Development and Application of Carbonate Dissolution Test Equipment under Thermal–Hydraulic–Chemical Coupling Condition
Abstract
1. Introduction
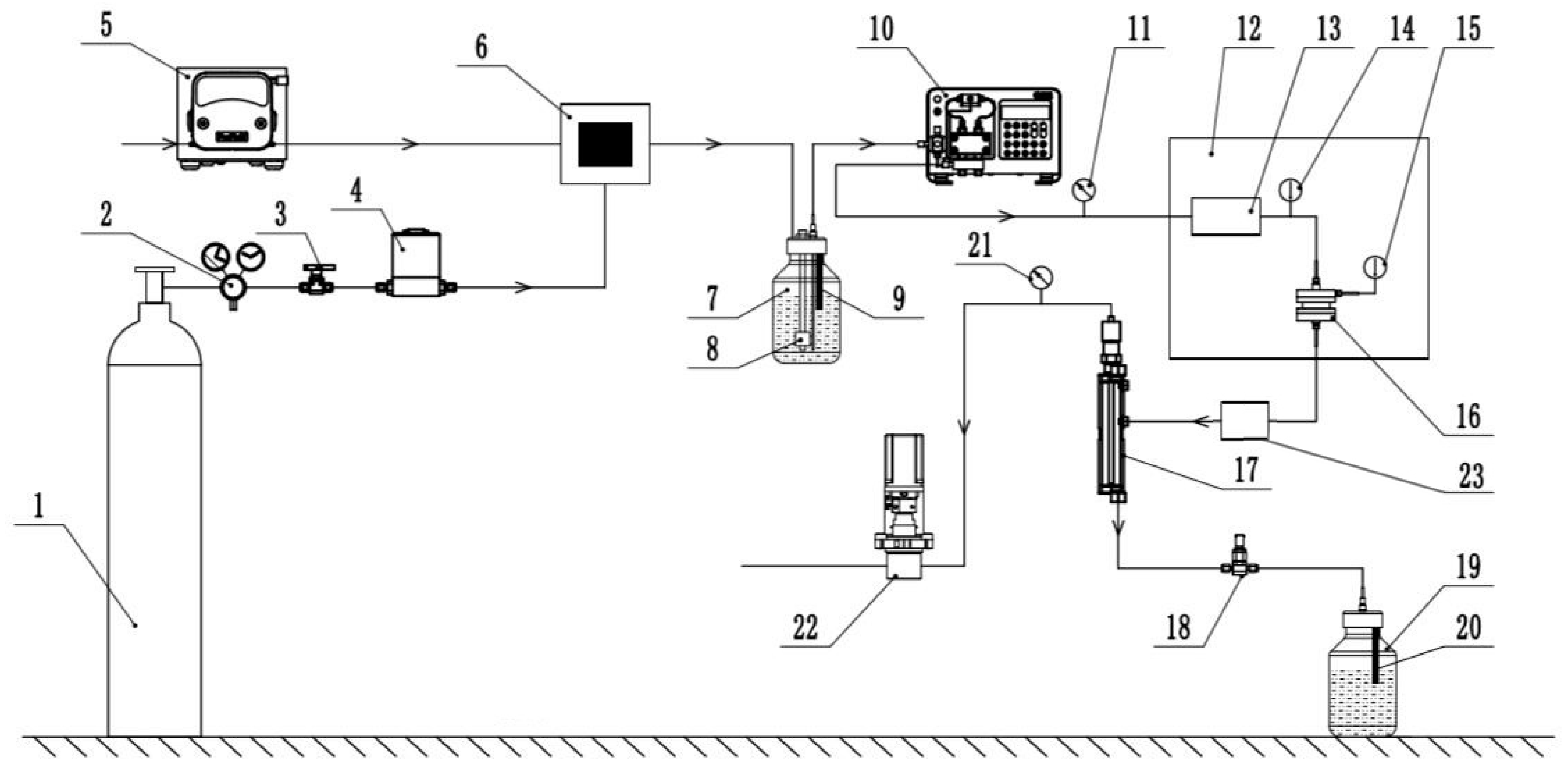
2. Test Equipment and Apparatus
2.1. Carbonated Water Preparation System
2.2. Dissolution Reaction System
2.3. Solution Receiving System
3. Materials and Experimental Methodology
3.1. Test Sample
3.1.1. Rock Samples
3.1.2. Sample of Solution
3.2. Test Plan
3.2.1. Test Conditions
3.2.2. Test Steps
4. Results and Discussion
4.1. Macro-Feature Analysis
4.2. Analysis of Dissolution Rate Change
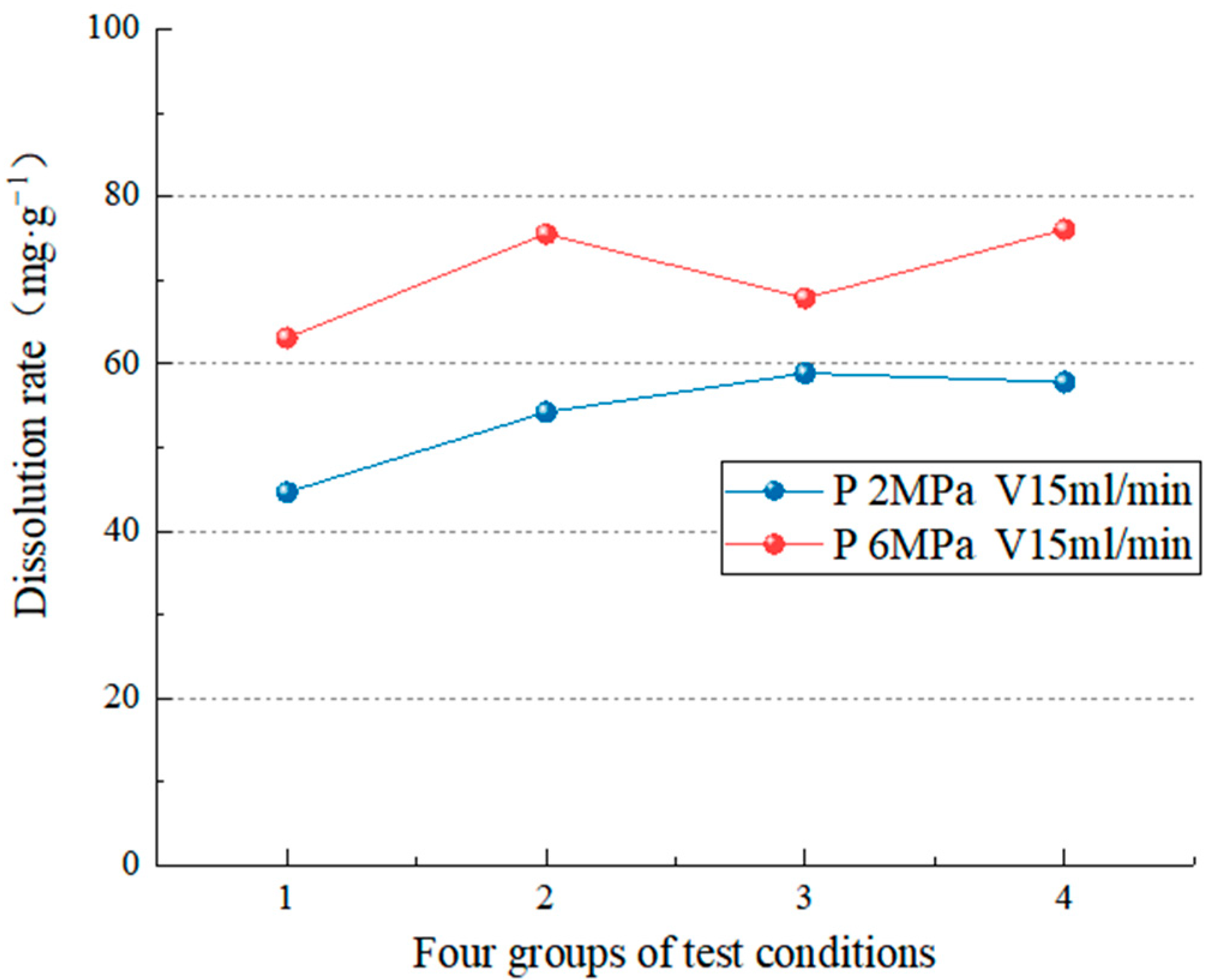
4.2.1. Dissolution Characteristics under Different Hydrodynamic Pressures
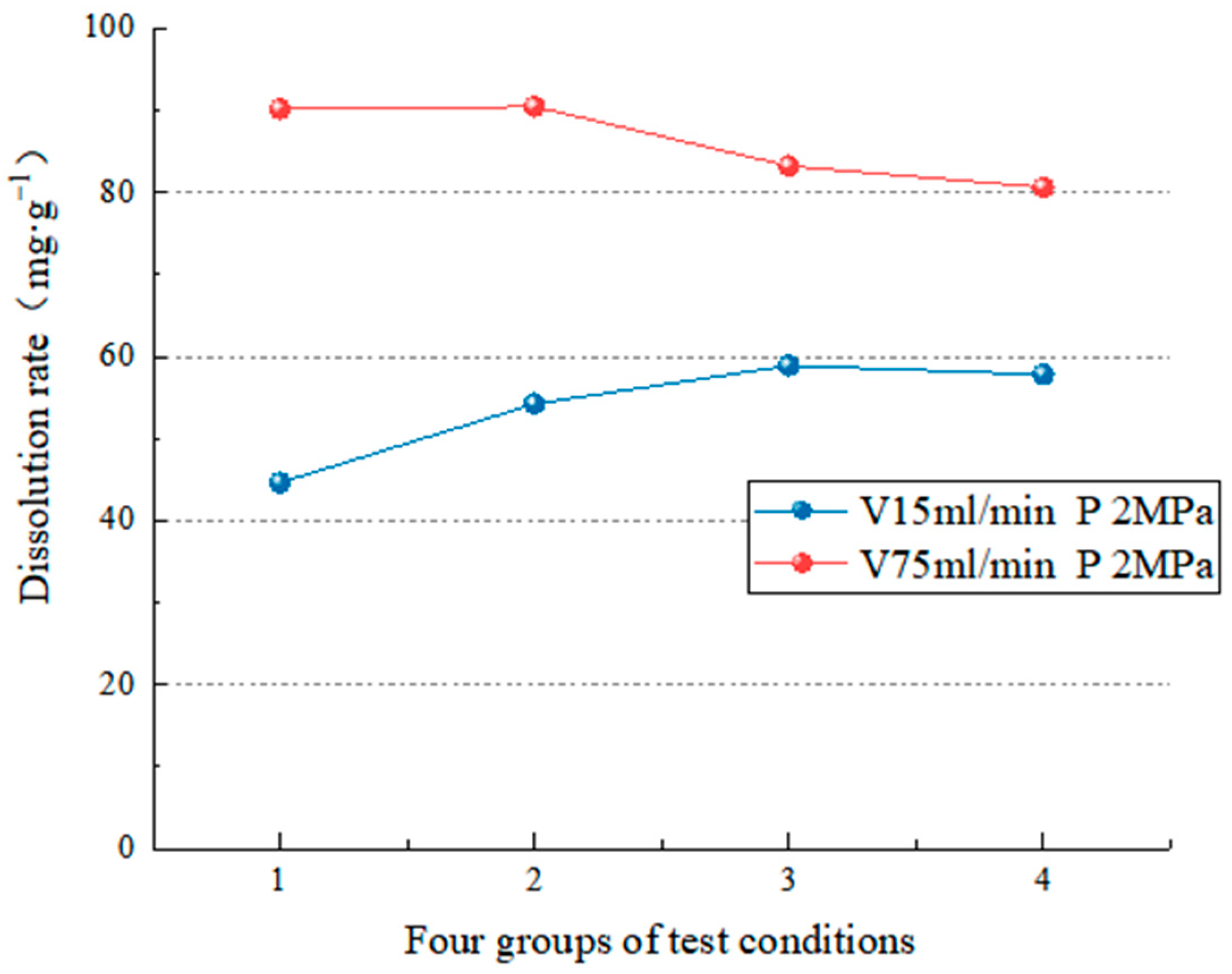
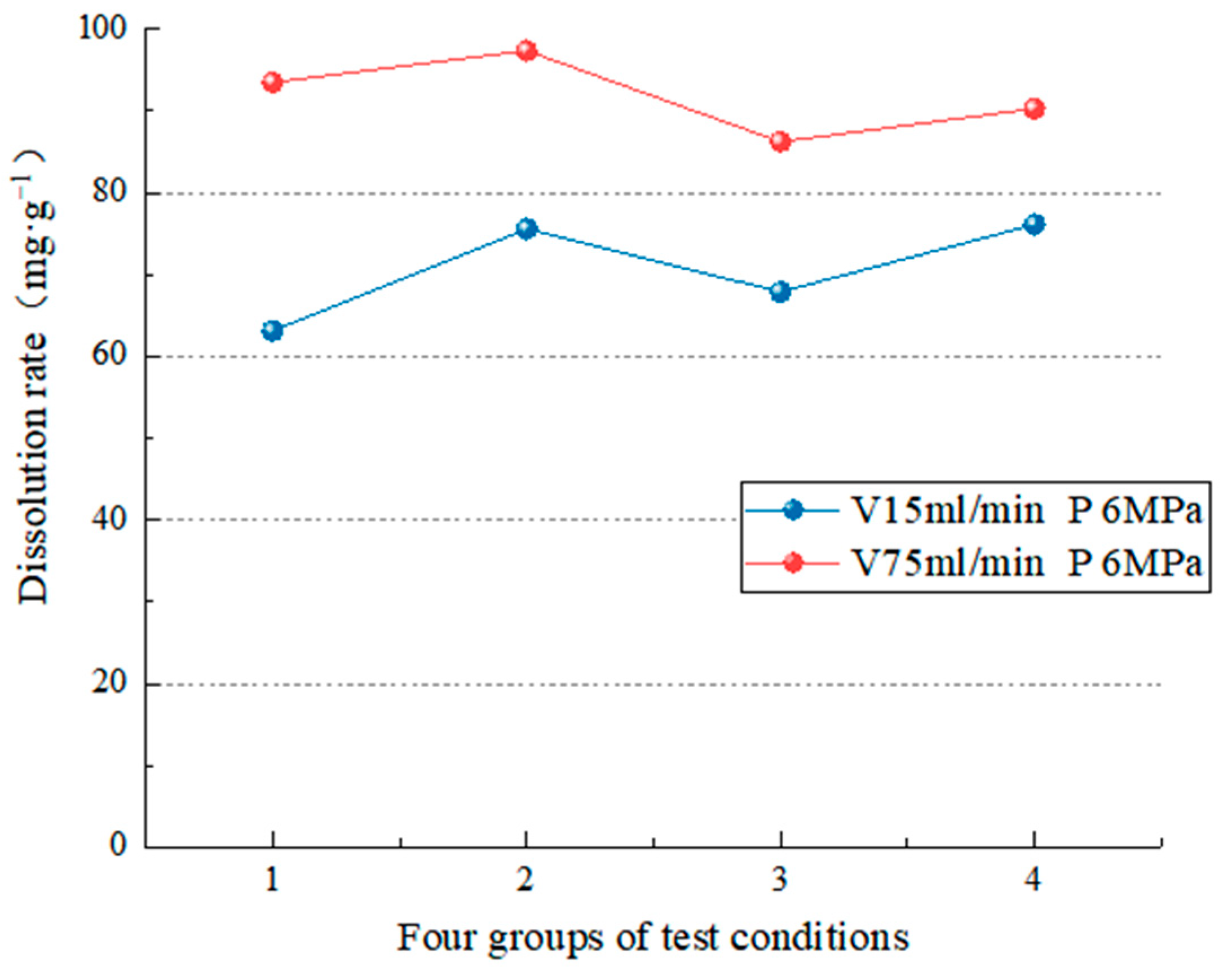
4.2.2. Dissolution Characteristics under Different Water Flow Velocities
4.3. Analysis of the Chemical Composition of Water
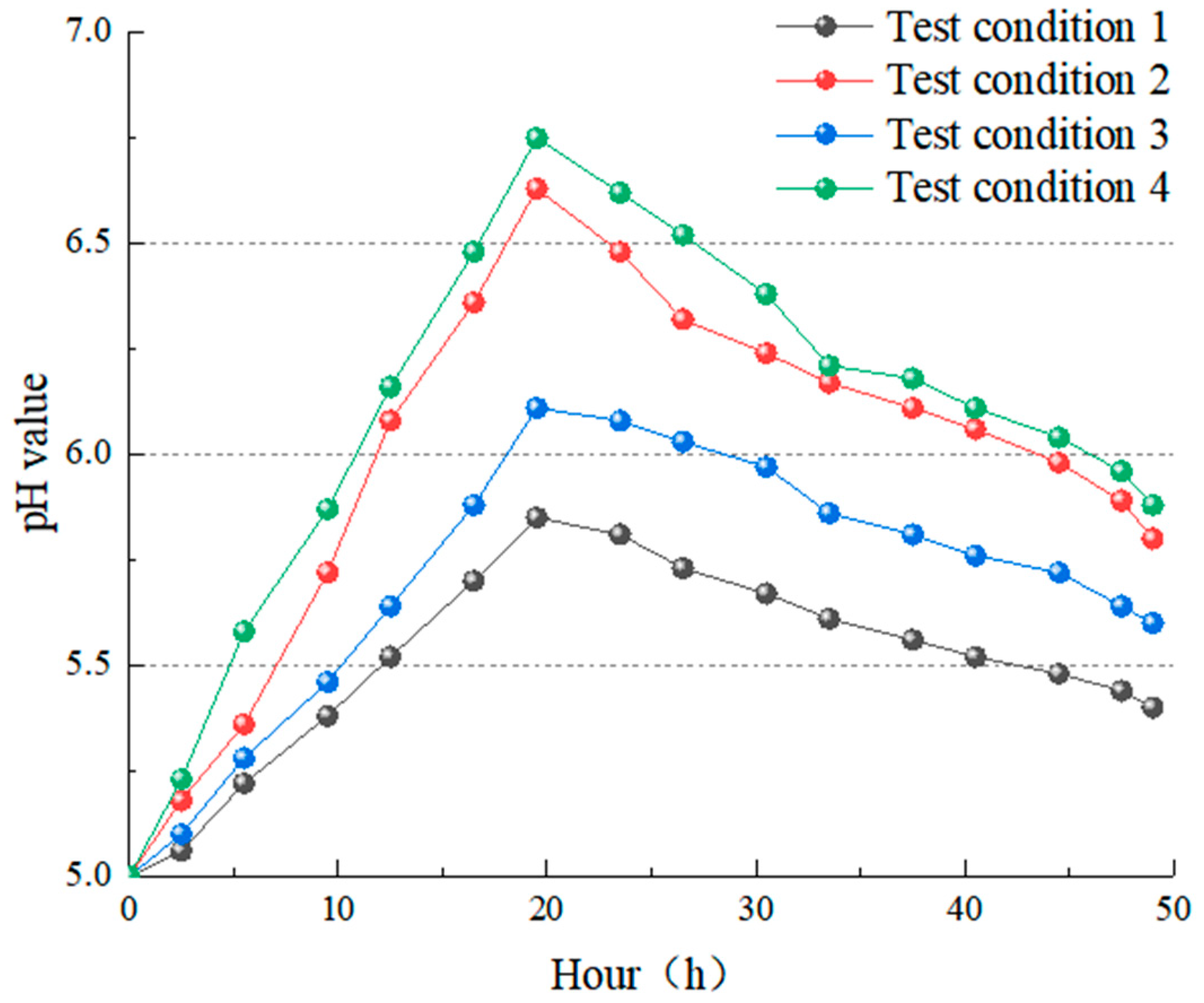
4.3.1. The Change Rule of pH Value
4.3.2. The Change Law of Electrical Conductivity
4.3.3. Change Rule of Ca2+ Concentration
5. Summary and Conclusions
- Self-designed and manufactured a set of carbonate rock hydrodynamic pressure dissolution test equipment simulating multifactor coupling conditions, which outperforms the previously closed dissolution test equipment. First, the smaller gas–liquid mixing microreactor was used for the first time in dissolution equipment. The micro-mixing technology ensured rapid and complete mixing of CO2 and aqueous solution. Second, the exact configuration of carbonic acid solutions with different concentrations was completed online in real time, ensuring safe, continuous, stable, and efficient gas–liquid mixing, breaking the limitation of pH parameter selection and the stability of previous test instruments, and significantly reducing the volume of carbonated water preparation device [26,37,50,51]. Next, the test system employs computer control software to control and set related devices and parameters, which reduces the difficulty of using the instrument, ensures numerical value accuracy, and enables intuitive observation. Third, the pressure and hydrodynamic device realize the combined regulation of solution dynamic water pressure and speed, raises and stabilizes the liquid pressure via the back pressure valve and adjusts the flow rate via the high-pressure constant-current infusion pump to ensure that the solution can complete the flow rate adjustment at different pressures. It breaks through the previous test hydrodynamic system that cannot be regulated simultaneously with the pressure system [26,51,52,53,55];
- The test simulates carbonate rock’s dissolution effect in different environmental factors (such as water chemical conditions, temperature conditions, dynamic water pressure conditions, and water flow speed conditions). In the analysis of the carbonate rock dissolution rate, the dissolution amount of the carbonate rock is directly proportional to the water flow speed [37], the dynamic water pressure [19,25,39,40], and the influence of the flow rate on the dissolution rate is more significant than the dynamic water pressure [38]. When the water flow rate is high (75 mL/min), the change in hydrodynamic pressure slightly impacts the dissolution rate (the average dissolution rate is 5.68 mg/g). However, when the flow rate is low (15 mL/min), the hydrodynamic pressure change significantly (the average dissolution rate is 16.76 mg/g). In addition, when the hydrodynamic pressure is low (2 MPa), the change in water velocity significantly impacts the dissolution rate (the average dissolution rate is 32.23 mg/g). On the other hand, when the hydrodynamic pressure is high (6 MPa), the change in water velocity has little impact (the average dissolution rate is 21.15 mg/g). To realize the coupling of water flow speed and dynamic water pressure, it breaks through the coupling of two factors that cannot be achieved by previous test, and also lack of research of the dissolution effect and the development law under the coupling action;
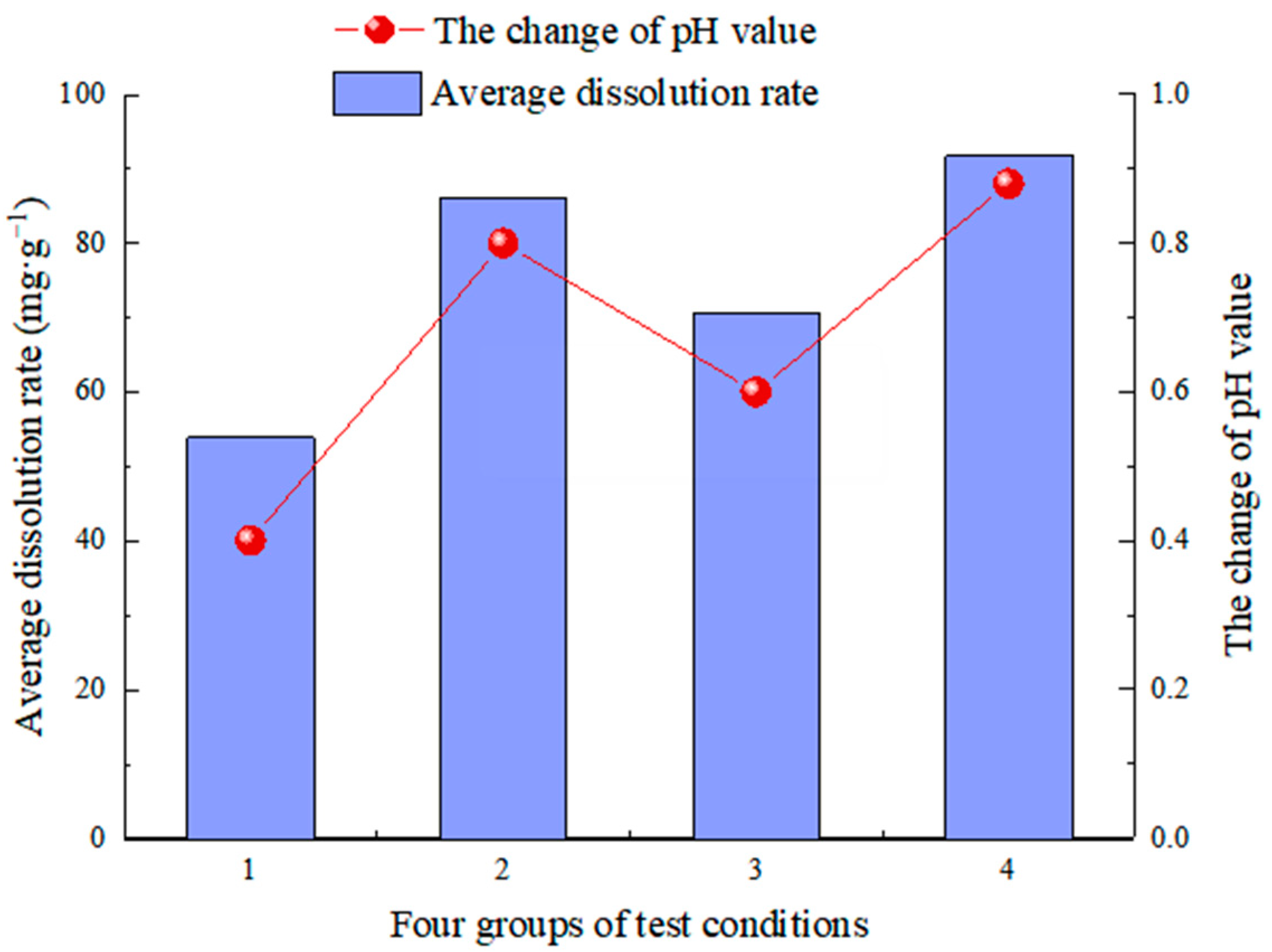
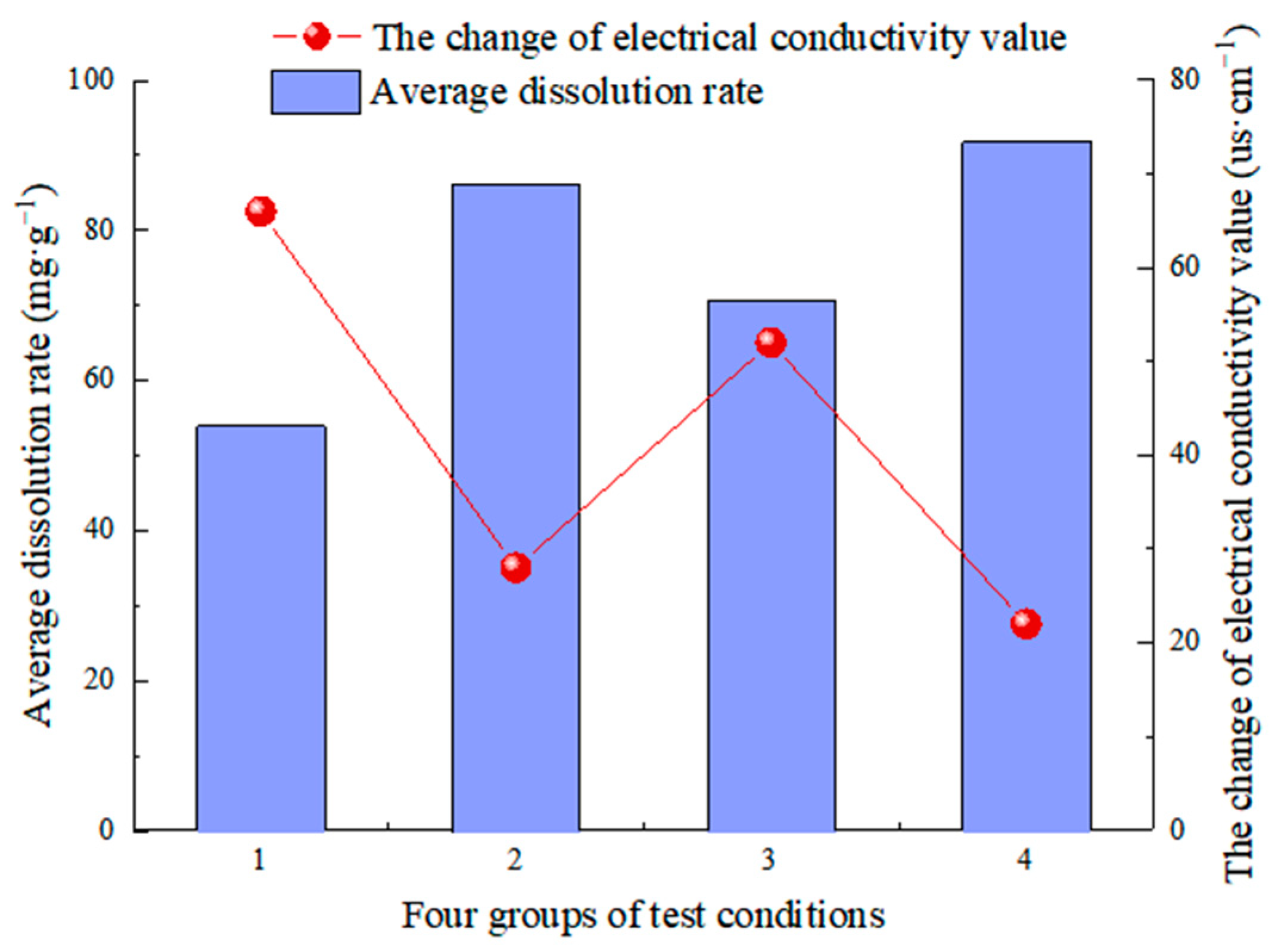
- CO2 aqueous solution significantly influences limestone dissolution and is the most important carbonate dissolution medium in the near-surface supergene condition. Following dissolution, visible dissolution grooves and grooves appear on the surface of rock samples, along with new calcium carbonate deposits. The changes in the solution’s pH value, conductivity value, and Ca2+ ion concentration show the dissolution rate and degree. The values after dissolution are greater than those before the test. The pH values were changed at +0.40, +0.80, +0.60, and +0.88, which are directly proportional to the change in the dissolution rate [57,58]. The conductivity values were +66 µs/cm, +28 µs/cm, +52 µs/cm and +22 µs/cm, and the Ca2+ ion content was +13.05, +6.48 and +10.25, respectively, which were inversely proportional to the change in dissolution rate [55]. The pH value generally follows the law of “first increasing and then decreasing, tending to be stable with a little fluctuation” during the test, which can effectively reflect the dissolution process of limestone under actual conditions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morse, J.W.; Arvidson, R.S.; Lüttge, A. Calcium Carbonate Formation and Dissolution. Chem. Rev. 2007, 107, 342–381. [Google Scholar] [CrossRef] [PubMed]
- Burdige, D.J.; Hu, X.; Zimmerman, R.C. The Widespread Occurrence of Coupled Carbonate Dissolution/Reprecipitation in Surface Sediments on the Bahamas Bank. Am. J. Sci. 2010, 310, 492–521. [Google Scholar] [CrossRef]
- Lunstrum, A.; Berelson, W. CaCO3 Dissolution in Carbonate-Poor Shelf Sands Increases with Ocean Acidification and Porewater Residence Time. Geochim. Cosmochim. Acta 2022, 329, 168–184. [Google Scholar] [CrossRef]
- Cai, W.-J.; Feely, R.A.; Testa, J.M.; Li, M.; Evans, W.; Alin, S.R.; Xu, Y.-Y.; Pelletier, G.; Ahmed, A.; Greeley, D.J.; et al. Natural and Anthropogenic Drivers of Acidification in Large Estuaries. Annu. Rev. Mar. Sci. 2021, 13, 23–55. [Google Scholar] [CrossRef] [PubMed]
- Araújo, R.E.B.; La Bruna, V.; Rustichelli, A.; Bezerra, F.H.R.; Xavier, M.M.; Audra, P.; Barbosa, J.A.; Antonino, A.C.D. Structural and Sedimentary Discontinuities Control the Generation of Karst Dissolution Cavities in a Carbonate Sequence, Potiguar Basin, Brazil. Mar. Pet. Geol. 2021, 123, 104753. [Google Scholar] [CrossRef]
- Zhang, C. Carbonate Rock Dissolution Rates in Different Landuses and Their Carbon Sink Effect. Chin. Sci. Bull. 2011, 56, 3759–3765. [Google Scholar] [CrossRef]
- Eisenlohr, L.; Meteva, K.; Gabrovšek, F.; Dreybrodt, W. The inhibiting action of intrinsic impurities in natural calcium carbonate minerals to their dissolution kinetics in aqueous H2O-CO2 solutions. Geochim. Cosmochim. Acta 1999, 63, 989–1002. [Google Scholar] [CrossRef]
- Sjöberg, E.L.; Rickard, D.T. Temperature dependence of calcite dissolution kinetics between 1 and 62 °C at pH 2.7 to 8.4 in aqueous solutions. Geochim. Cosmochim. Acta 1984, 48, 485–493. [Google Scholar] [CrossRef]
- Amrhein, C.; Jurinak, J.J.; Moore, W.M. Kinetics of calcite dissolution as affected by carbon dioxide partial pressure. Soil Sci. Soc. Am. J. 1985, 49, 1393–1398. [Google Scholar] [CrossRef]
- Buhmann, D.; Dreybrodt, W. Calcite dissolution kinetics in the system H2O-CO2—CaCO3 with participation of foreign ions. Chem. Geol. 1987, 64, 89–102. [Google Scholar] [CrossRef]
- Ma, K.; Liu, G. Three-Dimensional Discontinuous Deformation Analysis of Failure Mechanisms and Movement Characteristics of Slope Rockfalls. Rock Mech. Rock Eng. 2022, 55, 275–296. [Google Scholar] [CrossRef]
- Plummer, L.N.; Wigley, T.M.L.; Parkhurst, D.L. The kinetics of calcite dissolution in CO2-water systems at 5 to 60 °C and 0.0 to 1.0 atm CO2. Am. J. Sci. 1978, 278, 179–216. [Google Scholar] [CrossRef]
- Plummer, L.N.; Wigley, T.M.L.; Parkhurst, D.L. Critical review of the kinetics of calcite dissolution and precipitation. Chem. Model. Aqueous Syst. 1979, 93, 537–573. [Google Scholar]
- Alkattan, M.; Oelkers, E.H.; Dandurand, J.L.; Schott, J. An experimental study of calcite and limestone dissolution rates as a function of pH from −1 to 3 and temperature from 25 to 80 °C. Chem. Geol. 1998, 151, 199–214. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Golubev, S.V.; Schott, J. Dissolution kinetics of calcite, dolomite and magnesite at 25 °C and 0 to 50 atm pCO2. Chem. Geol. 2005, 217, 239–255. [Google Scholar] [CrossRef]
- Sjöberg, E.L.; Rickard, D. The Influence of Experimental Design on the Rate of Calcite Dissolution. Geochim. Cosmochim. Acta 1983, 47, 2281–2285. [Google Scholar] [CrossRef]
- Weyl, P.K. The change in solubility of calcium carbonate with temperature and carbon dioxide content. Geochim. Cosmochim. Acta 1959, 17, 214–225. [Google Scholar] [CrossRef]
- Schott, J.; Brantley, S.; Crerar, D.; Guy, C.; Borcsik, M.; Willaime, C. Dissolution kinetics of strained calcite. Geochim. Cosmochim. Acta 1989, 53, 373–382. [Google Scholar] [CrossRef]
- Rosenbauer, R.J.; Koksalan, T.; Palandri, J.L. Experimental Investigation of CO2–Brine–Rock Interactions at Elevated Temperature and Pressure: Implications for CO2 Sequestration in Deep-Saline Aquifers. Fuel Process. Technol. 2005, 86, 1581–1597. [Google Scholar] [CrossRef]
- Peng, J.; Wang, X.; Han, H.; Yin, S.; Xia, Q.; Li, B. Simulation for the Dissolution Mechanism of Cambrian Carbonate Rocks in Tarim Basin, NW China. Pet. Explor. Dev. 2018, 45, 431–441. [Google Scholar] [CrossRef]
- Valencia, F.L.; Laya, J.C. Deep-Burial Dissolution in an Oligocene-Miocene Giant Carbonate Reservoir (Perla Limestone), Gulf of Venezuela Basin: Implications on Microporosity Development. Mar. Pet. Geol. 2020, 113, 104144. [Google Scholar] [CrossRef]
- Taheri, M.; Ghobadi, M.H.; Yari, M.; Taheri, K. Laboratory Simulation of Karst Media Dissolution: An Experimental Approach and a Case Study. Carbonates Evaporites 2018, 33, 301–314. [Google Scholar] [CrossRef]
- Dove, P.M.; Crerar, D.A. Kinetics of quartz dissolution in electrolyte solutions using a hydrothermal mixed flow reactor. Geochim. Cosmochim. Acta 1990, 54, 955–969. [Google Scholar] [CrossRef]
- Herman, J.S.; White, W.B. Dissolution Kinetics of Dolomite: Effects of Lithology and Fluid Flow Velocity. Geochim. Cosmochim. Acta 1985, 49, 2017–2026. [Google Scholar] [CrossRef]
- Liu, Q.; Lu, Y.; Zhang, F. Laboratory Simulation Experiment on Dissolution of Limestone under Hydrodynamic Pressure. Carbonates Evaporites 2013, 28, 3–11. [Google Scholar] [CrossRef]
- Shao, D.M. Study on Dissolution Test of Ordovician Carbonate Rock in North-China Typical Coalfields. Master’s Thesis, China Coal Research Institute, Beijing, China, 2009. [Google Scholar]
- Shih, S.-M.; Lin, J.-P.; Shiau, G.-Y. Dissolution Rates of Limestones of Different Sources. J. Hazard. Mater. 2000, 79, 159–171. [Google Scholar] [CrossRef]
- Garing, C.; Gouze, P.; Kassab, M.; Riva, M.; Guadagnini, A. Anti-Correlated Porosity–Permeability Changes During the Dissolution of Carbonate Rocks: Experimental Evidences and Modeling. Transp. Porous Media 2015, 107, 595–621. [Google Scholar] [CrossRef]
- Liu, Z.; Yuan, D.; Dreybrodt, W. Comparative Study of Dissolution Rate-Determining Mechanisms of Limestone and Dolomite. Environ. Geol. 2005, 49, 274–279. [Google Scholar] [CrossRef]
- Jora, M.Z.; Nunes de Souza, R.; Lucas-Oliveira, E.; Speglich, C.; Bonagamba, T.J.; Sabadini, E. Static Acid Dissolution of Carbonate Outcrops Investigated by 1H NMR and X-Ray Tomography. J. Pet. Sci. Eng. 2021, 207, 109124. [Google Scholar] [CrossRef]
- Lyu, Q.; Ranjith, P.; Long, X.; Ji, B. Experimental Investigation of Mechanical Properties of Black Shales after CO2-Water-Rock Interaction. Materials 2016, 9, 663. [Google Scholar] [CrossRef]
- Hankermeyer, C.R.; Ohashi, K.L.; Delaney, D.C.; Ross, J.; Constantz, B.R. Dissolution Rates of Carbonated Hydroxyapatite in Hydrochloric Acid. Biomaterials 2002, 23, 743–750. [Google Scholar] [CrossRef]
- Gautelier, M.; Schott, J.; Oelkers, E.H. An Experimental Study of Dolomite Dissolution Rates at 80 °C as a Function of Chemical Affinity and Solution Composition. Chem. Geol. 2007, 242, 509–517. [Google Scholar] [CrossRef]
- Yoo, H.; Kim, Y.; Lee, W.; Lee, J. An Experimental Study on Acid-Rock Reaction Kinetics Using Dolomite in Carbonate Acidizing. J. Pet. Sci. Eng. 2018, 168, 478–494. [Google Scholar] [CrossRef]
- Huang, H.; Chen, Z.H.; Wang, T.; Luo, Z.H.; Zhang, L.; Wang, J.; Xiang, C.J.; Sun, B.T.; Wang, Y. Groundwater source identification incarbonate-hosted deposit using hydrogeochemistry, hydrogen and oxygen isotope method. Hydrogeol. Eng. Geol. 2019, 46, 19–26. [Google Scholar]
- Fan, M.; Jiang, X.Q.; Liu, W.X.; Zhang, J.Y.; Chen, H.Y. Dissolution of carbonate rocks in CO2 solution under the different temperatures. Acta Sedimentol. Sin. 2007, 25, 825–830. [Google Scholar]
- Gao, J.W. Experimental Simulation of Dissolution of Carbonate Rocks in Acid Solution. Liaoning Chem. Ind. 2016, 45, 254–256. [Google Scholar]
- Shao, D.M. Influence of temperature on dissolution rate in Ordovician carbonate rock in different water flow rate. Coal Geol. Explor. 2012, 40, 62–65. [Google Scholar]
- Yang, Y.K.; Liu, B.; Qin, S.; Luo, P.; Zhang, D.M.; Zhou, M.H.; Shi, K.B.; Tian, Y.J. Re-recognition of deep carbonate dissolution based on the observation of in-situ simulation experiment. Acta Sci. Nat. Univ. Pekin. 2014, 50, 316–322. (In Chinese) [Google Scholar]
- Shou, J.F.; She, M.; Shen, A.J. Experimental study of carbonate dissolution modification effect under deep conditions. Bull. Mineral. Petrol. Geochem. 2016, 35, 860–867. [Google Scholar]
- Taheri, M.; Ghobadi, M.H.; Mohseni, H.; Feyzi, M. Dissolution Behavior of Carbonate Rocks under Various Physicochemical Conditions: A Case Study; the Taleh Zang Formation, West of Iran. Carbonates Evaporites 2021, 36, 47. [Google Scholar] [CrossRef]
- Gautelier, M.; Oelkers, E.H.; Schott, J. An Experimental Study of Dolomite Dissolution Rates as a Function of pH from −0.5 to 5 and Temperature from 25 to 80 °C. Chem. Geol. 1999, 157, 13–26. [Google Scholar] [CrossRef]
- Taheri, M.; Nikudel, M.R.; Khamehchiyan, M.; Taheri, K. Laboratory Simulation of Karst Development in Carbonate Rocks Containing Insoluble Substances: A Case Study from West Iran. Bull. Eng. Geol. Environ. 2016, 75, 53–62. [Google Scholar] [CrossRef]
- Sjöberg, E.L.; Rickard, D.T. Calcite Dissolution Kinetics: Surface Speciation and the Origin of the Variable pH Dependence. Chem. Geol. 1984, 42, 119–136. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Wang, F.G.; Li, C.S.; Wu, F.S.; Cao, Y.Q. Carbonate Karst Erosion Experiment Research of Guizhou Huangguoshu Tunnel. Coal Technol. 2016, 35, 310–312. [Google Scholar]
- Mazzullo, S.J.; Harris, P.M. Mesogenetic Dissolution: Its Role in Porosity Development in Carbonate Reservoir. AAPG Bull. 1992, 76, 607–620. [Google Scholar]
- Buijse, M.; de Boer, P.; Breukel, B.; Burgos, G. Organic acids in carbonate acidizing. In Proceedings of the SPE European Formation Damage Conference, The Hague, The Netherlands, 13–14 May 2003. [Google Scholar]
- She, M.; Shou, J.F.; Shen, A.J.; He, X.Y.; Zhu, Y.; Wang, Y.; Zhang, T.F. Simulation experiment of the dissolution effect of buried organic acid fluid on dolomite reservoir. J. China Univ. Pet. (Nat. Sci.) 2014, 38, 10–17. [Google Scholar]
- Yang, J.J. Experimental simulation of carbonate dissolution process under temperature and pressure conditions. Acta Sedimentol. Sin. 1995, 13, 49–54. [Google Scholar]
- Wang, W.; Huang, K.J.; Bao, Z.Y.; Qiang, Y.X. Dissolution dynamics characteristics of different types of granular limestone reservoirs. Pet. Explor. Dev. 2011, 38, 495–502. [Google Scholar]
- Fan, M.; He, Z.L.; Li, Z.M.; Yu, L.J.; Zhang, W.T.; Liu, W.X.; Jiang, X.Q. Dissolution window of carbonate rocks and its geological significance. Oil Gas Geol. 2011, 32, 499–505. [Google Scholar]
- Jiang, X.Q.; Wang, S.Y.; Fan, M.; Zhang, J.Y.; Guan, H.L.; Bao, Y.J. Experimental study of carbonate dissolution in buried rock environment. Pet. Geol. Exp. 2008, 30, 643–646. [Google Scholar]
- She, M.; Shou, J.F.; Shen, A.J.; Zhu, Y.; Zheng, X.P. Experimental simulation of dissolution for carbonate rocks in organic acid under the conditions from epigenesis to deep burial environments. Geochimica 2014, 43, 276–286. [Google Scholar]
- Huang, S.Y.; Song, C.R. Dission and ambient temperature of carbonate rock. Carsologica Sin. 1987, 6, 287–296. [Google Scholar]
- Su, R. Experimental research on carbonate rock erosion by the Maocun in Guilin. Master’s Thesis, China University of Geosciences, Beijing, China, 2018. [Google Scholar]
- Liu, Z.H.; Dreybrodt, W. The DBL model and prediction of calcite dissolution/precipitation rates. Carsologica Sin. 1998, 17, 3–9. [Google Scholar]
- Huo, J.X.; Du, J.N.; Ma, F.H.; Song, H.Z. Experiment Research on Limestone Dissolution Kinetics in Open System by Measuring Conductivity. Water Resour. Power. 2018, 36, 74–78. [Google Scholar]
- Lin, Y.; Ren, H.X.; Wu, Y.Z.; Jia, F.J.; Liu, P.; Liang, J.L. Experimental Simulation of the Carbonate Dissolution Process under Different Occurrence Conditions. Hydrogeol. Eng. Geol. 2021, 48, 15–26. [Google Scholar]







| Mineral Composition | Mineral Content (%) |
|---|---|
| Quartz | 1.5 |
| Calcite | 96.5 |
| Else | 2.0 |
| Chemical Components | wt (%) |
|---|---|
| SiO2 | 0.532 |
| CaO | 55.41 |
| Fe2O3 | 0.206 |
| CO2 | 43.54 |
| MgO | 0.050 |
| Al2O3 | 0.053 |
| SrO | 0.052 |
| Cl | 0.040 |
| Loss on ignition | 0.117 |
| Test Batch Number | pH Value | Pressure (MPa) | Temperature (°C) | Current Speed (mL·min–1) | Duration (d) |
|---|---|---|---|---|---|
| 1 | 5 | 2 | 85 | 15 | 7 |
| 2 | 5 | 2 | 85 | 75 | 7 |
| 3 | 5 | 6 | 85 | 15 | 7 |
| 4 | 5 | 6 | 85 | 75 | 7 |
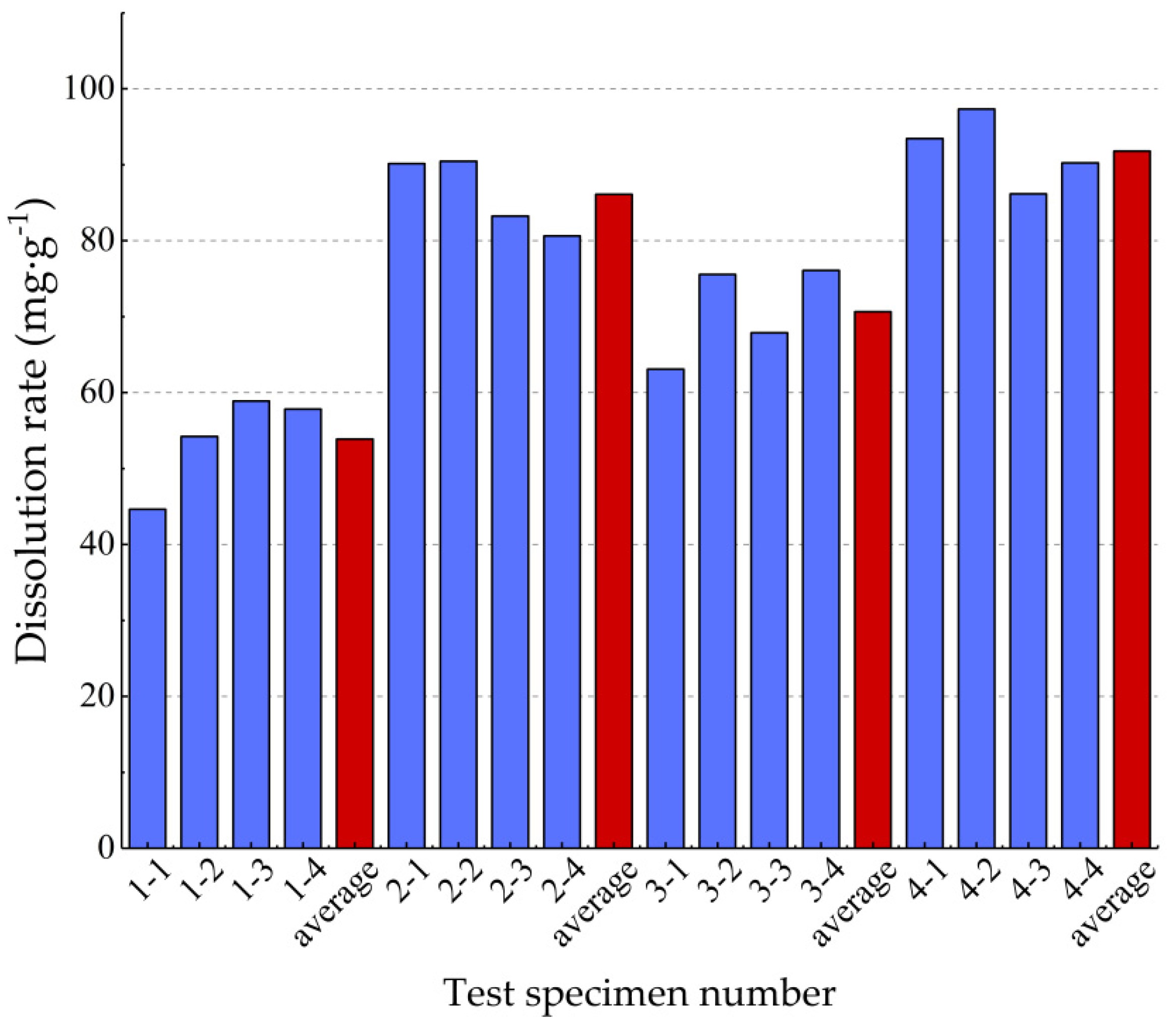
| Test Batch Number | Pressure (MPa) | Current Speed (mL·min–1) | Test Specimen Number | Before Dissolution Weight (g) | After Dissolution Weight (g) | Dissolution (g) | Dissolution Rate (mg·g−1) |
|---|---|---|---|---|---|---|---|
| 1 | 2 | 15 | 1-1 | 8.29 | 7.92 | 0.37 | 44.632 |
| 1-2 | 8.30 | 7.85 | 0.45 | 54.217 | |||
| 1-3 | 8.32 | 7.83 | 0.49 | 58.894 | |||
| 1-4 | 8.30 | 7.82 | 0.48 | 57.821 | |||
| average value | 8.30 | 7.86 | 0.45 | 53.89 | |||
| 2 | 2 | 75 | 2-1 | 8.32 | 7.57 | 0.63 | 90.144 |
| 2-2 | 8.29 | 7.54 | 0.75 | 90.470 | |||
| 2-3 | 8.29 | 7.60 | 0.69 | 83.233 | |||
| 2-4 | 8.31 | 7.64 | 0.67 | 80.626 | |||
| average value | 8.30 | 7.59 | 0.69 | 86.12 | |||
| 3 | 6 | 15 | 3-1 | 8.40 | 7.87 | 0.53 | 63.095 |
| 3-2 | 8.47 | 7.83 | 0.64 | 75.560 | |||
| 3-3 | 8.36 | 8.01 | 0.35 | 67.866 | |||
| 3-4 | 8.28 | 7.65 | 0.63 | 76.087 | |||
| average value | 8.38 | 7.84 | 0.54 | 70.65 | |||
| 4 | 6 | 75 | 4-1 | 8.24 | 7.47 | 0.77 | 93.447 |
| 4-2 | 8.22 | 7.42 | 0.80 | 97.324 | |||
| 4-3 | 8.27 | 7.64 | 0.63 | 86.179 | |||
| 4-4 | 8.20 | 7.46 | 0.74 | 90.243 | |||
| average value | 8.23 | 7.50 | 0.74 | 91.80 |
| Test Batch Number | Pressure (MPa) | Current Speed (mL·min–1) | Before Dissolution (µs·cm–1) | After Dissolution (µs·cm–1) | Variety (µs·cm–1) |
|---|---|---|---|---|---|
| 1 | 2 | 15 | 266 | 332 | +66 |
| 2 | 2 | 75 | 284 | 312 | +28 |
| 3 | 6 | 15 | 261 | 317 | +52 |
| 4 | 6 | 75 | 269 | 291 | +22 |
| Test Batch Number | Pressure (MPa) | Current Speed (mL·min−1) | Before Dissolution Ca2+ Ion Concentration (mg·L−1) | After Dissolution Ca2+ Ion Concentration (mg·L−1) | The ΔCa2+ Ion Concentration Was Precipitated (mg·L−1) |
|---|---|---|---|---|---|
| 1 | 2 | 15 | 17.68 | 30.73 | +13.05 |
| 2 | 2 | 75 | 29.02 | 35.50 | +6.48 |
| 3 | 6 | 15 | 27.24 | 37.49 | +10.25 |
| 4 | 6 | 75 | 27.00 | 33.34 | +6.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, J.; Chen, S.; Wang, J.; Chen, Z.; Zhang, J. Development and Application of Carbonate Dissolution Test Equipment under Thermal–Hydraulic–Chemical Coupling Condition. Materials 2022, 15, 7383. https://doi.org/10.3390/ma15207383
Meng J, Chen S, Wang J, Chen Z, Zhang J. Development and Application of Carbonate Dissolution Test Equipment under Thermal–Hydraulic–Chemical Coupling Condition. Materials. 2022; 15(20):7383. https://doi.org/10.3390/ma15207383
Chicago/Turabian StyleMeng, Jinzhu, Sili Chen, Junxiang Wang, Zhi Chen, and Jingyu Zhang. 2022. "Development and Application of Carbonate Dissolution Test Equipment under Thermal–Hydraulic–Chemical Coupling Condition" Materials 15, no. 20: 7383. https://doi.org/10.3390/ma15207383
APA StyleMeng, J., Chen, S., Wang, J., Chen, Z., & Zhang, J. (2022). Development and Application of Carbonate Dissolution Test Equipment under Thermal–Hydraulic–Chemical Coupling Condition. Materials, 15(20), 7383. https://doi.org/10.3390/ma15207383







