar]
- Ghorai, S.; Jalan, A.K.; Roy, M.; Das, A.; De, D. Tuning of accelerator and curing system in devulcanized green natural rubber compounds. Polym. Test. 2018, 69, 133–145. [Google Scholar] [CrossRef]
- Wu, Y.; Joseph, S.; Aluru, N. Effect of cross-linking on the diffusion of water, ions, and small molecules in hydrogels. J. Phys. Chem. B 2009, 113, 3512–3520. [Google Scholar] [CrossRef] [PubMed]
- Ismail, H.; Salmah; Nasir, M. The effect of dynamic vulcanization on mechanical properties and water absorption of silica and rubberwood filled polypropylene/natural rubber hybrid composites. Int. J. Polym. Mater. 2003, 52, 229–238. [Google Scholar] [CrossRef]
- McNaught, A.D.; Wilkinson, A. Compendium of Chemical Terminology; Blackwell Science Oxford: Oxford, UK, 1997. [Google Scholar]
- Chandler, Interfaces and the driving force of hydrophobic assembly. Nature 2005, 437, 640–647. [CrossRef]
- Valentín, J.; Carretero-González, J.; Mora-Barrantes, I.; Chassé, W.; Saalwachter, K. Uncertainties in the determination of cross-link density by equilibrium swelling experiments in natural rubber. Macromolecules 2008, 41, 4717–4729. [Google Scholar] [CrossRef]
- El Shafee, E.; Naguib, H. Water sorption in cross-linked poly (vinyl alcohol) networks. Polymer 2003, 44, 1647–1653. [Google Scholar] [CrossRef]
- Bajpai, A.; Giri, A. Water sorption behaviour of highly swelling (carboxy methylcellulose-g-polyacrylamide) hydrogels and release of potassium nitrate as agrochemical. Carbohydr. Polym. 2003, 53, 271–279. [Google Scholar] [CrossRef]




| Polymers | Purposes | Results | Reference |
|---|---|---|---|
| ENR/PP a blends | Effect of vulcanization system (sulfur, peroxide and a mixture of sulfur and peroxide) on properties. | The mechanical properties have been improved by the mixture of sulfur and peroxide cure system. | [12] |
| ENR | (1) The effect of accelerator/sulfur ratio on the scorch time; (2) Cure properties of unaccelerated sulfur vulcanization; (3) The effect of cure systems on cure properties, physical properties and thermal aging. | (1) t0.2 c: CV > SEV > EV; (2) t0.2: ENR-25 > ENR-50; (3) t90 d: EV > CV; crosslink density: CV > EV; SEV shows the highest thermal stability. | [13,14,15] |
| ENR/NR | Tensile properties. | A maximum tensile strength and elongation at break at 50% ENR for both ENR-25 /NR and ENR-50/NR blends. | [16] |
| ENR-30/XSBR b blends | Preparation and characterization. | t0.2 and t90: blends > ENR-30 300% modulus increased, tensile strength and elongation at break decreased. | [17] |
| HDPE e/NR/TPS blends | Effects of dynamic curing on the physical, mechanical and morphological properties. | Good dispersity, improved tensile strength. | [18] |
| Reclaimed ground tire rubber | Curing, mechanical and thermal properties. | TMTD sample—highest crosslink density. | [19] |
| ENR/PAA f | Physical properties. | The moisture content and absorption were increased, the elongation at break and toluene resistance were improved. | [20] |
| TPS/Polylactic acid/NR | The effects of dynamic vulcanization on the vulcanization system. | The addition of HVA-2 g and DCP h curatives showed an increase in crosslinking density and better tensile properties. Both of the vulcanization systems delayed biodegradability. | [21] |
| Sample | ENR [wt. %] | TPS [wt. %] |
|---|---|---|
| 1 | 0 | 100 |
| 2 | 30 | 70 |
| 3 | 50 | 50 |
| 4 | 70 | 30 |
| 5 | 100 | 0 |
| Accelerator | Chemical Name | Chemical Structure |
|---|---|---|
| CBS | N-cyclohexylbenzothiazole-2-sulphenamide | 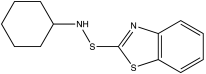 |
| MBT | 2-mercaptobenzothiazole | 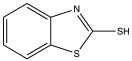 |
| TBBS | N-tert-butylbenzothiazole-2-sulphenamide | 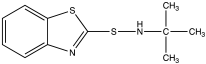 |
| I [phr] | II [phr] | III [phr] | |
|---|---|---|---|
| ENR/TPS | 100 | 100 | 100 |
| ZnO | 5 | 5 | 5 |
| Stearic acid | 2 | 2 | 2 |
| S | 2.5 | 2.5 | 2.5 |
| CBS | 1 | 0 | 0 |
| MBT | 0 | 1 | 0 |
| TBBS | 0 | 0 | 1 |
| CV [phr] | SEV [phr] | EV [phr] | |
|---|---|---|---|
| ENR/TPS | 100 | 100 | 100 |
| ZnO | 5 | 5 | 5 |
| Stearic acid | 2 | 2 | 2 |
| S | 2.5 | 1.7 | 0.8 |
| CBS | 1 | 2 | 3.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Z.; Čadek, D.; Jindrová, M.; Kadeřábková, A.; Kuta, A. Physical Properties and Biodegradability Evaluation of Vulcanized Epoxidized Natural Rubber/Thermoplastic Potato Starch Blends. Materials 2022, 15, 7478. https://doi.org/10.3390/ma15217478
Cai Z, Čadek D, Jindrová M, Kadeřábková A, Kuta A. Physical Properties and Biodegradability Evaluation of Vulcanized Epoxidized Natural Rubber/Thermoplastic Potato Starch Blends. Materials. 2022; 15(21):7478. https://doi.org/10.3390/ma15217478
Chicago/Turabian StyleCai, Zhejing, Drahomír Čadek, Michaela Jindrová, Alena Kadeřábková, and Antonín Kuta. 2022. "Physical Properties and Biodegradability Evaluation of Vulcanized Epoxidized Natural Rubber/Thermoplastic Potato Starch Blends" Materials 15, no. 21: 7478. https://doi.org/10.3390/ma15217478
APA StyleCai, Z., Čadek, D., Jindrová, M., Kadeřábková, A., & Kuta, A. (2022). Physical Properties and Biodegradability Evaluation of Vulcanized Epoxidized Natural Rubber/Thermoplastic Potato Starch Blends. Materials, 15(21), 7478. https://doi.org/10.3390/ma15217478








