Study of Temperature on the Corrosion Behavior of Antibacterial Steel by a Large−Scale Multiphase Flow Corrosion Test Loop
Abstract
1. Introduction
2. Experiment
Material and Solution
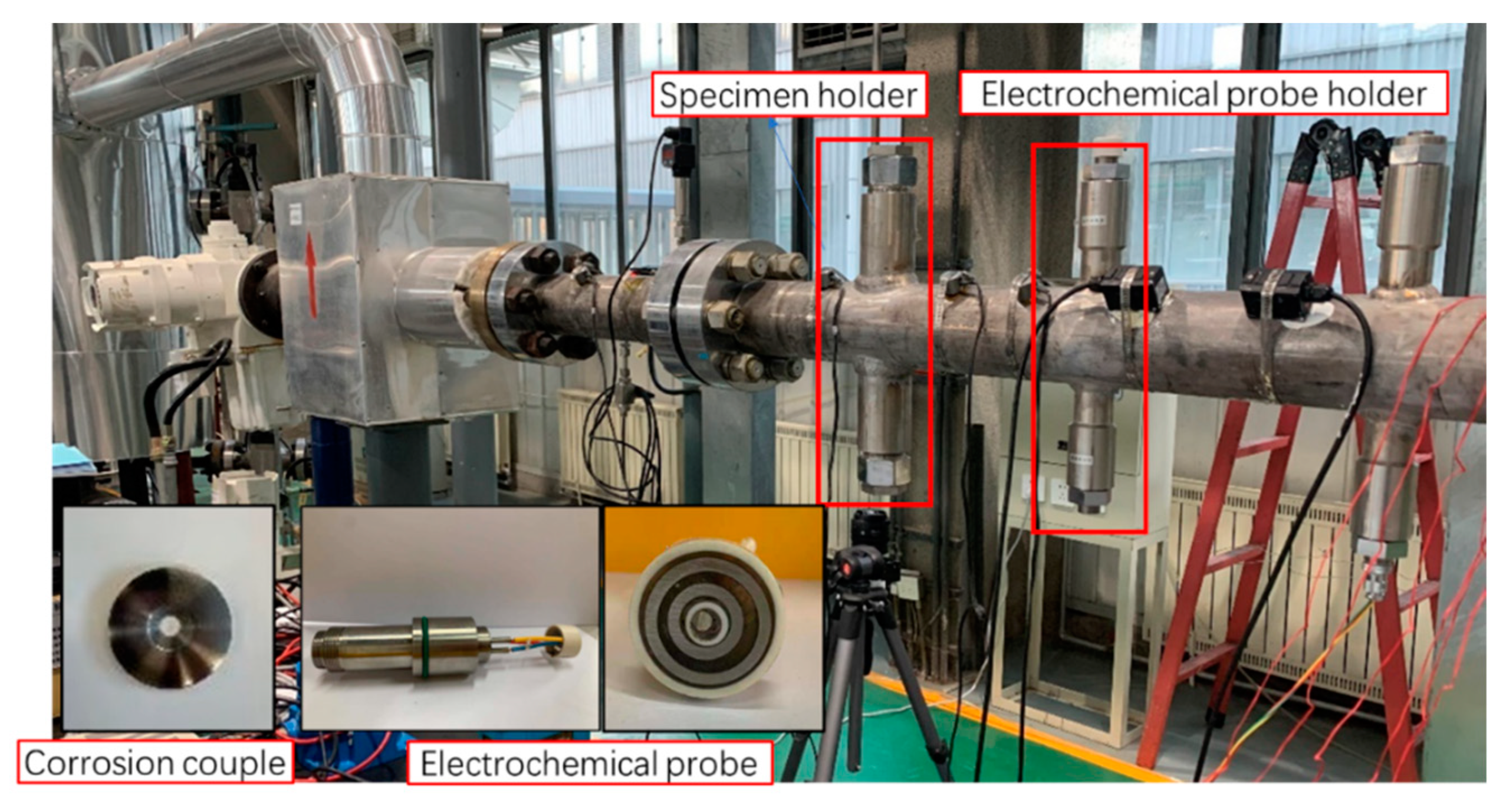
3. Flow Loop Test
4. Results
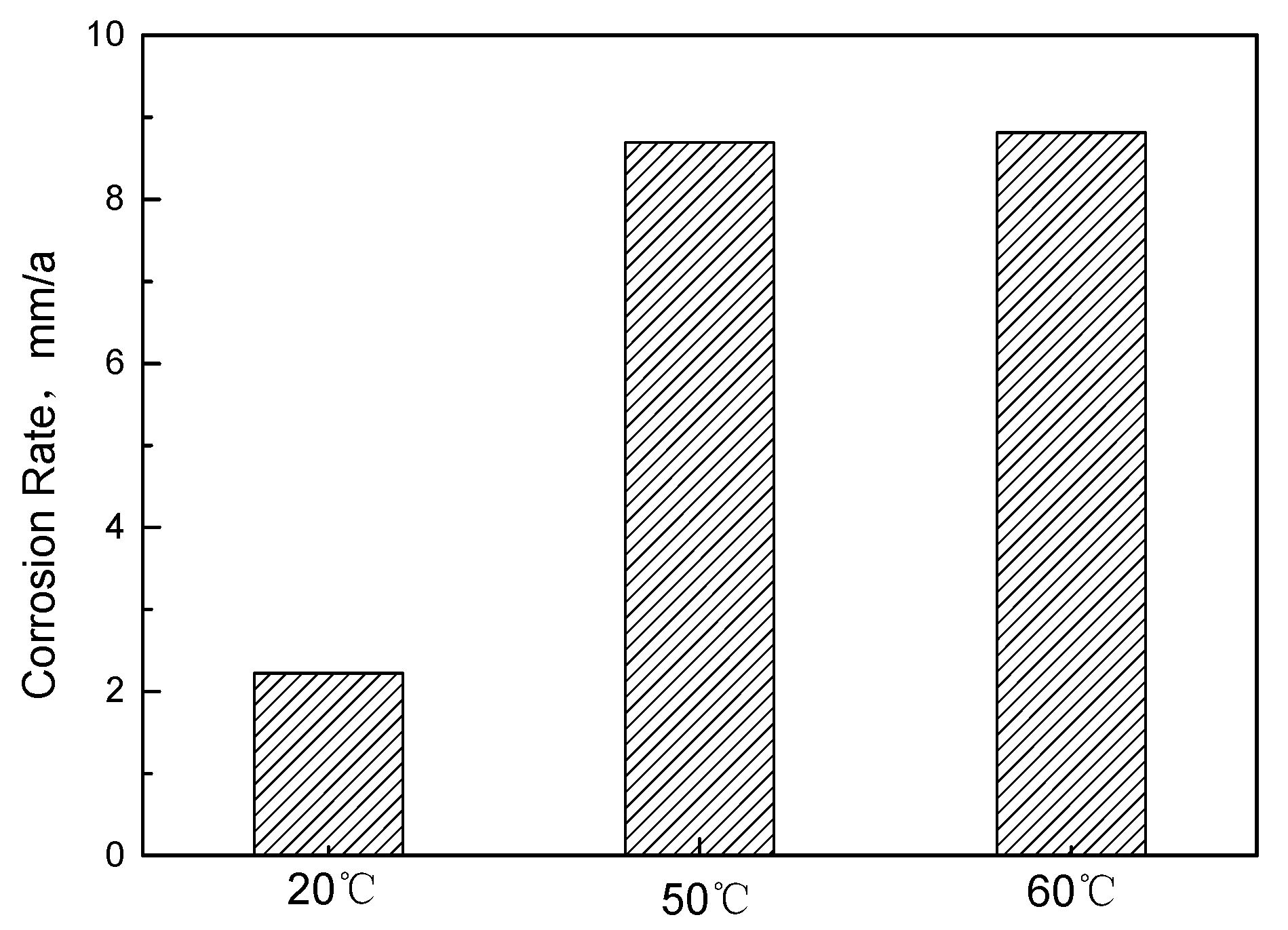
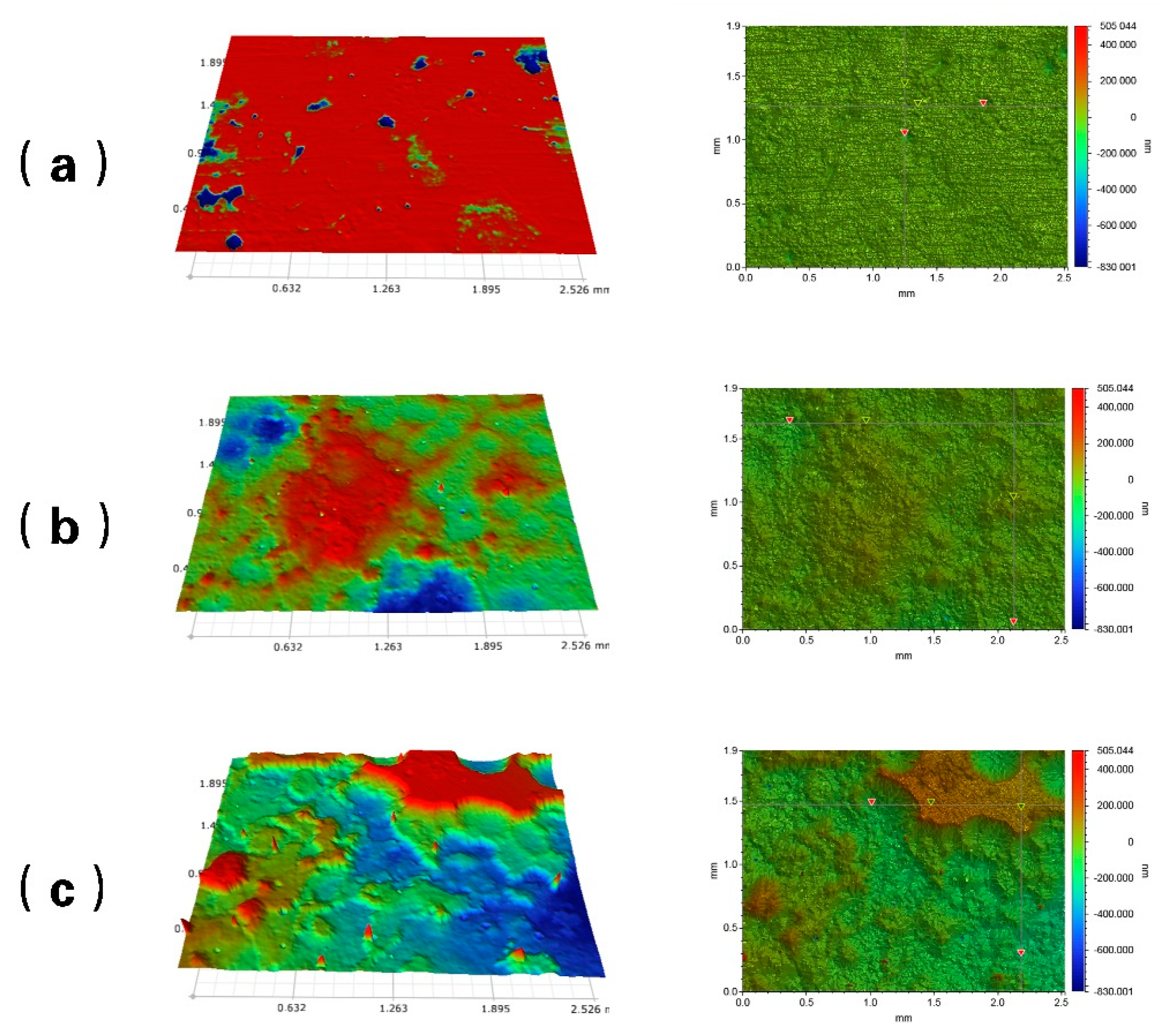
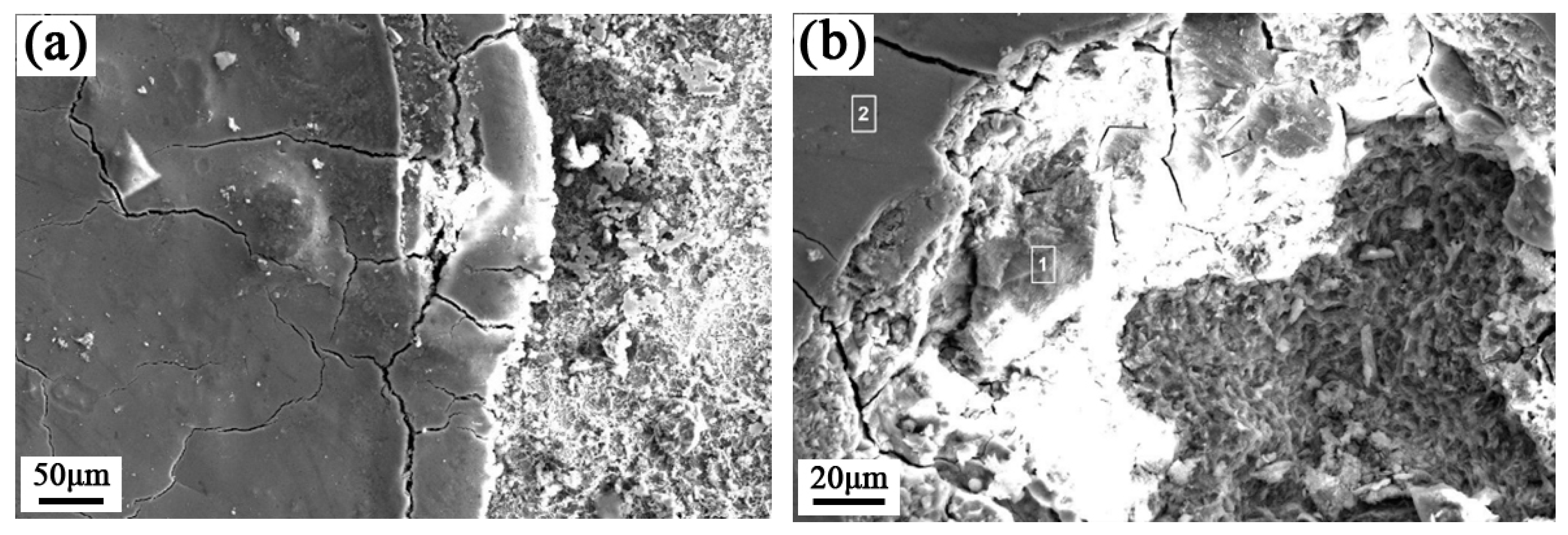
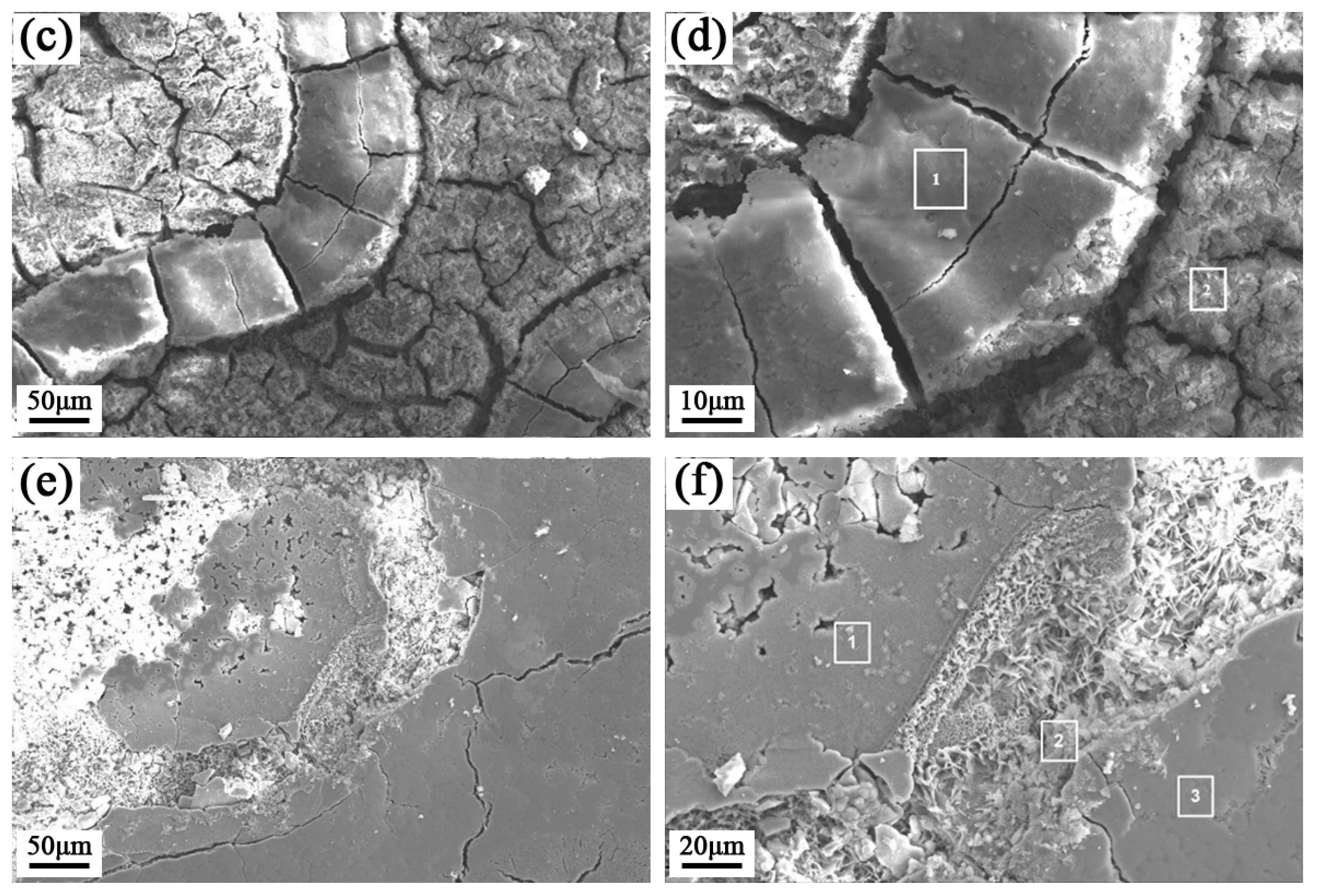
Corrosion Weight Loss Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, J.L.; Lin, H.L.; Yang, C.G.; Yang, K. Application and research status of antibacterial Cu−bearing stainless steel. China Metall. 2022, 32, 26–41. [Google Scholar]
- Huang, Y.; Liu, S.J.; Jiang, C.Y. Microbiologically influenced corrosion and mechanisms. Microbiol. China 2017, 44, 1699–1713. [Google Scholar]
- Huang, X.H.; Yu, H.; Wei, F.; Xi, M.M.; Liu, B.; Niu, A.J. Properties of X70 Grade Anti−bacterial HFW Welded Pipe. Pet. Tubul. Goods Instrum. 2022, 8, 19–23. [Google Scholar]
- Li, M.; Nan, L.; Xu, D.; Ren, G.; Yang, K. Antibacterial Performance of a Cu−bearing Stainless Steel against Microorganisms in Tap Water. J. Mater. Sci. Technol. 2015, 31, 243–251. [Google Scholar] [CrossRef]
- Nan, L.; Liu, Y.; Lü, M.; Yang, K. Study on antibacterial mechanism of copper−bearing austenitic antibacterial stainless steel by atomic force microscopy. J. Mater. Sci. Mater. Med. 2008, 19, 3057–3062. [Google Scholar] [CrossRef]
- González−Arévalo, N.; Velázquez, J.; Díaz−Cruz, M.; Cervantes−Tobón, A.; Terán, G.; Hernández−Sanchez, E.; Capula−Colindres, S. Influence of aging steel on pipeline burst pressure prediction and its impact on failure probability estimation. Eng. Fail. Anal. 2020, 120, 104950. [Google Scholar] [CrossRef]
- Jia, R.; Unsal, T.; Xu, D.; Lekbach, Y.; Gu, T. Microbiologically influenced corrosion and current mitigation strategies: A state of the art review. Int. Biodeterior. Biodegrad. 2018, 137, 42–58. [Google Scholar] [CrossRef]
- Vigneron, A.; Head, I.M.; Tsesmetzis, N. Damage to offshore production facilities by corrosive microbial biofilms. Appl. Microbiol. Biotechnol. 2018, 102, 2525–2533. [Google Scholar] [CrossRef]
- Farelas, F.; Galicia, M.; Brown, B.; Nesic, S.; Castaneda, H. Evolution of dissolution processes at the interface of carbon steel corroding in a CO2 environment studied by EIS. Corros. Sci. 2010, 52, 509–517. [Google Scholar] [CrossRef]
- Kermani, M.B.; Morshed, A. Carbon Dioxide Corrosion in Oil and Gas Production—A Compendium. Corrosion 2003, 59, 659–683. [Google Scholar] [CrossRef]
- Ezuber, H.M. Influence of temperature and thiosulfate on the corrosion behavior of steel in chloride solutions saturated in CO2. Mater. Des. 2009, 30, 3420–3427. [Google Scholar] [CrossRef]
- Zheng, P.F.; Wang, X.P.; Zhao, L.; Li, Q. Research and Application of Oilfield Water Injection Development Technology. Appl. Technol. 2013, 5, 162–168. [Google Scholar]
- Li, Y. Application and perspective of multiphase flow metering technologies for ocean oil and gas exploitation. J. Tsinghua Univ. (Sci Technol.) 2014, 54, 88–96. [Google Scholar]
- Mendorf, M.; Nachtrodt, H.; Mescher, A.; Ghaini, A.; Agar, D.W. Design and Control Techniques for the Numbering−up of Capillary Micro−reactors with Uniform Multiphase Flow Distribution. Ind. Eng. Chem. Res. 2010, 49, 10908–10916. [Google Scholar] [CrossRef]
- Robole, B.; Kvandal, H.K.; Schüller, R.B. The Norsk Hydro Multi Phase Flow Loop. A high pressure flow loop for real three−phase hydrocarbon systems. Flow Meas. Instrum. 2006, 17, 163–170. [Google Scholar] [CrossRef]
- Sun, Y.H.; Nesic, S. A parametric study and modeling on localized CO2 corrosion in horizontal wet gas flow. In Proceedings of the Corrosion 2004, NACE, New Orleans, LA, USA, 28 March–1 April 2004. Paper No: 04380. [Google Scholar]
- Nesic, S. Effects of Multiphase Flow on Internal CO2 Corrosion of Mild Steel Pipelines. Energy Fuels 2012, 26, 4098–4111. [Google Scholar] [CrossRef]
- Hong, T.; Sun, Y.; Jepson, W. Study on corrosion inhibitor in large pipelines under multiphase flow using EIS. Corros. Sci. 2002, 44, 101–112. [Google Scholar] [CrossRef]
- Nyborg, R.; Dugstad, A.; Martin, T. Top of Line Corrosion With High CO2 And Traces of H2S. In Proceedings of the Corrosion 2009, NACE, Atlanta, GE, USA, 22–26 March 2009. Paper No: 09283. [Google Scholar]
- Villarreal, J.; Laverde, D.; Fuentes, C. Carbon−steel corrosion in multiphase slug flow and CO2. Corros. Sci 2006, 48, 2363–2379. [Google Scholar] [CrossRef]
- Li, W.Q.; Yu, D.; Wu, H.H.; Li, Q.P.; Yao, H.Y.; Gong, J.; Chao, J.I. Design and Construction of a High Pressure Experimental Flow Loop for Hydrate /Wax Deposition. Res. Explor. Lab. 2011, 30, 13–16. [Google Scholar]
- Yu, Y.; Fan, X.H.; Zhang, Y.N.; Zhu, J.Y.; Chen, L.J.; Liu, Z.W.; Zhang, L. Top of Line Corrosion Behavior of X60 Pipeline Steel in CO2 Wet Gas Environment with High Cl−Concentration and High Gas Flow Rate. Mater. Prot. 2021, 54, 59–63. [Google Scholar]
- Seo, B.; Kanematsu, H.; Nakamoto, M.; Miyabayashi, Y.; Tanaka, T. Corrosion resistance and antibacterial activity of a surface coating created by super−spread wetting of liquid copper on laser−ablated carbon steel. Surf. Coat. Technol. 2022, 445, 128706. [Google Scholar] [CrossRef]
- Yu, H.B.; Chen, X.; Liu, Q.P.; Qi, Y.M.; Zhang, Z.H.; Li, Y.C. Anti Microbiological Corrosion Performance of Cu−Containing Antibacterial Pipeline Steel. Corros. Prot. 2020, 41, 10–15. [Google Scholar]
- Sun, W.D. Study on Prediction and Evaluation of Internal Corrosion of Submarine Pipeline for Imported Crude Oil. Corros. Prot. Petrochem. Ind. 2020, 37, 7–10. [Google Scholar]
- Chen, L.Q.; Sun, Y.L.; Chen, C.F.; Zheng, S.Q. Corrosion Behavior of N80 Steel in Simulated Environment of Natural Gaso−line−Mineralized Water−CO2 Multiphase Flow. Oil Field Equip. 2010, 39, 55–59. [Google Scholar]
- Wang, B.; Wang, Y.; Li, Q.P.; Li, H.X.; Zhang, L.; Lu, M.X. Effect of chromium on the corrosion behaviour of low Cr−bearing alloy steel under an extremely high flow rate. RSC Adv. 2020, 10, 35302–35309. [Google Scholar] [CrossRef]
- Lin, X.Q.; Liu, W.; Wu, F.; Xu, C.C. Effect of O2 on corrosion of 3Cr steel in high temperature and high pressure CO2−O2 en−vironment. Appl. Surf. Sci. 2015, 329, 104–115. [Google Scholar] [CrossRef]
- Zhu, C.M.; Liu, G.Z.; Dong, S.X.; Li, X.B.; Dong, B.J.; Liu, W. Effect of temperature on corrosion of 3Cr steel in CO2−O2 environment. J. Iron Steel Res. 2019, 31, 573–581. [Google Scholar]



| C | Cr | Mo | Si | Mn | Cu | V | Ni | Al | Ti | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.057 | 1.970 | 0.170 | 0.160 | 0.290 | 0.560 | 0.072 | 0.59 | 0.021 | 0.022 | Bal. |
| NaHCO3 | Na2SO4 | MgCl2 | NaCl | CaCl2 | SrCl2 |
|---|---|---|---|---|---|
| 32.98 | 92.38 | 427.77 | 17,065.67 | 19,037.2 | 2476.68 |
| No. | T (°C) | PCO2 (MPa) | O2 (ppb) | Velocity (ms−1) | t (h) |
|---|---|---|---|---|---|
| 1 | 20 | 5 | 155 | 0.75 | 168 |
| 2 | 50 | 5 | 155 | 0.75 | 168 |
| 3 | 60 | 5 | 155 | 0.75 | 168 |
| T/°C | Area | O | Cr | Fe | Ca | Cu | Cl |
|---|---|---|---|---|---|---|---|
| 20 | 1 | 15.20 | 1.90 | 82.90 | − | − | − |
| 2 | 21.34 | − | 78.66 | − | − | − | |
| 50 | 1 | 16.69 | 0.85 | 81.48 | 0.99 | − | − |
| 2 | 28.53 | 31.10 | 15.05 | 10.13 | 15.19 | − | |
| 60 | 1 | 18.75 | 0.05 | 73.96 | − | 1.56 | 5.68 |
| 2 | 23.85 | 0.02 | 75.01 | − | − | 1.11 |
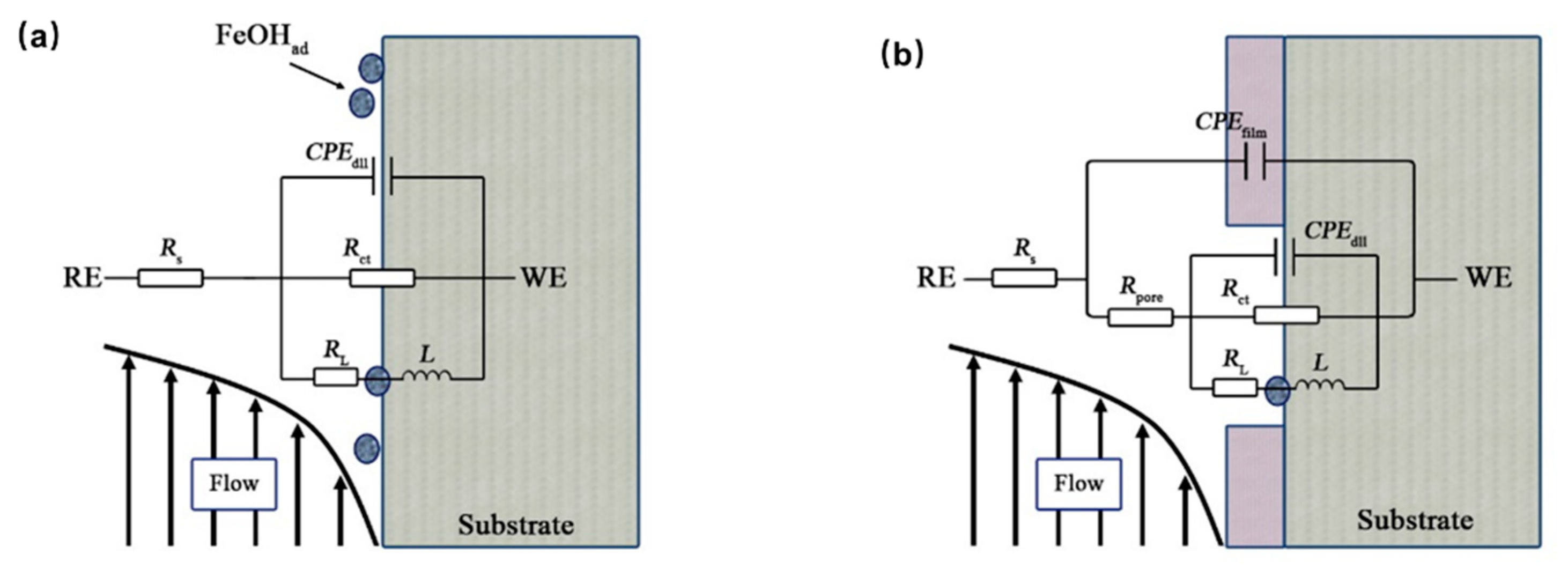
| Conditions | Rs (Ω·cm2) | CPEfilm (Ω−1∙sn∙cm−2) | CPEfilm−n | Rpore (Ω·cm2) | CPEdl (Ω−1∙sn∙cm−2) | CPEdl n | Rct (Ω·cm2) | RL (Ω⋅cm2) | L (H⋅cm−2) |
|---|---|---|---|---|---|---|---|---|---|
| 20 °C−24 h | 3.32 | 4.76 × 10−4 | 0.87 | 1.08 | 2.19 × 10−3 | 0.91 | 35.67 | 163.4 | 70.16 |
| 20 °C−48 h | 4.28 | 9.56 × 10−4 | 0.81 | 1.03 | 4.90 × 10−3 | 0.93 | 44.84 | 203.1 | 190.3 |
| 20 °C−72 h | 4.45 | 1.22 × 10−3 | 0.79 | 1.04 | 6.71 × 10−3 | 0.92 | 39.78 | 159.2 | 126.5 |
| 20 °C−96 h | 4.32 | 1.67 × 10−3 | 0.76 | 1.04 | 8.68 × 10−3 | 0.92 | 31.28 | 110.5 | 81.04 |
| 20 °C−120 h | 4.47 | 1.74 × 10−3 | 0.74 | 1.15 | 1.05 × 10−2 | 0.92 | 30.49 | 101.6 | 76.57 |
| 20 °C−144 h | 4.60 | 1.67 × 10−3 | 0.72 | 1.22 | 1.29 × 10−2 | 0.89 | 32.91 | 90.28 | 56.64 |
| 20 °C−168 h | 4.87 | 1.98 × 10−3 | 0.72 | 1.16 | 1.41 × 10−2 | 0.88 | 37.87 | 84.51 | 57.23 |
| 50 °C−24 h | 2.53 | − | − | − | 4.87 × 10−4 | 0.91 | 185.4 | 1271 | 4350 |
| 50 °C−48 h | 3.46 | − | − | − | 5.87 × 10−3 | 0.75 | 16.82 | 47.72 | 8.93 |
| 50 °C−72 h | 3.28 | − | − | − | 4.64 × 10−3 | 0.80 | 34.14 | 80.87 | 13.84 |
| 50 °C−96 h | 3.71 | 5.24 × 10−3 | 0.81 | 2.10 × 10−3 | 3.44 × 10−2 | 0.80 | 31.23 | 58.28 | 25.79 |
| 50 °C−120 h | 3.33 | 4.75 × 10−4 | 1.00 | 0.37 | 5.15 × 10−3 | 0.81 | 19.26 | 52.86 | 23.88 |
| 50 °C−144 h | 3.71 | 4.27 × 10−4 | 1.00 | 0.43 | 4.15 × 10−3 | 0.79 | 28.3 | 78.45 | 37.4 |
| 50 °C−168 h | 2.98 | − | − | − | 4.68 × 10−3 | 0.80 | 13.68 | 30.99 | 6.54 |
| 60 °C−24 h | 1.88 | 7.03 × 10−5 | 1.00 | 0.96 | 4.18 × 10−4 | 0.86 | 103 | 502.6 | 96.61 |
| 60 °C−72 h | 3.15 | − | − | − | 1.72 × 10−3 | 0.74 | 32.29 | 63.07 | 29.82 |
| 60 °C−96 h | 3.11 | − | − | − | 1.97 × 10−3 | 0.72 | 28.12 | 53.89 | 14.66 |
| 60 °C−120 h | 2.82 | 2.30 × 10−3 | 0.70 | 10.16 | 9.79 × 10−4 | 0.36 | 8.32 | 1.63 | 0.9 |
| 60 °C−144 h | 3.29 | 4.43 × 10−5 | 1.00 | 0.71 | 1.94 × 10−3 | 0.73 | 24.45 | 59.15 | 16.37 |
| 60 °C−168 h | 3.14 | 4.96 × 10−5 | 1.00 | 0.78 | 1.75 × 10−3 | 0.75 | 21.34 | 44.99 | 10.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, Y.; Wen, L.; Kong, W.; Yang, Y.; Zhu, J.; Liu, F.; Jin, Y. Study of Temperature on the Corrosion Behavior of Antibacterial Steel by a Large−Scale Multiphase Flow Corrosion Test Loop. Materials 2022, 15, 7472. https://doi.org/10.3390/ma15217472
Zhang Y, Zhang Y, Wen L, Kong W, Yang Y, Zhu J, Liu F, Jin Y. Study of Temperature on the Corrosion Behavior of Antibacterial Steel by a Large−Scale Multiphase Flow Corrosion Test Loop. Materials. 2022; 15(21):7472. https://doi.org/10.3390/ma15217472
Chicago/Turabian StyleZhang, Yunan, Yongqiang Zhang, Lei Wen, Wei Kong, Yinghua Yang, Jinyang Zhu, Fuhai Liu, and Ying Jin. 2022. "Study of Temperature on the Corrosion Behavior of Antibacterial Steel by a Large−Scale Multiphase Flow Corrosion Test Loop" Materials 15, no. 21: 7472. https://doi.org/10.3390/ma15217472
APA StyleZhang, Y., Zhang, Y., Wen, L., Kong, W., Yang, Y., Zhu, J., Liu, F., & Jin, Y. (2022). Study of Temperature on the Corrosion Behavior of Antibacterial Steel by a Large−Scale Multiphase Flow Corrosion Test Loop. Materials, 15(21), 7472. https://doi.org/10.3390/ma15217472








