Proton Transport in the Gadolinium-Doped Layered Perovskite BaLaInO4
Abstract
1. Introduction
2. Materials and Methods
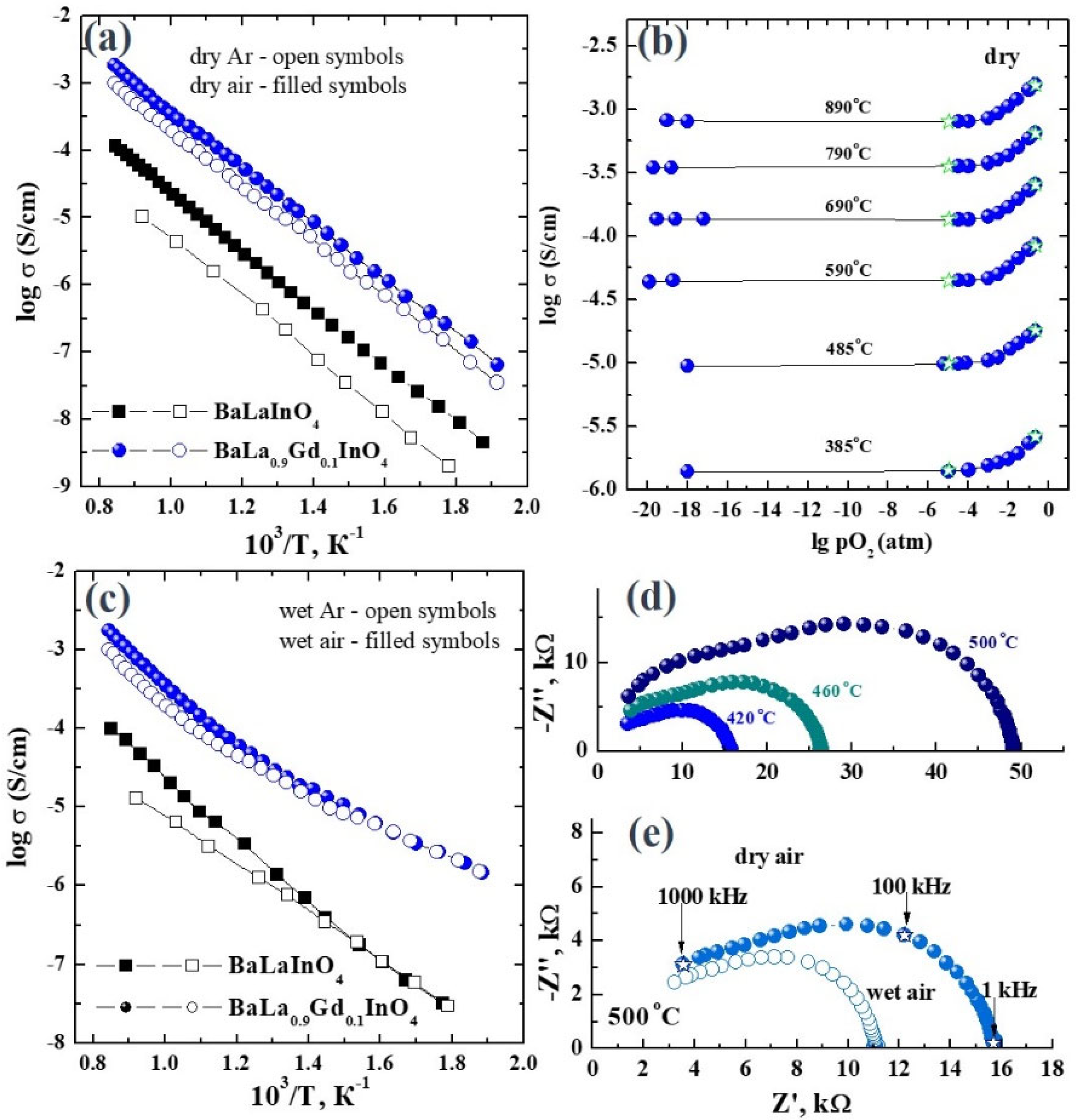
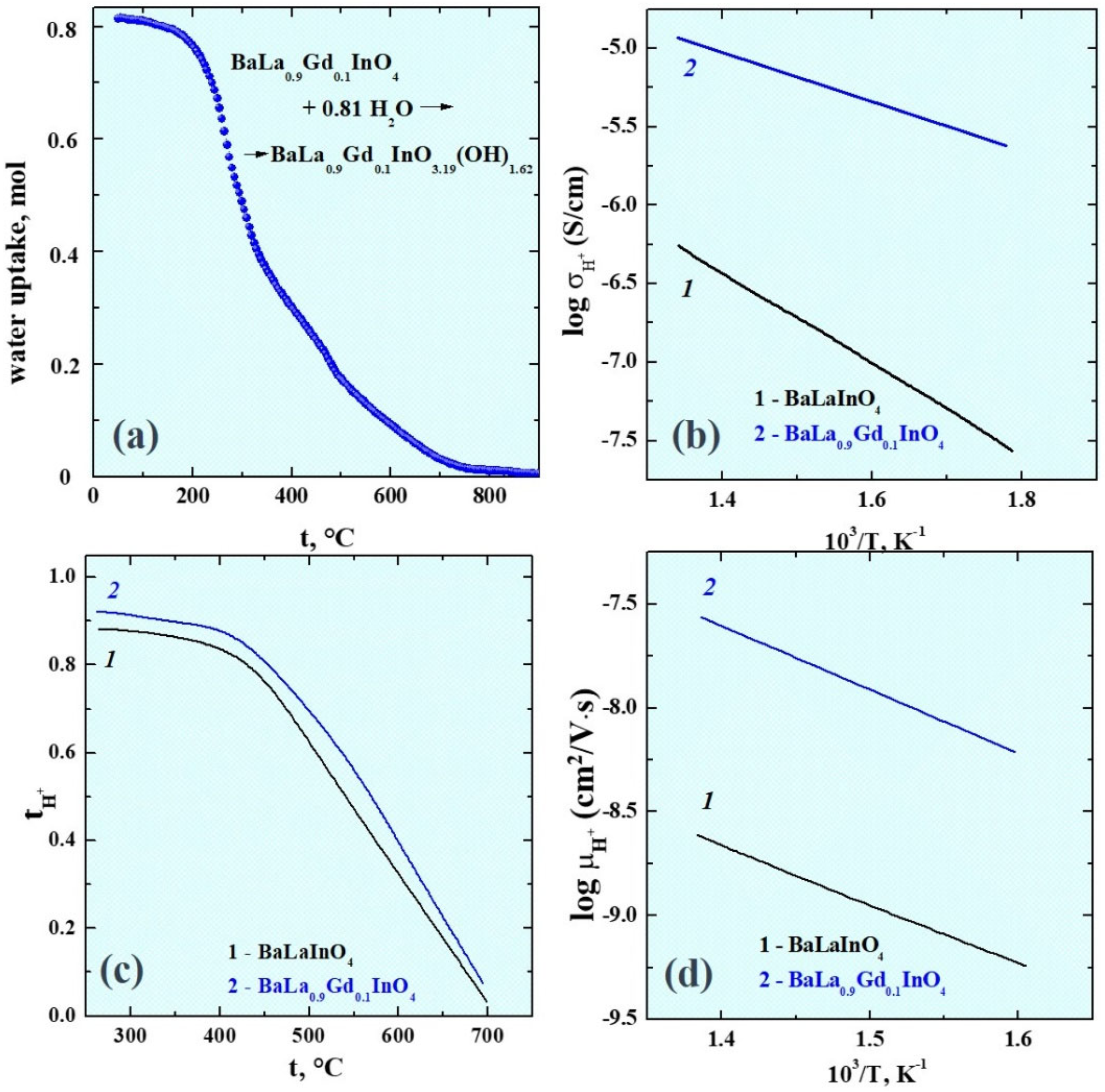
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Q.; Domen, K. Particulate Photocatalysts for Light-Driven Water Splitting: Mechanisms, Challenges, and Design Strategies. Chem. Rev. 2020, 120, 919–985. [Google Scholar] [CrossRef] [PubMed]
- Tarasova, N.; Animitsa, I.; Galisheva, A.; Medvedev, D. Layered and hexagonal perovskites as novel classes of proton-conducting solid electrolytes: A focus review. Electrochem. Mater. Technol. 2022, 1, 20221004. [Google Scholar] [CrossRef]
- Wu, F.; Maier, J.; Yu, Y. Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chem. Soc. Rev. 2020, 49, 1569–1614. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Gogotsi, Y. Perspectives for electrochemical capacitors and related devices. Nat. Mater. 2020, 19, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Guo, Y.; Zhou, X.; Shi, W.; Yu, G. Materials for solar-powered water evaporation. Nat. Rev. Mater. 2020, 5, 388–401. [Google Scholar] [CrossRef]
- Oses, C.; Toher, C.; Curtarolo, S. High-entropy ceramics. Nat. Rev. Mater. 2020, 5, 295–309. [Google Scholar] [CrossRef]
- Beretta, D.; Neophytou, N.; Hodges, J.M.; Kanatzidis, M.G.; Narducci, D.; Martin-Gonzalez, M.; Beekman, M.; Balke, B.; Ceretti, G.; Tremel, W.; et al. Thermoelectrics: From history, a window to the future. Mater. Sci. Eng. R Rep. 2019, 13, 100501. [Google Scholar] [CrossRef]
- Alberi, K.; Nardelli, M.B.; Zakutaev, A.; Mitas, L.; Curtarolo, S.; Jain, A.; Fornari, M.; Marzari, N.; Takeuchi, I.; Green, M.L.; et al. The 2019 materials by design roadmap. J. Phys. D Appl. Phys. 2019, 52, 013001. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhao, L.-D. Seeking new, highly effective thermoelectrics. Science 2020, 367, 1196–1197. [Google Scholar] [CrossRef]
- Boyd, C.C.; Cheacharoen, R.; Leijtens, T.; McGehee, M.D. Understanding Degradation Mechanisms and Improving Stability of Perovskite Photovoltaics. Chem. Rev. 2019, 119, 3418–3451. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, J.-W.; Jung, H.S.; Shin, H.; Park, N.-G. High-Efficiency Perovskite Solar Cells. Chem. Rev. 2020, 120, 7867–7918. [Google Scholar] [CrossRef] [PubMed]
- Grancini, G.; Nazeeruddin, M.K. Dimensional tailoring of hybrid perovskites for photovoltaics. Nat. Rev. Mater. 2019, 4, 4–22. [Google Scholar] [CrossRef]
- Fu, Y.; Zhu, H.; Chen, J.; Hautzinger, M.P.; Zhu, X.-Y.; Jin, S. Metal halide perovskite nanostructures for optoelectronic applications and the study of physical properties. Nat. Rev. Mater. 2019, 4, 169–188. [Google Scholar] [CrossRef]
- Yin, W.-J.; Weng, B.; Ge, J.; Sun, Q.; Li, Z.; Yan, Y. Oxide perovskites, double perovskites and derivatives for electrocatalysis, photocatalysis, and photovoltaics. Energy Environ. Sci. 2019, 12, 442–462. [Google Scholar] [CrossRef]
- Zhao, C.; Li, Y.; Zhang, W.; Zheng, Y.; Lou, X.; Yu, B.; Chen, J.; Chen, Y.; Liu, M.; Wang, J. Heterointerface engineering for enhancing the electrochemical performance of solid oxide cells. Energy Environ. Sci. 2020, 13, 53–85. [Google Scholar] [CrossRef]
- Papac, M.; Stevanović, V.; Zakutaev, A.; O’Hayre, R. Triple ionic–electronic conducting oxides for next-generation electrochemical devices. Nat. Mater. 2021, 20, 301–313. [Google Scholar] [CrossRef]
- Abd Aziz, A.J.; Baharuddin, N.A.; Somalu, M.R.; Muchtar, A. Review of composite cathodes for intermediate-temperature solid oxide fuel cell applications. Ceram. Int. 2020, 46, 23314–23351. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, F.; Xia, C. A review on cathode processes and materials for electro-reduction of carbon dioxide in solid oxide electrolysis cells. J. Power Sources 2021, 4931, 229713. [Google Scholar] [CrossRef]
- Mather, G.C.; Muñoz-Gil, D.; Zamudio-García, J.; Porras-Vázquez, J.M.; Marrero-López, D.; Pérez-Coll, D. Perspectives on cathodes for protonic ceramic fuel cells. Appl. Sci. 2021, 11, 5363. [Google Scholar] [CrossRef]
- Li, X.; Kuang, X.; Sun, J. Rare earth elements based oxide ion conductors. Inorg. Chem. Front. 2021, 8, 1374–1398. [Google Scholar] [CrossRef]
- Tasleem, S.; Tahir, M. Recent progress in structural development and band engineering of perovskites materials for photocatalytic solar hydrogen production: A review. Int. J. Hydrogen Energy 2020, 45, 19078–19111. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, J.; Deng, Y.; Qian, Y.; Jia, X.; Ma, M.; Yang, C.; Liu, K.; Wang, Z.; Qu, S.; et al. The application of perovskite materials in solar water splitting. J. Semicond. 2020, 41, 011701. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, G.; Wang, L.; Irvine, J.T.S. Inorganic perovskite photocatalysts for solar energy utilization. Chem. Soc. Rev. 2016, 45, 5951–5984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, J.; Gong, J. Tantalum-based semiconductors for solar water splitting. Chem. Soc. Rev. 2014, 43, 4395–4422. [Google Scholar] [CrossRef]
- Tarasova, N.; Animitsa, I.; Galisheva, A. Electrical properties of new protonic conductors Ba1+xLa1−xInO4−0.5x with Ruddlesden-Popper structure. J. Solid State Electrochem. 2020, 24, 1497–1508. [Google Scholar] [CrossRef]
- Tarasova, N.; Galisheva, A.; Animitsa, I. Improvement of oxygen-ionic and protonic conductivity of BaLaInO4 through Ti doping. Ionics 2020, 26, 5075–5088. [Google Scholar] [CrossRef]
- Tarasova, N.; Galisheva, A.; Animitsa, I. Ba2+/Ti4+- co-doped layered perovskite BaLaInO4: The structure and ionic (O2−, H+) conductivity. Int. J. Hydrogen Energy 2021, 46, 16868–16877. [Google Scholar] [CrossRef]
- Tarasova, N.; Animitsa, I.; Galisheva, A. Effect of acceptor and donor doping on the state of protons in block-layered structures based on BaLaInO4. Solid State Commun. 2021, 323, 14093. [Google Scholar] [CrossRef]
- Tarasova, N.; Galisheva, A.; Animitsa, I.; Anokhina, I.; Gilev, A.; Cheremisina, P. Novel mid-temperature Y3+ → In3+ doped proton conductors based on the layered perovskite BaLaInO4. Ceram. Int. 2022, 48, 15677–15685. [Google Scholar] [CrossRef]
- Tarasova, N.; Animitsa, I.; Galisheva, A.; Korona, D.; Davletbaev, K. Novel proton-conducting layered perovskite based on BaLaInO4 with two different cations in B-sublattice: Synthesis, hydration, ionic (O2−, H+) conductivity. Int. J. Hydrogen Energy 2022, 47, 18972–18982. [Google Scholar] [CrossRef]
- Kato, S.; Ogasawara, M.; Sugai, M.; Nakata, S. Synthesis and oxide ion conductivity of new layered perovskite La1-xSr1+xInO4-d. Solid State Ion. 2002, 149, 53–57. [Google Scholar] [CrossRef]
- Troncoso, L.; Alonso, J.A.; Aguadero, A. Low activation energies for interstitial oxygen conduction in the layered perovskites La1+xSr1-xInO4+d. J. Mater. Chem. A 2015, 3, 17797–17803. [Google Scholar] [CrossRef]
- Troncoso, L.; Alonso, J.A.; Fernández-Díaz, M.T.; Aguadero, A. Introduction of interstitial oxygen atoms in the layered perovskite LaSrIn1-xBxO4+δ system (B = Zr, Ti). Solid State Ion. 2015, 282, 82–87. [Google Scholar] [CrossRef]
- Troncoso, L.; Mariño, C.; Arce, M.D.; Alonso, J.A. Dual oxygen defects in layered La1.2Sr0.8-xBaxInO4+d (x = 0.2, 0.3) oxide-ion conductors: A neutron diffraction study. Materials 2019, 12, 1624. [Google Scholar] [CrossRef] [PubMed]
- Troncoso, L.; Arce, M.D.; Fernández-Díaz, M.T.; Mogni, L.V.; Alonso, J.A. Water insertion and combined interstitial-vacancy oxygen conduction in the layered perovskites La1.2Sr0.8-xBaxInO4+δ. New J. Chem. 2019, 43, 6087–6094. [Google Scholar] [CrossRef]
- Fujii, K.; Esaki, Y.; Omoto, K.; Yashima, M.; Hoshikawa, A.; Ishigaki, T.; Hester, J.R. New perovskite-related structure family of oxide-ion conducting materials NdBaInO4. Chem. Mater. 2014, 26, 2488–2491. [Google Scholar] [CrossRef]
- Fujii, K.; Shiraiwa, M.; Esaki, Y.; Yashima, M.; Kim, S.J.; Lee., S. Improved oxide-ion conductivity of NdBaInO4 by Sr doping. J. Mater. Chem. A 2015, 3, 11985. [Google Scholar] [CrossRef]
- Ishihara, T.; Yan, Yu.; Sakai, T.; Ida, S. Oxide ion conductivity in doped NdBaInO4. Solid State Ion. 2016, 288, 262–265. [Google Scholar] [CrossRef]
- Yang, X.; Liu, S.; Lu, F.; Xu, J.; Kuang, X. Acceptor doping and oxygen vacancy migration in layered perovskite NdBa1-nO4-based mixed conductors. J. Phys. Chem. C 2016, 120, 6416–6426. [Google Scholar] [CrossRef]
- Fijii, K.; Yashima, M. Discovery and development of BaNdInO4 -A brief review. J. Ceram. Soc. Jpn. 2018, 126, 852–859. [Google Scholar] [CrossRef]
- Zhou, Y.; Shiraiwa, M.; Nagao, M.; Fujii, K.; Tanaka, I.; Yashima, M.; Baque, L.; Basbus, J.F.; Mogni, L.V.; Skinner, S.J. Protonic conduction in the BaNdInO4 structure achieved by acceptor doping. Chem. Mater. 2021, 33, 2139–2146. [Google Scholar] [CrossRef] [PubMed]
- Shiraiwa, M.; Kido, T.; Fujii, K.; Yashima, M. High-temperature proton conductors based on the (110) layered perovskite BaNdScO4. J. Mater. Chem. A 2021, 9, 8607. [Google Scholar] [CrossRef]
- Tarasova, N.; Animitsa, I. Materials AIILnInO4 with Ruddlesden-Popper structure for electrochemical applications: Relationship between ion (oxygen-ion, proton) conductivity, water uptake and structural changes. Materials 2022, 15, 114. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Allred, A.L. Electronegativity values from thermochemical data. J. Inorg. Nucl. Chem. 1961, 17, 215–221. [Google Scholar] [CrossRef]

| Composition | a, Å | b, Å | c, Å | Unit Cell Volume, (Å3) |
|---|---|---|---|---|
| BaLaInO4 | 12.932(3) | 5.906(0) | 5.894(2) | 450.19(5) |
| BaLa0.9Gd0.1InO4 | 12.988(5) | 5.908(1) | 5.910(8) | 453.92(8) |
| Element | Ba | La | Nd | In |
|---|---|---|---|---|
| Content, atomic % | 33.5 ± 0.7 (33.3) | 29.8 ± 0.6 (30.0) | 3.2 ± 0.1 (3.3) | 33.5 ± 0.7 (33.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarasova, N.; Bedarkova, A.; Animitsa, I. Proton Transport in the Gadolinium-Doped Layered Perovskite BaLaInO4. Materials 2022, 15, 7351. https://doi.org/10.3390/ma15207351
Tarasova N, Bedarkova A, Animitsa I. Proton Transport in the Gadolinium-Doped Layered Perovskite BaLaInO4. Materials. 2022; 15(20):7351. https://doi.org/10.3390/ma15207351
Chicago/Turabian StyleTarasova, Nataliia, Anzhelika Bedarkova, and Irina Animitsa. 2022. "Proton Transport in the Gadolinium-Doped Layered Perovskite BaLaInO4" Materials 15, no. 20: 7351. https://doi.org/10.3390/ma15207351
APA StyleTarasova, N., Bedarkova, A., & Animitsa, I. (2022). Proton Transport in the Gadolinium-Doped Layered Perovskite BaLaInO4. Materials, 15(20), 7351. https://doi.org/10.3390/ma15207351







