Gallium Oxide for Gas Sensor Applications: A Comprehensive Review
Abstract
1. Introduction
2. Polymorphs and Crystal Structures of Ga2O3
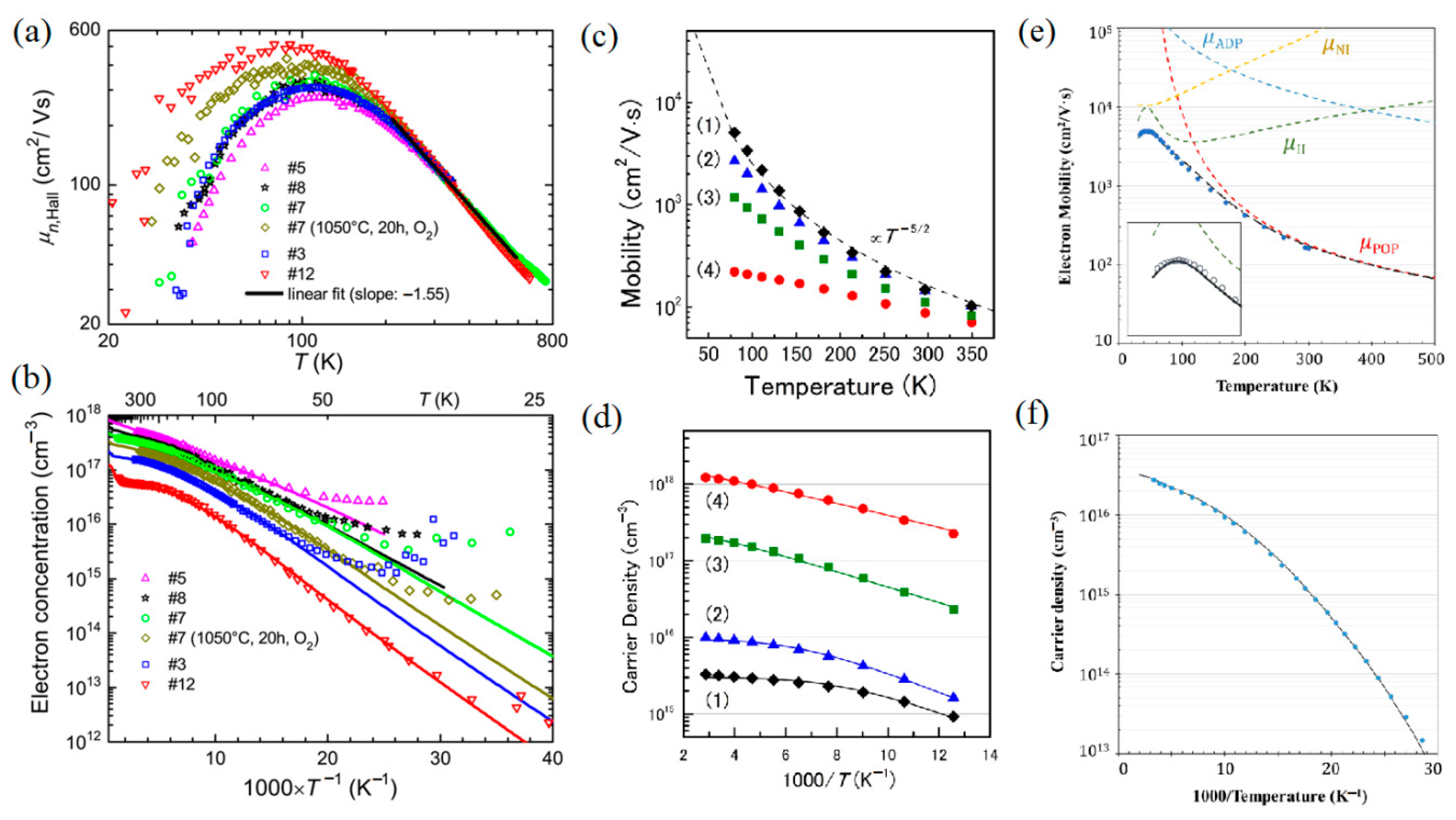
3. Electrical Properties of Ga2O3
4. Preparation Methods of Ga2O3 Gas-Sensing Material
4.1. Bulk Single-Crystal Growth
4.2. Magnetron Sputtering
4.3. Chemical Vapor Deposition
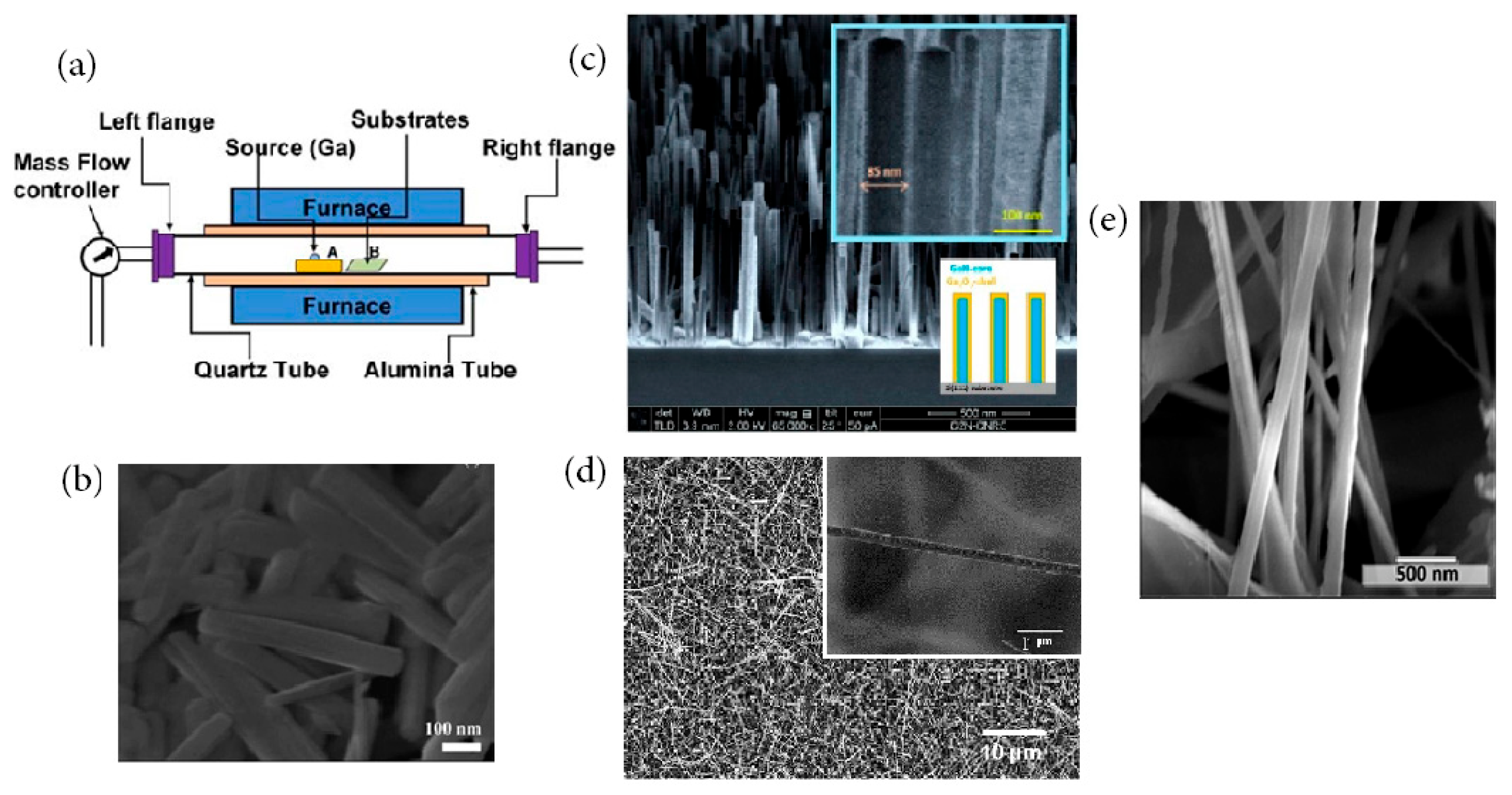
4.4. Sol–Gel Synthesis
4.5. Hydrothermal Synthesis
4.6. Other Methods
5. Ga2O3-Based Gas Sensors
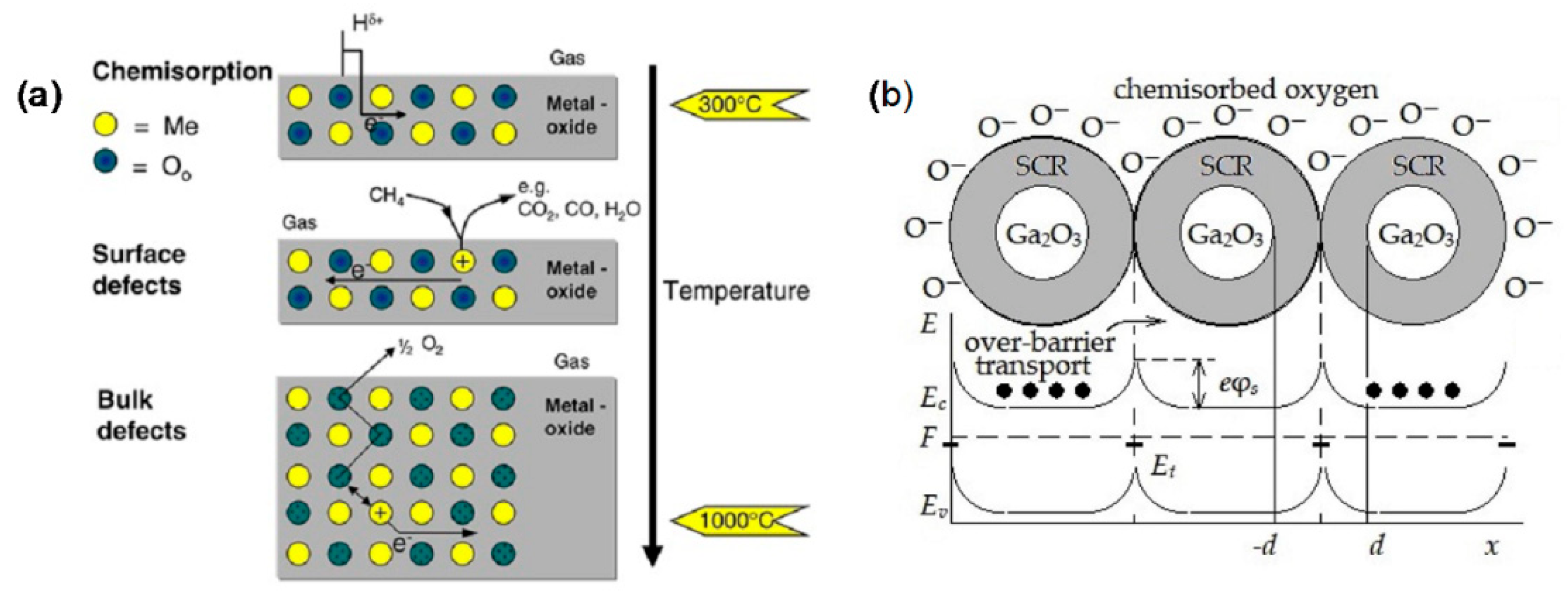
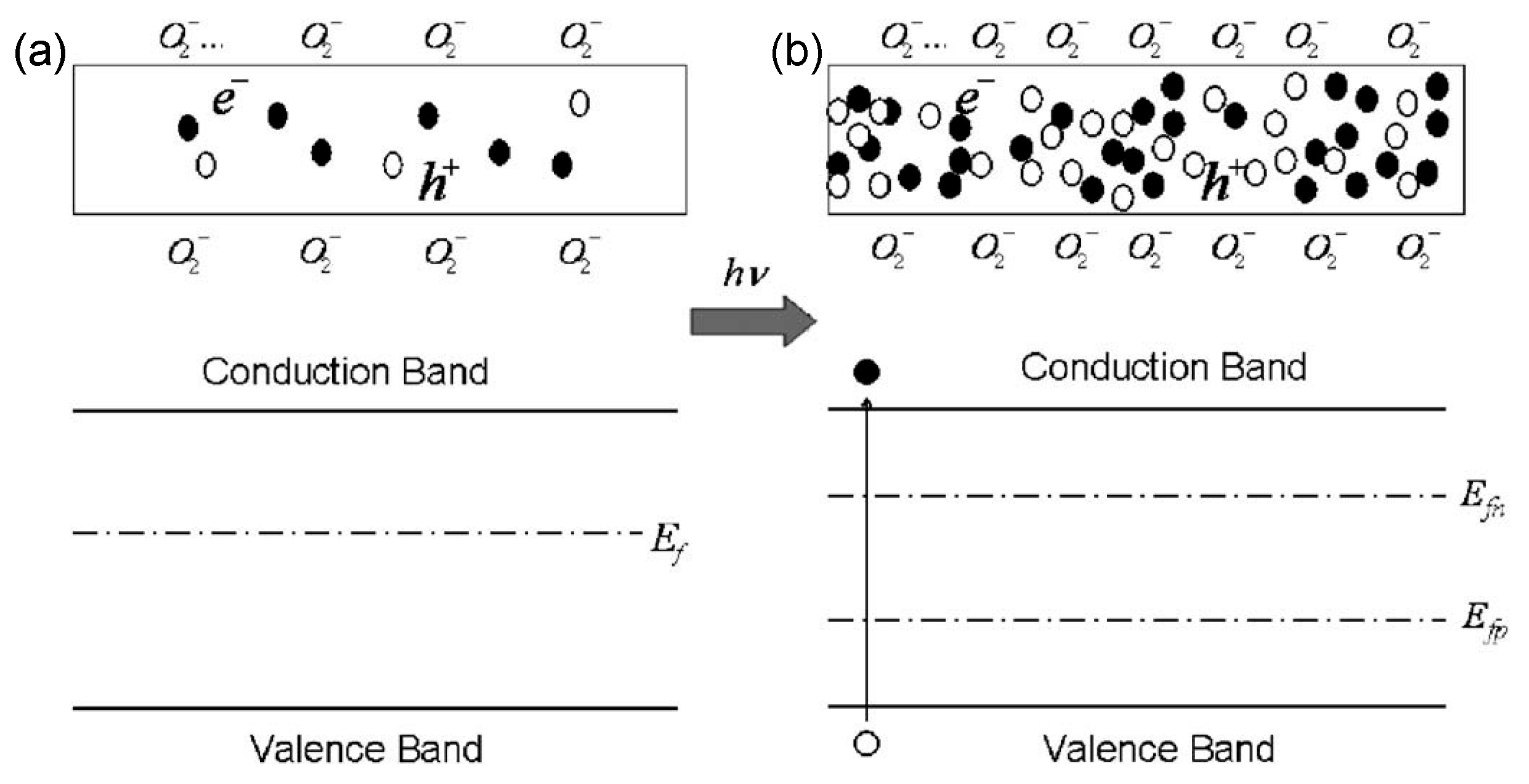
5.1. Sensing Mechanisms
5.2. Evaluation Criteria
5.3. Classification of Ga2O3-Based Gas Sensors
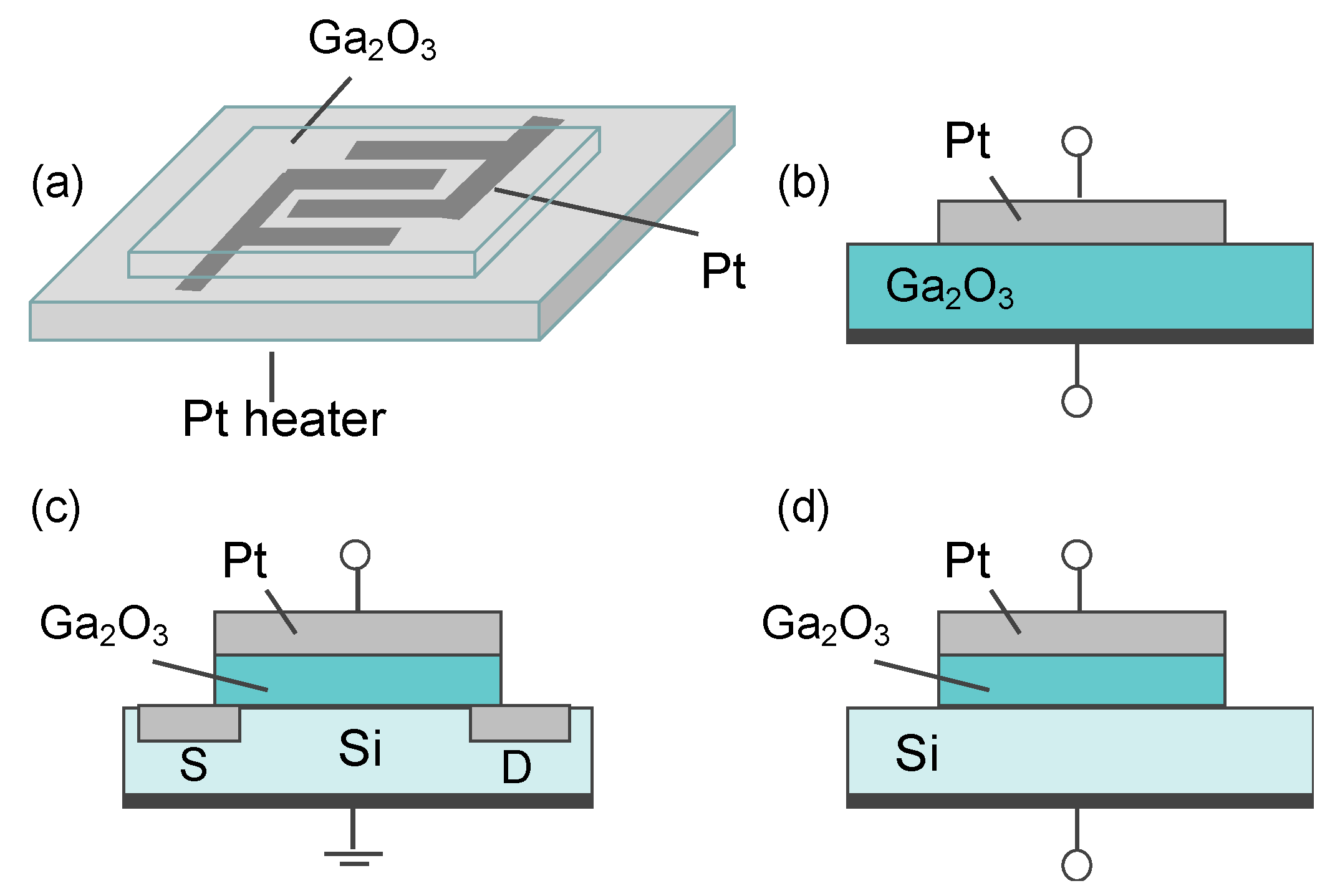
5.3.1. Electrical Gas Sensors
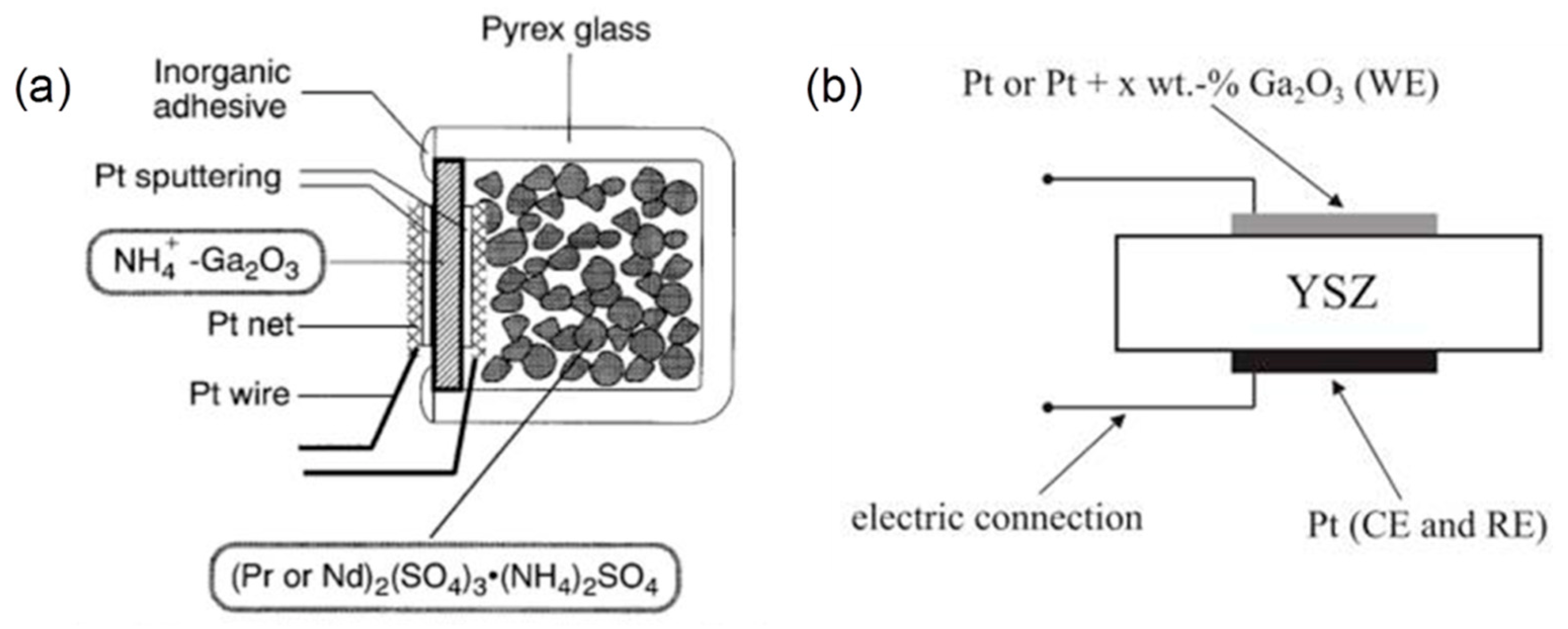
5.3.2. Electrochemical Gas Sensors
5.3.3. Optical Gas Sensors
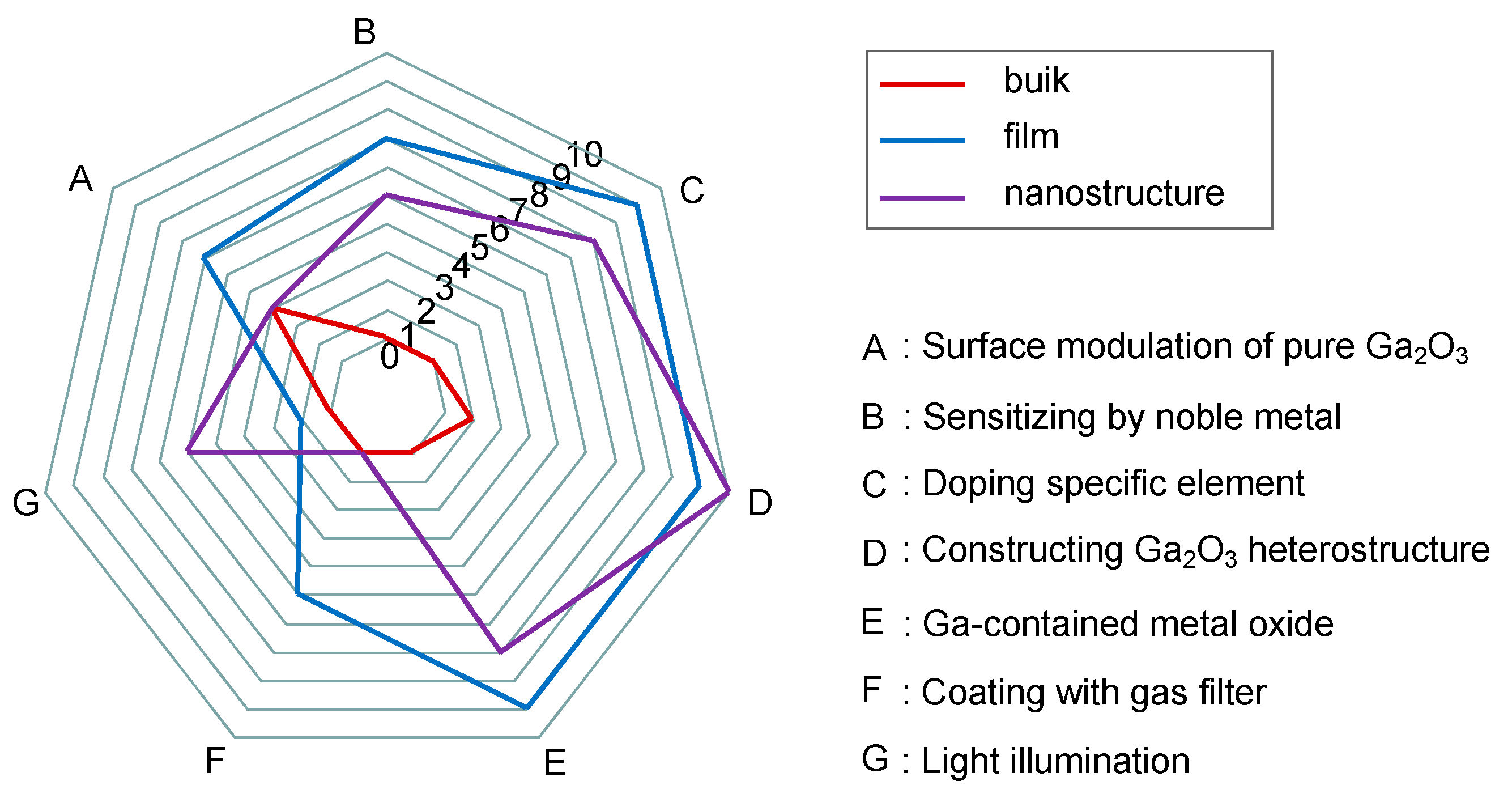
5.4. Performance Enhancement Strategies
5.4.1. Surface Modulation of Pure Ga2O3
5.4.2. Sensitizing by Noble Metal
5.4.3. Doping Specific Element
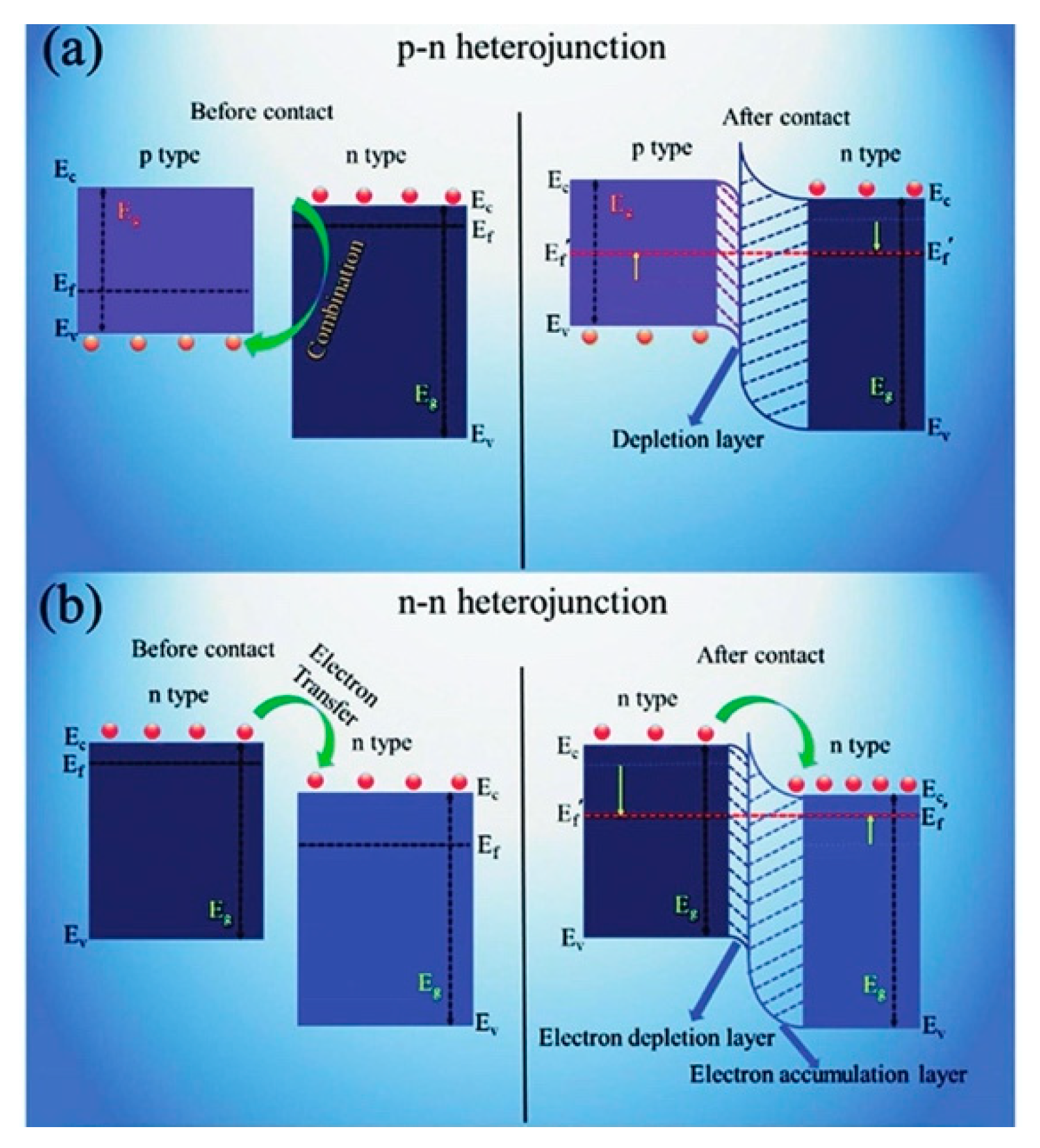
5.4.4. Constructing Ga2O3 Heterostructure
5.4.5. Ga-Contained Metal Oxide
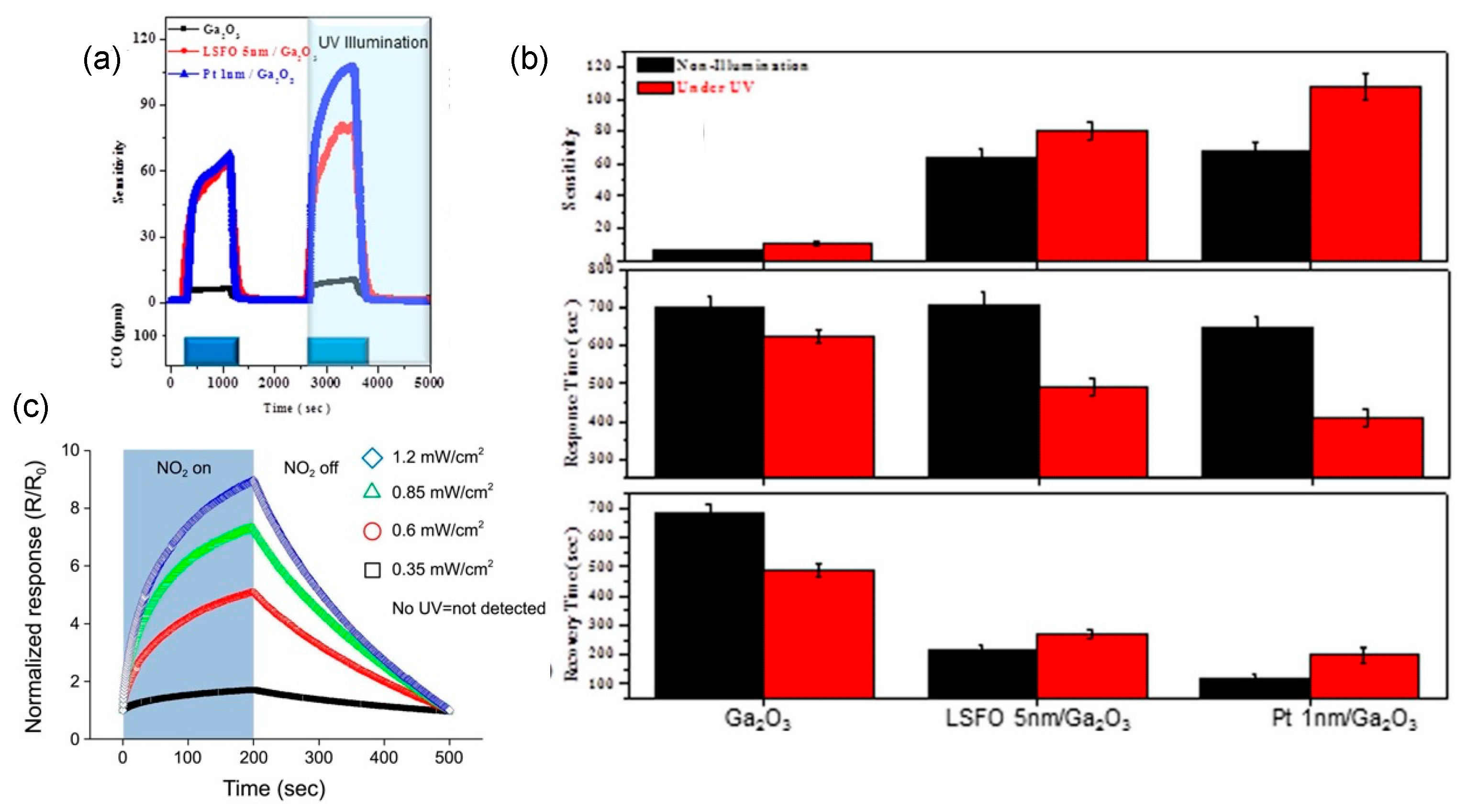
5.4.6. Coating with Gas Filter
5.4.7. Light Illumination
6. Conclusions and Outlook
- (i)
- Construction of hybrid structures with two-dimensional (2D) materials and organic polymers. Two-dimensional materials are regarded as having promising potential for gas-sensing devices owing to their large surface-to-volume ratio and high surface sensitivity [231]. The integration of 2D layers with Ga2O3, especially a 2D Ga2O3 monolayer [232], can form a van der Waals heterojunction without constraints on the chemical bonding and interfacial lattice matching, which will be expected to widen the building blocks for novel applications with unprecedented properties and excellent performance. The energy band alignment of such van der Waals heterojunction could be precisely designed by selecting suitable 2D materials, such as transition metal dichalcogenides with different band gaps and working functions so as to optimize the gas selectivity and performance of the Ga2O3 gas sensor. Additionally, organic semiconductors offer a viable alternative to conventional inorganic semiconductors in gas sensor applications because of their unusual electrical properties, diversity, large area, and potentially low cost [233]. They show good sensitivities toward many gases or vapors, ranging from organic solvents to inorganic gases. Moreover, it allows for the inexpensive fabrication of novel Ga2O3 gas sensors using organic polymers as a platform, owing to their porosity, mechanical flexibility, environmental stability, and solution processability. Therefore, more research should be conducted to fabricate Ga2O3-based gas sensors by constructing heterostructures with 2D materials and organic polymers, which may result in some novel features and potential applications.
- (ii)
- Combinations with DFT calculations and machine learning. Hitherto, quite few theoretical investigations have been devoted to understanding the interaction behaviors between gas molecules and Ga2O3 in the field of gas sensors. DFT calculations [232,234,235] have been performed to study the adsorption, dissociation, and diffusion characteristics of gas molecules on the surface of Ga2O3. It is suggested that the calculated adsorption energy, electron structures of oxygen vacancy, and charge transfer are highly significant in defining the performance of a sensor. Large adsorption energy and significant charge transfer indicate high sensitivity and selectivity towards a specific gas. Alongside DFT, machine learning is considered as an effective data processing approach for developing smart devices with the ability to deal with selectivity and drift problems [236]. The machine learning technique involves data processing of sensor output, dimensionality reduction, and then training a system/network for the predictions. Therefore, DFT and machine learning can be implemented as a powerful tool for studying gas-sensing behavior and provide valuable suggestions and predictions of gas-sensing materials and target gas species.
- (iii)
- Development of optical sensors. Compared to electrical characteristic gas sensors, more attention should be paid on the less-explored sensors using the promising optical properties of Ga2O3. In a pioneer study on Ga2O3-based optical gas sensors, Reiprich et al. [122] pointed out that the spectral composition of photoluminescence showed a strong intensity difference upon exposure to various gases at room temperature indirectly caused by the negatively charged oxygen ions in Ga2O3. Optical gas sensors offer a number of advantages, such as fast responses, minimal drift and high gas specificity [187]. Gas detection can be made in real time and in situ by adopting optical sensors. With the help of the rapid developed technology of Ga2O3 optical devices, it is possible to design Ga2O3-based optical gas sensors that can measure gas concentrations in the ppm or ppb range with zero cross-response to other gases and high temporal resolution. In this way, optical gas-sensing fills a gap between low-cost electrical/electrochemical sensors with inferior performance and high-end analytic equipment.
Funding
Acknowledgments
Conflicts of Interest
References
- Tsao, J.Y.; Chowdhury, S.; Hollis, M.A.; Jena, D.; Johnson, N.M.; Jones, K.A.; Kaplar, R.J.; Rajan, S.; Van de Walle, C.G.; Bellotti, E.; et al. Ultrawide-bandgap semiconductors: Research opportunities and challenges. Adv. Electron. Mater. 2018, 4, 1600501. [Google Scholar] [CrossRef]
- Higashiwaki, M.; Sasaki, K.; Kuramata, A.; Masui, T.; Yamakoshi, S. Gallium oxide (Ga2O3) metal-semiconductor field-effect transistors on single-crystal β-Ga2O3 (010) substrates. Appl. Phys. Lett. 2012, 100, 013504. [Google Scholar] [CrossRef]
- Pearton, S.; Ren, F.; Mastro, M. Gallium Oxide: Technology, Devices and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 439–464. [Google Scholar] [CrossRef]
- Higashiwaki, M.; Fujita, S. Gallium Oxide: Materials Properties, Crystal Growth, and Devices; Springer Nature: Cham, Switzerland, 2020; pp. 1–11. [Google Scholar] [CrossRef]
- Xue, H.; He, Q.; Jian, G.; Long, S.; Pang, T.; Liu, M. An overview of the ultrawide bandgap Ga2O3 semiconductor-based Schottky barrier diode for power electronics application. Nanoscale Res. Lett. 2018, 13, 290. [Google Scholar] [CrossRef]
- Liu, Z.; Li, P.; Zhi, Y.; Wang, X.; Chu, X.; Tang, W. Review of gallium oxide based field-effect transistors and Schottky barrier diodes. Chin. Phys. B 2019, 28, 017105. [Google Scholar] [CrossRef]
- Dong, H.; Xue, H.; He, Q.; Qin, Y.; Jian, G.; Long, S.; Liu, M. Progress of power field effect transistor based on ultra-wide bandgap Ga2O3 semiconductor material. J. Semicond. 2019, 40, 011802. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, L.; Tang, X.; Hu, J.; Sun, J.; Zhang, Y.; Zhang, Y. Progress of ultra-wide bandgap Ga2O3 semiconductor materials in power MOSFETs. IEEE Trans. Power Electron. 2020, 35, 5157–5179. [Google Scholar] [CrossRef]
- Singh, R.; Lenka, T.R.; Panda, D.K.; Velpula, R.T.; Jain, B.; Bui, H.Q.T.; Nguyen, H.P.T. The dawn of Ga2O3 HEMTs for high power electronics—A review. Mater. Sci. Semicond. Process. 2020, 119, 105216. [Google Scholar] [CrossRef]
- Wong, M.H.; Higashiwaki, M. Vertical β-Ga2O3 power transistors: A review. IEEE Trans. Electron Dev. 2020, 67, 3925–3937. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R. Nanofunctional gallium oxide (Ga2O3) nanowires/nanostructures and their applications in nanodevices. Phys. Status Solidi RRL 2013, 7, 781–792. [Google Scholar] [CrossRef]
- Higashiwaki, M.; Sasaki, K.; Murakami, H.; Kumagai, Y.; Koukitu, A.; Kuramata, A.; Masui, T.; Yamakoshi, S. Recent progress in Ga2O3 power devices. Semicond. Sci. Technol. 2016, 31, 034001. [Google Scholar] [CrossRef]
- Mastro, M.A.; Kuramata, A.; Calkins, J.; Kim, J.; Ren, F.; Pearton, S.J. Opportunities and future directions for Ga2O3. ECS J. Solid State Sci. Technol. 2017, 6, P356–P359. [Google Scholar] [CrossRef]
- Pearton, S.J.; Yang, J.; Cary IV, P.H.; Ren, F.; Kim, J.; Tadjer, M.J.; Mastro, M.A. A review of Ga2O3 materials, processing, and devices. Appl. Phys. Rev. 2018, 5, 011301. [Google Scholar] [CrossRef]
- Galazka, Z. β-Ga2O3 for wide-bandgap electronics and optoelectronics. Semicond. Sci. Technol. 2018, 33, 113001. [Google Scholar] [CrossRef]
- Baldini, M.; Galazka, Z.; Wagner, G. Recent progress in the growth of β-Ga2O3 for power electronics applications. Mater. Sci. Semicond. Process. 2018, 78, 132–146. [Google Scholar] [CrossRef]
- Higashiwaki, M.; Jessen, G.H. The dawn of gallium oxide microelectronics. Appl. Phys. Lett. 2018, 112, 060401. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, J.; Zhang, C.; Feng, Q.; Zhao, S.; Ma, P.; Hao, Y. A review of the most recent progresses of state-of-art gallium oxide power devices. J. Semicond. 2019, 40, 011803. [Google Scholar] [CrossRef]
- Xu, J.; Zheng, W.; Huang, F. Gallium oxide solar-blind ultraviolet photodetectors: A review. J. Mater. Chem. C 2019, 7, 8753–8770. [Google Scholar] [CrossRef]
- Kim, J.; Pearton, S.J.; Fares, C.; Yang, J.; Ren, F.; Kim, S.; Polyakov, A.Y. Radiation damage effects in Ga2O3 materials and devices. J. Mater. Chem. C 2019, 7, 10–24. [Google Scholar] [CrossRef]
- Qin, Y.; Long, S.; Dong, H.; He, Q.; Jian, G.; Zhang, Y.; Hou, X.; Tan, P.; Zhang, Z.; Lv, H.; et al. Review of deep ultraviolet photodetector based on gallium oxide. Chin. Phys. B 2019, 28, 018501. [Google Scholar] [CrossRef]
- Guo, D.; Guo, Q.; Chen, Z.; Wu, Z.; Li, P.; Tang, W. Review of Ga2O3-based optoelectronic devices. Mater. Today Phys. 2019, 11, 100157. [Google Scholar] [CrossRef]
- Chen, X.; Ren, F.; Gu, S.; Ye, J. Review of gallium-oxide-based solar-blind ultraviolet photodetectors. Photonics Res. 2019, 7, 381. [Google Scholar] [CrossRef]
- Saikumar, A.K.; Nehate, S.D.; Sundaram, K.B. Review-RF sputtered films of Ga2O3. ECS J. Solid State Sci. Technol. 2019, 8, Q3064–Q3078. [Google Scholar] [CrossRef]
- Tadjer, M.J.; Lyons, J.L.; Nepal, N.; Freitas, J.A., Jr.; Koehler, A.D.; Foster, G.M. Review-theory and characterization of doping and defects in β-Ga2O3. ECS J. Solid State Sci. Technol. 2019, 8, Q3187–Q3194. [Google Scholar] [CrossRef]
- Afzal, A. β-Ga2O3 nanowires and thin films for metal oxide semiconductor gas sensors: Sensing mechanisms and performance enhancement strategies. J. Mater. 2019, 5, 542–557. [Google Scholar] [CrossRef]
- Zhai, H.; Wu, Z.; Fang, Z. Recent progress of Ga2O3-based gas sensors. Ceram. Int. 2022, 48, 24213–24233. [Google Scholar] [CrossRef]
- McCluskey, M.D. Point defects in Ga2O3. J. Appl. Phys. 2020, 127, 101101. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, J.; Qi, D.; Chen, L.; Zhang, K.H.L. Recent progress on the electronic structure, defect, and doping properties of Ga2O3. APL Mater. 2020, 8, 020906. [Google Scholar] [CrossRef]
- Bosi, M.; Mazzolini, P.; Seravalli, L.; Fornari, R. Ga2O3 polymorphs: Tailoring the epitaxial growth conditions. J. Mater. Chem. C 2020, 8, 10975–10992. [Google Scholar] [CrossRef]
- Hou, X.; Zou, Y.; Ding, M.; Qin, Y.; Zhang, Z.; Ma, X.; Tan, P.; Yu, S.; Zhou, X.; Zhao, X.; et al. Review of polymorphous Ga2O3 materials and their solar-blind photodetector applications. J. Phys. D Appl. Phys. 2021, 54, 043001. [Google Scholar] [CrossRef]
- Tak, B.R.; Kumar, S.; Kapoor, A.K.; Wang, D.; Li, X.; Sun, H.; Singh, R. Recent advances in the growth of gallium oxide thin films employing various growth techniques—A review. J. Phys. D Appl. Phys. 2021, 54, 453002. [Google Scholar] [CrossRef]
- Blevins, J.; Yang, G. On optical properties and scintillation performance of emerging Ga2O3: Crystal growth, emission mechanisms and doping strategies. Mater. Res. Bull. 2021, 144, 111494. [Google Scholar] [CrossRef]
- Yuan, Y.; Hao, W.; Mu, W.; Wang, Z.; Chen, X.; Liu, Q.; Xu, G.; Wang, C.; Zhou, H.; Zou, Y.; et al. Toward emerging gallium oxide semiconductors: A roadmap. Fundam. Res. 2021, 1, 697–716. [Google Scholar] [CrossRef]
- Huang, H.-C.; Ren, Z.; Chan, C.; Li, X. Wet etch, dry etch, and MacEtch of β-Ga2O3: A review of characteristics and mechanism. J. Mater. Res. 2021, 36, 4756–4770. [Google Scholar] [CrossRef]
- Galazka, Z.; Ganschow, S.; Irmscher, K.; Klimm, D.; Albrecht, M.; Schewski, R.; Pietsch, M.; Schulz, T.; Dittmar, A.; Kwasniewski, A.; et al. Bulk single crystals of β-Ga2O3 and Ga-based spinels as ultra-wide bandgap transparent semiconducting oxides. Prog. Cryst. Growth Charact. Mater. 2021, 67, 100511. [Google Scholar] [CrossRef]
- Sharma, R.; Law, M.E.; Ren, F.; Polyakov, A.Y.; Pearton, S.J. Diffusion of dopants and impurities in β-Ga2O3. J. Vac. Sci. Technol. A 2021, 39, 060801. [Google Scholar] [CrossRef]
- Cooke, J.; Sensale-Rodriguez, B.; Ghadbeigi, L. Methods for synthesizing β-Ga2O3 thin films beyond epitaxy. J. Phys. Photonics 2021, 3, 032005. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Xu, S.; Zhang, C.; Feng, Q.; Zhang, Y.; Ning, J.; Zhao, S.; Zhou, H.; Hao, Y. Progress in state-of-the-art technologies of Ga2O3 devices. J. Phys. D Appl. Phys. 2021, 54, 243001. [Google Scholar] [CrossRef]
- Kim, H. Control and understanding of metal contacts to β-Ga2O3 single crystals: A review. SN Appl. Sci. 2022, 4, 27. [Google Scholar] [CrossRef]
- Fleischer, M.; Meixner, H. Gallium oxide thin films: A new material for high-temperature oxygen sensors. Sens. Actuators B Chem. 1991, 4, 437–441. [Google Scholar] [CrossRef]
- Fleischer, M.; Meixner, H. Oxygen sensing with long-term stable Ga2O3 thin films. Sens. Actuators B Chem. 1991, 5, 115–119. [Google Scholar] [CrossRef]
- Malik, R.; Tomer, V.K.; Mishra, Y.K.; Lin, L. Functional gas sensing nanomaterials: A panoramic view. Appl. Phys. Rev. 2020, 7, 021301. [Google Scholar] [CrossRef]
- Herbstein, F.H. Diversity amidst similarity: A multidisciplinary approach to phase relationships, solvates, and polymorphs. Cryst. Growth Des. 2004, 4, 1419–1429. [Google Scholar] [CrossRef]
- Roy, R.; Hill, V.; Osborn, E. Polymorphism of Ga2O3 and the system Ga2O3-H2O. J. Am. Chem. Soc. 1952, 74, 719–722. [Google Scholar] [CrossRef]
- Yoshioka, S.; Hayashi, H.; Kuwabara, A.; Oba, F.; Matsunaga, K.; Tanaka, I. Structures and energetics of Ga2O3 polymorphs. J. Phys. Condens. Matter 2007, 19, 346211. [Google Scholar] [CrossRef]
- Zinkevich, M.; Aldinger, F. Thermodynamic assessment of the gallium-oxygen system. J. Am. Ceram. Soc. 2004, 87, 683–691. [Google Scholar] [CrossRef]
- Playford, H.Y.; Hannon, A.C.; Barney, E.R.; Walton, R.I. Structures of uncharacterised polymorphs of gallium oxide from total neutron diffraction. Chem. Eur. J. 2013, 19, 2803–2813. [Google Scholar] [CrossRef]
- Cora, I.; Mezzadri, F.; Boschi, F.; Bosi, M.; Caplovicova, M.; Calestani, G.; Dodony, I.; Pecza, B.; Fornari, R. The real structure of ε-Ga2O3 and its relation to κ-phase. CrystEngComm 2017, 19, 1509–1516. [Google Scholar] [CrossRef]
- Kim, J.; Tahara, D.; Miura, Y.; Kim, B.G. First-principle calculations of electronic structures and polar properties of (κ, ε)-Ga2O3. Appl. Phys. Express 2018, 11, 061101. [Google Scholar] [CrossRef]
- Nishinaka, H.; Ueda, O.; Tahara, D.; Ito, Y.; Ikenaga, N.; Hasuike, N.; Yoshimoto, M. Single-domain and atomically flat surface of κ-Ga2O3 thin films on FZ-grown ε-GaFeO3 substrates via step-flow growth mode. ACS Omega 2020, 5, 29585–29592. [Google Scholar] [CrossRef]
- Jang, S.; Jung, S.; Kim, J.; Ren, F.; Pearton, S.J.; Baik, K.H. Hydrogen sensing characteristics of Pt Schottky diodes on (01) and (010) Ga2O3 single crystals. ECS J. Solid State Sci. Technol. 2018, 7, Q3180–Q3182. [Google Scholar] [CrossRef]
- Fleischer, M.; Handrider, W.; Meixner, H. Stability of semiconducting gallium oxide thin films. Thin Solid Films 1990, 190, 93–102. [Google Scholar] [CrossRef]
- Ueda, N.; Hosono, H.; Waseda, R.; Kawazoe, H. Synthesis and control of conductivity of ultraviolet transmitting β-Ga2O3 single crystals. Appl. Phys. Lett. 1997, 70, 3561–3563. [Google Scholar] [CrossRef]
- Varley, J.B.; Weber, J.R.; Janotti, A.; Van de Walle, C.G. Oxygen vacancies and donor impurities in β-Ga2O3. Appl. Phys. Lett. 2010, 97, 142106. [Google Scholar] [CrossRef]
- Feng, Z.; Bhuiyan, A.F.M.A.U.; Karim, M.R.; Zhao, H. MOCVD homoepitaxy of Si-doped (010) β-Ga2O3 thin films with superior transport properties. Appl. Phys. Lett. 2019, 114, 250601. [Google Scholar] [CrossRef]
- Fleischer, M.; Meixner, H. Electron mobility in single and polycrystalline Ga2O3. J. Appl. Phys. 1993, 74, 300–305. [Google Scholar] [CrossRef]
- Irmscher, K.; Galazka, Z.; Pietsch, M.; Uecker, R.; Fornari, R. Electrical properties of β-Ga2O3 single crystals grown by the Czochralski method. J. Appl. Phys. 2011, 110, 063720. [Google Scholar] [CrossRef]
- Ma, N.; Tanen, N.; Verma, A.; Guo, Z.; Luo, T.; Xing, H.; Jena, D. Intrinsic electron mobility limits in β-Ga2O3. Appl. Phys. Lett. 2016, 109, 212101. [Google Scholar] [CrossRef]
- Goto, K.; Konishi, K.; Murakami, H.; Kumagai, Y.; Monemar, B.; Higashiwaki, M.; Kuramata, A.; Yamakoshi, S. Halide vapor phase epitaxy of Si doped β-Ga2O3 and its electrical properties. Thin Solid Films 2018, 666, 182–184. [Google Scholar] [CrossRef]
- Spencer, J.A.; Mock, A.L.; Jacobs, A.G.; Schubert, M.; Zhang, Y.; Tadjer, M.J. A review of band structure and material properties of transparent conducting and semiconducting oxides: Ga2O3, Al2O3, In2O3, ZnO, SnO2, CdO, NiO, CuO, and Sc2O3. Appl. Phys. Rev. 2022, 9, 011315. [Google Scholar] [CrossRef]
- Ogita, M.; Higo, K.; Nakanishi, Y.; Hatanaka, Y. Ga2O3 thin film for oxygen sensor at high temperature. Appl. Surf. Sci. 2001, 175–176, 721–725. [Google Scholar] [CrossRef]
- Ogita, M.; Yuasa, S.; Kobayashi, K.; Yamada, Y.; Nakanishi, Y.; Hatanaka, Y. Presumption and improvement for gallium oxide thin film of high temperature oxygen sensors. Appl. Surf. Sci. 2003, 212–213, 397–401. [Google Scholar] [CrossRef]
- Sui, Y.X.; Liang, H.L.; Chen, Q.S.; Huo, W.X.; Du, X.L.; Mei, Z.X. Room-temperature ozone sensing capability of IGZO-decorated amorphous Ga2O3 films. ACS Appl. Mater. Interfaces 2020, 12, 8929–8934. [Google Scholar] [CrossRef] [PubMed]
- Almaev, A.V.; Nikolaev, V.I.; Stepanov, S.I.; Pechnikov, A.I.; Chernikov, E.V.; Davletkildeev, N.A.; Sokolov, D.V.; Yakovlev, N.N.; Kalygina, V.M.; Kopyev, V.V.; et al. Hydrogen influence on electrical properties of Pt-contacted α-Ga2O3/ε-Ga2O3 structures grown on patterned sapphire substrates. J. Phys. D Appl. Phys. 2020, 53, 414004. [Google Scholar] [CrossRef]
- Almaev, A.; Nikolaev, V.; Butenko, P.; Stepanov, S.; Pechnikov, A.; Yakovlev, N.; Sinyugin, I.; Shapenkov, S.; Scheglov, M. Gas sensors based on pseudohexagonal phase of gallium oxide. Phys. Status Solidi B 2022, 259, 2100306. [Google Scholar] [CrossRef]
- Bartic, M.; Baban, C.; Suzuki, H.; Ogita, M.; Isai, M. β-gallium oxide as oxygen gas sensors at a high temperature. J. Am. Ceram. Soc. 2007, 90, 2879–2884. [Google Scholar] [CrossRef]
- Nakagomi, S.; Kaneko, M.; Kokubun, Y. Hydrogen sensitive Schottky diode based on β-Ga2O3 single crystal. Sens. Lett. 2011, 9, 31–35. [Google Scholar] [CrossRef]
- Nakagomi, S.; Ikeda, M.; Kokubun, Y. Comparison of hydrogen sensing properties of Schottky diodes based on SiC and β-Ga2O3 single crystal. Sens. Lett. 2011, 9, 616–620. [Google Scholar] [CrossRef]
- Bartic, M. Mechanism of oxygen sensing on β-Ga2O3 single-crystal sensors for high temperatures. Phys. Status Solidi A 2016, 213, 457–462. [Google Scholar] [CrossRef]
- Uhlendorf, J.; Galazka, Z.; Schmidt, H. Oxygen diffusion in β-Ga2O3 single crystals at high temperatures. Appl. Phys. Lett. 2021, 119, 242106. [Google Scholar] [CrossRef]
- Macri, P.P.; Enzo, S.; Sberveglieri, G.; Groppelli, S.; Perego, C. Unknown Ga2O3 structural phase and related characteristics as active layers for O2 sensors. Appl. Surf. Sci. 1993, 65–66, 277–282. [Google Scholar] [CrossRef]
- Fleischer, M.; Meixner, H. A selective CH4 sensor using semiconducting Ga2O3 thin films based on temperature switching of multigas reactions. Sens. Actuators B Chem. 1995, 25, 544–547. [Google Scholar] [CrossRef]
- Bausewein, A.; Hacker, B.; Fleischer, M.; Meixner, H. Effects of palladium dispersions on gas-sensitive conductivity of semiconducting Ga2O3 thin-film ceramics. J. Am. Ceram. Soc. 1997, 80, 317–323. [Google Scholar] [CrossRef]
- Schwebel, T.; Fleischer, M.; Meixner, H.; Kohl, C.-D. CO-sensor for domestic use based on high temperature stable Ga2O3 thin films. Sens. Actuators B Chem. 1998, 49, 46–51. [Google Scholar] [CrossRef]
- Josepovits, V.K.; Krafcsik, O.; Kiss, G.; Perczel, I.V. Effect of gas adsorption on the surface structure of β-Ga2O3 studied by XPS and conductivity measurements. Sens. Actuators B Chem. 1998, 48, 373–375. [Google Scholar] [CrossRef]
- Flingelli, G.K.; Fleischer, M.; Meixner, H. Selective detection of methane in domestic environments using a catalyst sensor system based on Ga2O3. Sens. Actuators B Chem. 1998, 48, 258–262. [Google Scholar] [CrossRef]
- Fleischer, M.; Kornely, S.; Weh, T.; Frank, J.; Meixner, H. Selective gas detection with high-temperature operated metal oxides using catalytic filters. Sens. Actuators B Chem. 2000, 69, 205–210. [Google Scholar] [CrossRef]
- Lang, A.C.; Fleischer, M.; Meixner, H. Surface modifications of Ga2O3 thin film sensors with Rh, Ru and Ir clusters. Sens. Actuators B Chem. 2000, 66, 80–84. [Google Scholar] [CrossRef]
- Bene, R.; Pinter, Z.; Perczel, I.V.; Fleischer, M.; Reti, F. High-temperature semiconductor gas sensors. Vacuum 2001, 61, 275–278. [Google Scholar] [CrossRef]
- Frank, J.; Fleischer, M.; Zimmer, M.; Meixner, H. Ozone sensing using In2O3 modified Ga2O3 thin films. IEEE Sens. J. 2001, 1, 318–321. [Google Scholar] [CrossRef]
- Frank, J.; Meixner, H. Sensor system for indoor air monitoring using semiconducting metal oxides and IR-absorption. Sens. Actuators B Chem. 2001, 78, 298–302. [Google Scholar] [CrossRef]
- Kiss, G.; Pinter, Z.; Perczel, I.V.; Sassi, Z.; Reti, F. Study of oxide semiconductor sensor materials by selected methods. Thin Solid Films 2001, 391, 216–223. [Google Scholar] [CrossRef]
- Lampe, U.; Simon, E.; Pohle, R.; Fleischer, M.; Meixner, H.; Frerichs, H.-P.; Lehmann, M.; Kiss, G. GasFET for the detection of reducing gases. Sens. Actuators B Chem. 2005, 111–112, 106–110. [Google Scholar] [CrossRef]
- Stegmeier, S.; Fleischer, M.; Hauptmann, P. Influence of the morphology of platinum combined with β-Ga2O3 on the VOC response of work function type sensors. Sens. Actuators B Chem. 2010, 148, 439–449. [Google Scholar] [CrossRef]
- Stegmeier, S.; Fleischer, M.; Hauptmann, P. Thermally activated platinum as VOC sensing material for work function type gas sensors. Sens. Actuators B Chem. 2010, 144, 418–424. [Google Scholar] [CrossRef]
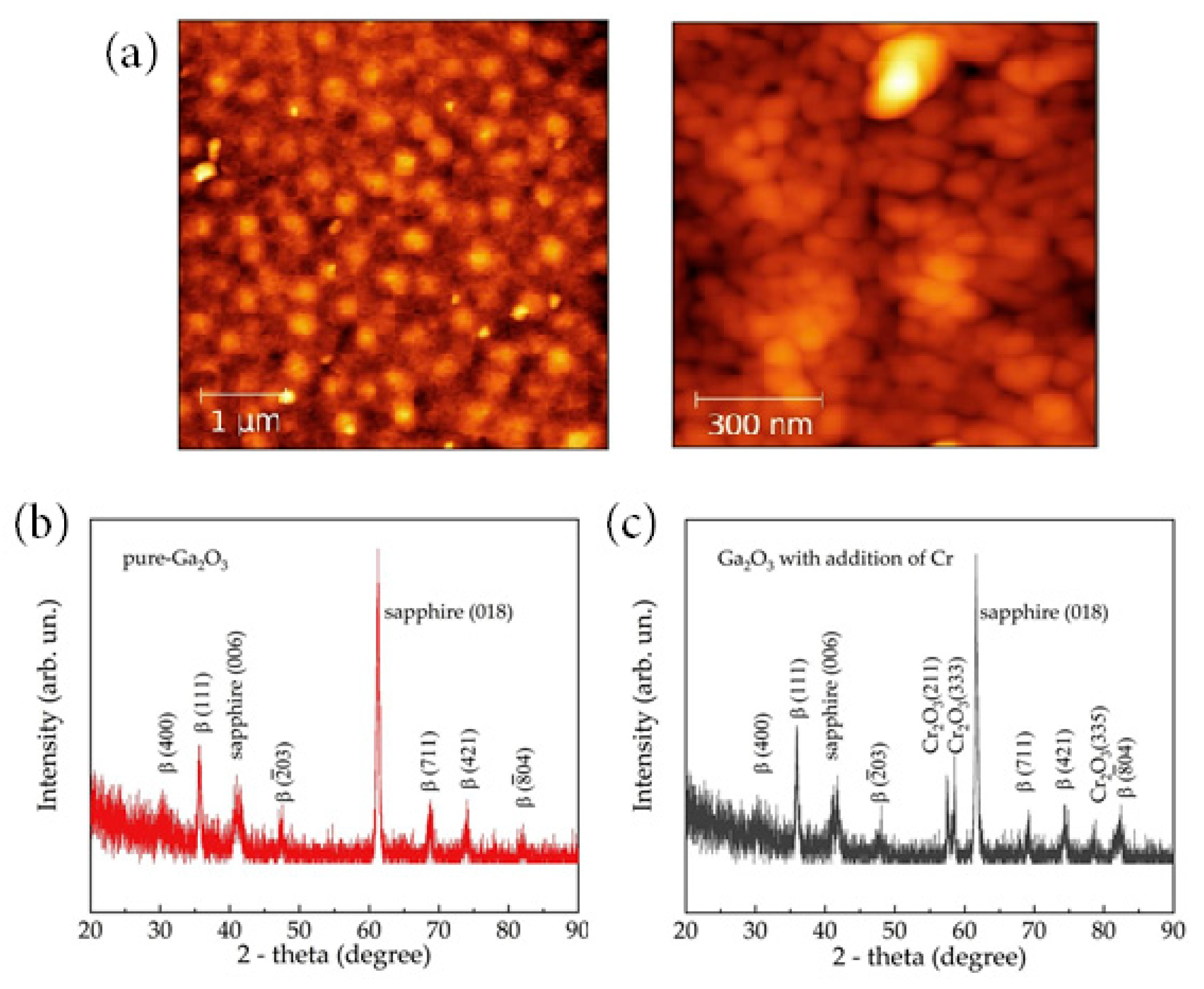
- Almaev, A.V.; Chernikov, E.V.; Davletkildeev, N.A.; Sokolov, D.V. Oxygen sensors based on gallium oxide thin films with addition of chromium. Superlattices Microstruct. 2020, 139, 106392. [Google Scholar] [CrossRef]
- Almaev, A.V.; Chernikov, E.V.; Novikov, V.V.; Kushnarev, B.O.; Yakovlev, N.N.; Chuprakova, E.V.; Oleinik, V.L.; Lozinskaya, A.D.; Gogova, D.S. Impact of Cr2O3 additives on the gas-sensitive properties of β-Ga2O3 thin films to oxygen, hydrogen, carbon monoxide, and toluene vapors. J. Vac. Sci. Technol. A 2021, 39, 023405. [Google Scholar] [CrossRef]
- Fleischer, M.; Giber, J.; Meixner, H. H2-induced changes in electrical conductance of β-Ga2O3 thin-film systems. Appl. Phys. A 1992, 54, 560–566. [Google Scholar] [CrossRef]
- Fleischer, M.; Meixner, H. Improvements in Ga2O3 sensors for reducing gases. Sens. Actuators B Chem. 1993, 13, 259–263. [Google Scholar] [CrossRef]
- Fleischer, M.; Hollbauer, L.; Meixner, H. Effect of the sensor structure on the stability of Ga2O3 sensors for reducing gases. Sens. Actuators B Chem. 1994, 18–19, 119–124. [Google Scholar] [CrossRef]
- Fleischer, M.; Meixner, H. In situ Hall measurements at temperatures up to 1100 degrees C with selectable gas atmospheres. Meas. Sci. Technol. 1994, 5, 580–583. [Google Scholar] [CrossRef]
- Fleischer, M.; Wagner, V.; Hacker, B.; Meixner, H. Comparison of a.c. and d.c. measurement techniques using semiconducting Ga2O3 sensors. Sens. Actuators B Chem. 1995, 26–27, 85–88. [Google Scholar] [CrossRef]
- Fleischer, M.; Meixner, H. Sensitive, selective and stable CH4 detection using semiconducting Ga2O3 thin films. Sens. Actuators B Chem. 1995, 26–27, 81–84. [Google Scholar] [CrossRef]
- Fleischer, M.; Seth, M.; Kohl, C.-D.; Meixner, H. A selective H2 sensor implemented using Ga2O3 thin-films which are covered with a gas-filtering SiO2 layer. Sens. Actuators B Chem. 1996, 35–36, 297–302. [Google Scholar] [CrossRef]
- Fleischer, M.; Seth, M.; Koh, C.-D.; Meixner, H. A study of surface modification at semiconducting Ga2O3 thin film sensors for enhancement of the sensitivity and selectivity. Sens. Actuators B Chem. 1996, 35–36, 290–296. [Google Scholar] [CrossRef]
- Reti, F.; Fleischer, M.; Perczel, I.V.; Meixner, H.; Giber, J. Detection of reducing gases in air by β-Ga2O3 thin films using self-heated and externally (oven-) heated operation modes. Sens. Actuators B Chem. 1996, 34, 378–382. [Google Scholar] [CrossRef]
- Baban, C.-I.; Toyoda, Y.; Ogita, M. High temperature oxygen sensor using a Pt-Ga2O3-Pt sandwich structure. Jpn. J. Appl. Phys. 2004, 43, 7213–7216. [Google Scholar] [CrossRef]
- Baban, C.; Toyoda, Y.; Ogita, M. Oxygen sensing at high temperatures using Ga2O3 films. Thin Solid Films 2005, 484, 369–373. [Google Scholar] [CrossRef]
- Bartic, M.; Toyoda, Y.; Baban, C.-I.; Ogita, M. Oxygen sensitivity in gallium oxide thin films and single crystals at high temperatures. Jpn. J. Appl. Phys. 2006, 45, 5186–5188. [Google Scholar] [CrossRef]
- Manandhar, S.; Battu, A.K.; Devaraj, A.; Shutthanandan, V.; Thevuthasan, S.; Ramana, C.V. Rapid response high temperature oxygen sensor based on titanium doped gallium oxide. Sci. Rep. 2020, 10, 178. [Google Scholar] [CrossRef]
- Frank, J.; Fleischer, M.; Meixner, H. Electrical doping of gas-sensitive, semiconducting Ga2O3 thin films. Sens. Actuators B Chem. 1996, 34, 373–377. [Google Scholar] [CrossRef]
- Ogita, M.; Saika, N.; Nakanishi, Y.; Hatanaka, Y. Ga2O3 thin films for high-temperature gas sensors. Appl. Surf. Sci. 1999, 142, 188–191. [Google Scholar] [CrossRef]
- Juan, Y.M.; Chang, S.-J.; Hsueh, H.T.; Chen, T.C.; Huang, S.W.; Lee, Y.H.; Hsueh, T.J.; Wu, C.L. Self-powered hybrid humidity sensor and dual-band UV photodetector fabricated on back-contact photovoltaic cell. Sens. Actuators B Chem. 2015, 219, 43–49. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Z.; Karim, M.R.; Zhao, H. High-temperature low-pressure chemical vapor deposition of β-Ga2O3. J. Vac. Sci. Technol. A 2020, 38, 050806. [Google Scholar] [CrossRef]
- Yu, M.-F.; Atashbar, M.Z.; Chen, X. Mechanical and electrical characterization of β-Ga2O3 nanostructures for sensing applications. IEEE Sens. J. 2005, 5, 20–25. [Google Scholar] [CrossRef]
- Feng, P.; Xue, X.Y.; Liu, Y.G.; Wan, Q.; Wang, T.H. Achieving fast oxygen response in individual β-Ga2O3 nanowires by ultraviolet illumination. Appl. Phys. Lett. 2006, 89, 112114. [Google Scholar] [CrossRef]
- Huang, Y.; Yue, S.; Wang, Z.; Wang, Q.; Shi, C.; Xu, Z.; Bai, X.D.; Tang, C.; Gu, C. Preparation and electrical properties of ultrafine Ga2O3 nanowires. J. Phys. Chem. B 2006, 110, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yamazaki, T.; Shen, Y.; Kikuta, T.; Nakatani, N.; Li, Y.X. O2 and CO sensing of Ga2O3 multiple nanowire gas sensors. Sens. Actuators B Chem. 2008, 129, 666–670. [Google Scholar] [CrossRef]
- Arnold, S.P.; Prokes, S.M.; Perkins, F.K.; Zaghloul, M.E. Design and performance of a simple, room-temperature Ga2O3 nanowire gas sensor. Appl. Phys. Lett. 2009, 95, 103102. [Google Scholar] [CrossRef]
- Mazeina, L.; Picard, Y.N.; Maximenko, S.I.; Perkins, F.K.; Glaser, E.R.; Twigg, M.E.; Freitas, J.A., Jr.; Prokes, S.M. Growth of Sn-doped β-Ga2O3 nanowires and Ga2O3-SnO2 heterostructures for gas sensing applications. Cryst. Growth Des. 2009, 9, 4471–4479. [Google Scholar] [CrossRef]
- Ma, H.L.; Fan, D.W. Influence of oxygen pressure on structural and sensing properties of β-Ga2O3 nanomaterial by thermal evaporation. Chin. Phys. Lett. 2009, 26, 117302. [Google Scholar] [CrossRef]
- Cuong, N.D.; Park, Y.W.; Yoon, S.G. Microstructural and electrical properties of Ga2O3 nanowires grown at various temperatures by vapor-liquid-solid technique. Sens. Actuators B Chem. 2009, 140, 240–244. [Google Scholar] [CrossRef]
- Mazeina, L.; Perkins, F.K.; Bermudez, V.M.; Arnold, S.P.; Prokes, S.M. Functionalized Ga2O3 nanowires as active material in room temperature capacitance-based gas sensors. Langmuir 2010, 26, 13722–13726. [Google Scholar] [CrossRef] [PubMed]
- Mazeina, L.; Bermudez, V.M.; Perkins, F.K.; Arnold, S.P.; Prokes, S.M. Interaction of functionalized Ga2O3 NW-based room temperature gas sensors with different hydrocarbons. Sens. Actuators B Chem. 2010, 151, 114–120. [Google Scholar] [CrossRef]
- Ma, H.L.; Fan, D.W.; Niu, X.S. Preparation and NO2-gas sensing property of individual β-Ga2O3 nanobelt. Chin. Phys. B 2010, 19, 076102. [Google Scholar] [CrossRef]
- Jang, Y.-G.; Kim, W.-S.; Kim, D.-H.; Hong, S.-H. Fabrication of Ga2O3/SnO2 core-shell nanowires and their ethanol gas sensing properties. J. Mater. Res. 2011, 26, 2322–2327. [Google Scholar] [CrossRef]
- Nakagomi, S.; Sai, T.; Kokubun, Y. Hydrogen gas sensor with self temperature compensation based on β-Ga2O3 thin film. Sens. Actuators B Chem. 2013, 187, 413–419. [Google Scholar] [CrossRef]
- Nakagomi, S.; Yokoyama, K.; Kokubun, Y. Devices based on series-connected Schottky junctions and β-Ga2O3/SiC heterojunctions characterized as hydrogen sensors. J. Sens. Sens. Syst. 2014, 3, 231–239. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Luan, Q.; Chang, S.-J.; Jiao, Z.; Weng, W.Y.; Lin, Y.-H.; Hsu, C.L. Highly sensitive β-Ga2O3 nanowire isopropyl alcohol sensor. IEEE Sens. J. 2014, 14, 401–405. [Google Scholar] [CrossRef]
- Zhong, W.; Wang, P.W.; Han, X.B.; Yu, D.P. Gas sensor based on Ga2O3 nanowires. J. Chin. Electron Microsc. Soc. 2014, 33, 7–13. [Google Scholar] [CrossRef]
- Reiprich, J.; Isaac, N.A.; Schlag, L.; Hopfeld, M.; Ecke, G.; Stauden, T.; Pezoldt, J.; Jacobs, H.O. Corona discharge assisted growth morphology switching of tin-doped gallium oxide for optical gas sensing applications. Cryst. Growth Des. 2019, 19, 6945–6953. [Google Scholar] [CrossRef]
- Krawczyk, M.; Wozniak, P.S.; Szukiewicz, R.; Kuchowicz, M.; Korbutowicz, R.; Teterycz, H. Morphology of Ga2O3 nanowires and their sensitivity to volatile organic compounds. Nanomaterials 2021, 11, 456. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jin, C.; An, S.; Lee, C. Fabrication and CO gas-sensing properties of Pt-functionalized Ga2O3 nanowires. Ceram. Int. 2012, 38, 3563–3567. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.; Jin, C.; Lee, C. Synthesis, structure, and room-temperature gas sensing of multiple-networked Pd-doped Ga2O3 nanowires. J. Korean Phys. Soc. 2012, 60, 1560–1564. [Google Scholar] [CrossRef]
- Jin, C.; Park, S.; Kim, H.; Lee, C. Ultrasensitive multiple networked Ga2O3-core/ZnO-shell nanorod gas sensors. Sens. Actuators B Chem. 2012, 161, 223–228. [Google Scholar] [CrossRef]
- Tsai, T.-Y.; Chang, S.-J.; Weng, W.-Y.; Liu, S.; Hsu, C.-L.; Hsueh, H.-T.; Hsueh, T.-J. β-Ga2O3 nanowires-based humidity sensors prepared on GaN/sapphire substrate. IEEE Sens. J. 2013, 13, 4891–4896. [Google Scholar] [CrossRef]
- An, S.; Park, S.; Mun, Y.; Lee, C. UV enhanced NO2 sensing properties of Pt functionalized Ga2O3 nanorods. Bull. Korean Chem. Soc. 2013, 34, 1632–1636. [Google Scholar] [CrossRef][Green Version]
- Park, S.H.; Kim, S.H.; Park, S.Y.; Lee, C. Synthesis and CO gas sensing properties of surface-nitridated Ga2O3 nanowires. RSC Adv. 2014, 4, 63402–63407. [Google Scholar] [CrossRef]
- Juan, Y.M.; Chang, S.-J.; Hsueh, H.T.; Wang, S.H.; Weng, W.Y.; Cheng, T.C.; Wu, C.L. Effects of humidity and ultraviolet characteristics on β-Ga2O3 nanowire sensor. RSC Adv. 2015, 5, 84776–84781. [Google Scholar] [CrossRef]
- Bui, Q.C.; Largeau, L.; Morassi, M.; Jegenyes, N.; Mauguin, O.; Travers, L.; Lafosse, X.; Dupuis, C.; Harmand, J.-C.; Tchernycheva, M.; et al. GaN/Ga2O3 core/shell nanowires growth: Towards high response gas sensors. Appl. Sci. 2019, 9, 3528. [Google Scholar] [CrossRef]
- Abdullah, Q.N.; Ahmed, A.R.; Ali, A.M.; Yam, F.K.; Hassan, Z.; Bououdina, M. Novel SnO2-coated β-Ga2O3 nanostructures for room temperature hydrogen gas sensor. Int. J. Hydrogen Energy 2021, 46, 7000–7010. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.; Sun, G.-J.; Lee, C. Synthesis, structure and ethanol sensing properties of Ga2O3-core/WO3-shell nanostructures. Thin Solid Films 2015, 591, 341–345. [Google Scholar] [CrossRef]
- Sprincean, V.; Caraman, M.; Spataru, T.; Fernandez, F.; Paladi, F. Influence of the air humidity on the electrical conductivity of the β-Ga2O3-GaS structure: Air humidity sensor. Appl. Phys. A 2022, 128, 303. [Google Scholar] [CrossRef]
- Girija, K.; Thirumalairajan, S.; Mastelaro, V.R.; Mangalaraj, D. Catalyst free vapor-solid deposition of morphologically different β-Ga2O3 nanostructure thin films for selective CO gas sensors at low temperature. Anal. Methods 2016, 8, 3224–3235. [Google Scholar] [CrossRef]
- Almaev, A.V.; Nikolaev, V.I.; Yakovlev, N.N.; Butenko, P.N.; Stepanov, S.I.; Pechnikov, A.I.; Scheglov, M.P.; Chernikov, E.V. Hydrogen sensors based on Pt/α-Ga2O3: Sn/Pt structures. Sens. Actuators B Chem. 2022, 364, 131904. [Google Scholar] [CrossRef]
- Yakovlev, N.N.; Nikolaev, V.I.; Stepanov, S.I.; Almaev, A.V.; Pechnikov, A.I.; Chernikov, E.V.; Kushnarev, B.O. Effect of oxygen on the electrical conductivity of Pt-contacted α-Ga2O3/ε(κ)-Ga2O3 MSM structures on patterned sapphire substrates. IEEE Sens. J. 2021, 21, 14636–14644. [Google Scholar] [CrossRef]
- Krawczyk, M.; Korbutowicz, R.; Szukiewicz, R.; Wozniak, P.S.; Kuchowicz, M.; Teterycz, H. P-type inversion at the surface of β-Ga2O3 epitaxial layer modified with Au nanoparticles. Sensors 2022, 22, 932. [Google Scholar] [CrossRef]
- Liu, K.W.; Sakurai, M.; Aono, M. One-step fabrication of β-Ga2O3-amorphous-SnO2 core-shell microribbons and their thermally switchable humidity sensing properties. J. Mater. Chem. 2012, 22, 12882. [Google Scholar] [CrossRef]
- Weng, T.-F.; Ho, M.-S.; Sivakumar, C.; Balraj, B.; Chung, P.-F. VLS growth of pure and Au decorated β-Ga2O3 nanowires for room temperature CO gas sensor and resistive memory applications. Appl. Surf. Sci. 2020, 533, 147476. [Google Scholar] [CrossRef]
- Aymerich, E.L.; Gil, G.D.; Moreno, M.; Pellegrino, P.; Rodriguez, A.R. Fabrication, characterization and performance of low power gas sensors based on (GaxIn1-x)2O3 nanowires. Sensors 2021, 21, 3342. [Google Scholar] [CrossRef]
- Ge, X.T.; Fang, D.R.; Liu, X.Q. The preparation and gas-sensing properties of Ga2O3-NiO complex oxide by sol-gel method. Acta Phys. Chim. Sin. 2005, 21, 10–15. [Google Scholar] [CrossRef]
- Mohammadi, M.R.; Fray, D.J. Semiconductor TiO2-Ga2O3 thin film gas sensors derived from particulate sol-gel route. Acta Mater. 2007, 55, 4455–4466. [Google Scholar] [CrossRef]
- Mohammadi, M.R.; Fray, D.J.; Ghorbani, M. Comparison of single and binary oxide sol-gel gas sensors based on titania. Solid State Sci. 2008, 10, 884–893. [Google Scholar] [CrossRef]
- Hou, Y.; Jayatissa, A.H. Low resistive gallium doped nanocrystalline zinc oxide for gas sensor application via sol-gel process. Sens. Actuators B Chem. 2014, 204, 310–318. [Google Scholar] [CrossRef]
- Trinchi, A.; Galatsis, K.; Wlodarski, W.; Li, Y.X. A Pt/Ga2O3-ZnO/SiC Schottky diode-based hydrocarbon gas sensor. IEEE Sens. J. 2003, 3, 548–553. [Google Scholar] [CrossRef]
- Trinchi, A.; Li, Y.X.; Wlodarski, W.; Kaciulis, S.; Pandolfi, L.; Russo, S.P.; Duplessis, J.; Viticoli, S. Investigation of sol-gel prepared Ga-Zn oxide thin films for oxygen gas sensing. Sens. Actuators A Phys. 2003, 108, 263–270. [Google Scholar] [CrossRef]
- Li, Y.X.; Trinchi, A.; Wlodarski, W.; Galatsis, K.; Kalantar-zadeh, K. Investigation of the oxygen gas sensing performance of Ga2O3 thin films with different dopants. Sens. Actuators B Chem. 2003, 93, 431–434. [Google Scholar] [CrossRef]
- Trinchi, A.; Kaciulis, S.; Pandolfi, L.; Ghantasala, M.K.; Li, Y.X.; Wlodarski, W.; Viticoli, S.; Comini, E.; Sberveglieri, G. Characterization of Ga2O3 based MRISiC hydrogen gas sensors. Sens. Actuators B Chem. 2004, 103, 129–135. [Google Scholar] [CrossRef]
- Trinchi, A.; Wlodarski, W.; Li, Y.X. Hydrogen sensitive Ga2O3 Schottky diode sensor based on SiC. Sens. Actuators B Chem. 2004, 100, 94–98. [Google Scholar] [CrossRef]
- Trinchi, A.; Wlodarski, W.; Faglia, G.; Ponzoni, A.; Comini, E.; Sberveglieri, G. High temperature hydrocarbon sensing with Pt-thin Ga2O3-SiC diodes. Mater. Sci. Forum 2005, 483–485, 1033–1036. [Google Scholar] [CrossRef]
- Trinchi, A.; Wlodarski, W.; Li, Y.X.; Faglia, G.; Sberveglieri, G. Pt/Ga2O3/SiC MRISiC devices: A study of the hydrogen response. J. Phys. D Appl. Phys. 2005, 38, 754–763. [Google Scholar] [CrossRef]
- Bagheri, M.; Khodadadi, A.A.; Mahjoub, A.R.; Mortazavi, Y. Strong effects of gallia on structure and selective responses of Ga2O3-In2O3 nanocomposite sensors to either ethanol, CO or CH4. Sens. Actuators B Chem. 2015, 220, 590–599. [Google Scholar] [CrossRef]
- Lin, H.-J.; Baltrus, J.P.; Gao, H.; Ding, Y.; Nam, C.-Y.; Ohodnicki, P.; Gao, P.-X. Perovskite nanoparticle-sensitized Ga2O3 nanorod arrays for CO detection at high temperature. ACS Appl. Mater. Interfaces 2016, 8, 8880–8887. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-J.; Gao, H.; Gao, P.-X. UV-enhanced CO sensing using Ga2O3-based nanorod arrays at elevated temperature. Appl. Phys. Lett. 2017, 110, 043101. [Google Scholar] [CrossRef]
- Chen, H.; Hu, J.B.; Li, G.-D.; Gao, Q.; Wei, C.D.; Zou, X.X. Porous Ga-In bimetallic oxide nanofibers with controllable structures for ultrasensitive and selective detection of formaldehyde. ACS Appl. Mater. Interfaces 2017, 9, 4692–4700. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Jia, C.W.; Kong, Q.; Fan, N.Y.; Chen, G.; Guan, H.T.; Dong, C.J. ZnO-decorated In/Ga oxide nanotubes derived from bimetallic In/Ga MOFs for fast acetone detection with high sensitivity and selectivity. ACS Appl. Mater. Interfaces 2020, 12, 26161. [Google Scholar] [CrossRef]
- Pilliadugula, R.; Krishnan, N. Gas sensing performance of GaOOH and β-Ga2O3 synthesized by hydrothermal method: A comparison. Mater. Res. Express 2018, 6, 025027. [Google Scholar] [CrossRef]
- Pilliadugula, R.; Krishnan, N.G. Effect of pH dependent morphology on room temperature NH3 sensing performances of β-Ga2O3. Mater. Sci. Semicond. Process. 2020, 112, 105007. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, S.S.; Liu, H.L.; Wang, S.H.; Pan, Q.J.; Yin, Y.D.; Zhang, G. P-type gas-sensing behavior of Ga2O3/Al2O3 nanocomposite with high sensitivity to NOx at room temperature. J. Alloys Compd. 2020, 814, 152284. [Google Scholar] [CrossRef]
- Zhang, B.; Lin, H.-J.; Gao, H.Y.; Lu, X.X.; Nam, C.-Y.; Gao, P.-X. Perovskite-sensitized β-Ga2O3 nanorod arrays for highly selective and sensitive NO2 detection at high temperature. J. Mater. Chem. A 2020, 8, 10845–10854. [Google Scholar] [CrossRef]
- Pilliadugula, R.; Gopalakrishnan, N. Room temperature ammonia sensing performances of pure and Sn doped β-Ga2O3. Mater. Sci. Semicond. Process. 2021, 135, 106086. [Google Scholar] [CrossRef]
- Demin, I.E.; Kozlov, A.G. In2O3-Ga2O3 thin films for ammonia sensors of petrochemical industry safety systems. AIP Conf. Proc. 2018, 2007, 050004. [Google Scholar] [CrossRef]
- Demin, I.E.; Kozlov, A.G. Selectivity of the gas sensor based on the 50% In2O3-50% Ga2O3 thin film in dynamic mode of operation. J. Phys. Conf. Ser. 2018, 944, 012027. [Google Scholar] [CrossRef]
- Demin, I.E. Increasing the selectivity of semiconductor gas sensors working at sinusoidal-varying temperature for machine industry safety systems. J. Phys. Conf. Ser. 2019, 1260, 032008. [Google Scholar] [CrossRef]
- Demin, I.E. Selection of methods for increasing gas selectivity on the example of a sensor system based on In2O3-Ga2O3 semiconductor films. J. Phys. Conf. Ser. 2019, 1210, 012032. [Google Scholar] [CrossRef]
- Demin, I.E. Reducing the energy consumption of semiconductor methane sensors for gas alarm systems. AIP Conf. Proc. 2019, 2141, 050020. [Google Scholar] [CrossRef]
- Wei, Z.H.; Akbari, M.K.; Hai, Z.Y.; Ramachandran, R.K.; Detavernier, C.; Verpoort, F.; Kats, E.; Xu, H.Y.; Hu, J.; Zhuiykov, S. Ultra-thin sub-10 nm Ga2O3-WO3 heterostructures developed by atomic layer deposition for sensitive and selective C2H5OH detection on ppm level. Sens. Actuators B Chem. 2019, 287, 147–156. [Google Scholar] [CrossRef]
- Jochum, W.; Penner, S.; Fottinger, K.; Kramer, R.; Rupprechter, G.; Klotzer, B. Hydrogen on polycrystalline β-Ga2O3: Surface chemisorption, defect formation, and reactivity. J. Catal. 2008, 256, 268–277. [Google Scholar] [CrossRef]
- Vorobyeva, N.; Rumyantseva, M.; Platonov, V.; Filatova, D.; Chizhov, A.; Marikutsa, A.; Bozhev, I.; Gaskov, A. Ga2O3 (Sn) oxides for high-temperature gas sensors. Nanomaterials 2021, 11, 2938. [Google Scholar] [CrossRef]
- Pandeeswari, R.; Jeyaprakash, B.G. High sensing response of β-Ga2O3 thin film towards ammonia vapours: Influencing factors at room temperature. Sens. Actuators B Chem. 2014, 195, 206–214. [Google Scholar] [CrossRef]
- Yan, J.-T.; Lee, C.-T. Improved detection sensitivity of Pt/β-Ga2O3/GaN hydrogen sensor diode. Sens. Actuators B Chem. 2009, 143, 192–197. [Google Scholar] [CrossRef]
- Lee, C.-T.; Yan, J.-T. Investigation of a metal-insulator-semiconductor Pt/mixed Al2O3 and Ga2O3 insulator/AlGaN hydrogen sensor. J. Electrochem. Soc. 2010, 157, J281. [Google Scholar] [CrossRef]
- Lee, C.-T.; Yan, J.-T. Sensing mechanisms of Pt/β-Ga2O3/GaN hydrogen sensor diodes. Sens. Actuators B Chem. 2010, 147, 723–729. [Google Scholar] [CrossRef]
- Shafiei, M.; Hoshyargar, F.; Motta, N.; O’Mullane, A.P. Utilizing p-type native oxide on liquid metal microdroplets for low temperature gas sensing. Mater. Des. 2017, 122, 288–295. [Google Scholar] [CrossRef]
- Saidi, S.A.; Tang, J.; Yang, J.; Han, J.; Daeneke, T.; O’Mullane, A.P.; Kalantar-Zadeh, K. Liquid metal-based route for synthesizing and tuning gas-sensing elements. ACS Sens. 2020, 5, 1177–1189. [Google Scholar] [CrossRef]
- Sivasankaran, B.R.; Balaji, M. Novel gallium oxide/reduced graphene oxide nanocomposite for ammonia gas sensing application. Mater. Lett. 2021, 288, 129386. [Google Scholar] [CrossRef]
- Ji, H.; Zeng, W.; Li, Y. Gas sensing mechanisms of metal oxide semiconductors: A focus review. Nanoscale 2019, 11, 22664–22684. [Google Scholar] [CrossRef]
- Fleischer, M.; Meixner, H. Fast gas sensors based on metal oxides which are stable at high temperatures. Sens. Actuators B Chem. 1997, 43, 1–10. [Google Scholar] [CrossRef]
- Fleischer, M.; Meixner, H. Thin-film gas sensors based on high-temperature-operated metal oxides. J. Vac. Sci. Technol. A 1999, 17, 1866–1872. [Google Scholar] [CrossRef]
- Hoefer, U.; Frank, J.; Fleischer, M. High temperature Ga2O3-gas sensors and SnO2-gas sensors: A comparison. Sens. Actuators B Chem. 2001, 78, 6–11. [Google Scholar] [CrossRef]
- Fleischer, M. Advances in application potential of adsorptive-type solid state gas sensors: High-temperature semiconducting oxides and ambient temperature GasFET devices. Meas. Sci. Technol. 2008, 19, 042001. [Google Scholar] [CrossRef]
- Pohle, R.; Weisbrod, E.; Hedler, H. Enhancement of MEMS-based Ga2O3 gas sensors by surface modifications. Proc. Eng. 2016, 168, 211–215. [Google Scholar] [CrossRef]
- Weisz, P.B.; Prater, C.D. Interpretation of measurements in experimental catalysis. Adv. Catal. 1954, 6, 143. [Google Scholar] [CrossRef]
- Jaaniso, R.; Tan, O.K. Semiconductor Gas Sensors; Woodhead Publishing: Duxford, UK, 2019; pp. 27–34. [Google Scholar] [CrossRef]
- Yamazoe, N. New approaches for improving semiconductor gas sensors. Sens. Actuators B Chem. 1991, 5, 7–19. [Google Scholar] [CrossRef]
- Korotcenkov, G. Handbook of Gas Sensor Materials; Springer: New York, NY, USA, 2013; pp. 49–116. [Google Scholar] [CrossRef]
- Frank, J.; Fleischer, M.; Meixner, H. Gas-sensitive electrical properties of pure and doped semiconducting Ga2O3 thick films. Sens. Actuators B Chem. 1998, 48, 318–321. [Google Scholar] [CrossRef]
- Frank, J.; Fleischer, M.; Meixner, H.; Feltz, A. Enhancement of sensitivity and conductivity of semiconducting Ga2O3 gas sensors by doping with SnO2. Sens. Actuators B Chem. 1998, 49, 110–114. [Google Scholar] [CrossRef]
- Schwebel, T.; Fleischer, M.; Meixner, H. A selective, temperature compensated O2 sensor based on Ga2O3 thin films. Sens. Actuators B Chem. 2000, 65, 176–180. [Google Scholar] [CrossRef]
- Fleischer, M.; Meixner, H. Sensing reducing gases at high temperatures using long-term stable Ga2O3 thin films. Sens. Actuators B Chem. 1992, 6, 257–261. [Google Scholar] [CrossRef]
- Hacker, B.; Fleischer, M.; Meixner, H. Topography and performance of gas-sensing devices: An AFM study. Scanning 1993, 15, 291–294. [Google Scholar] [CrossRef]
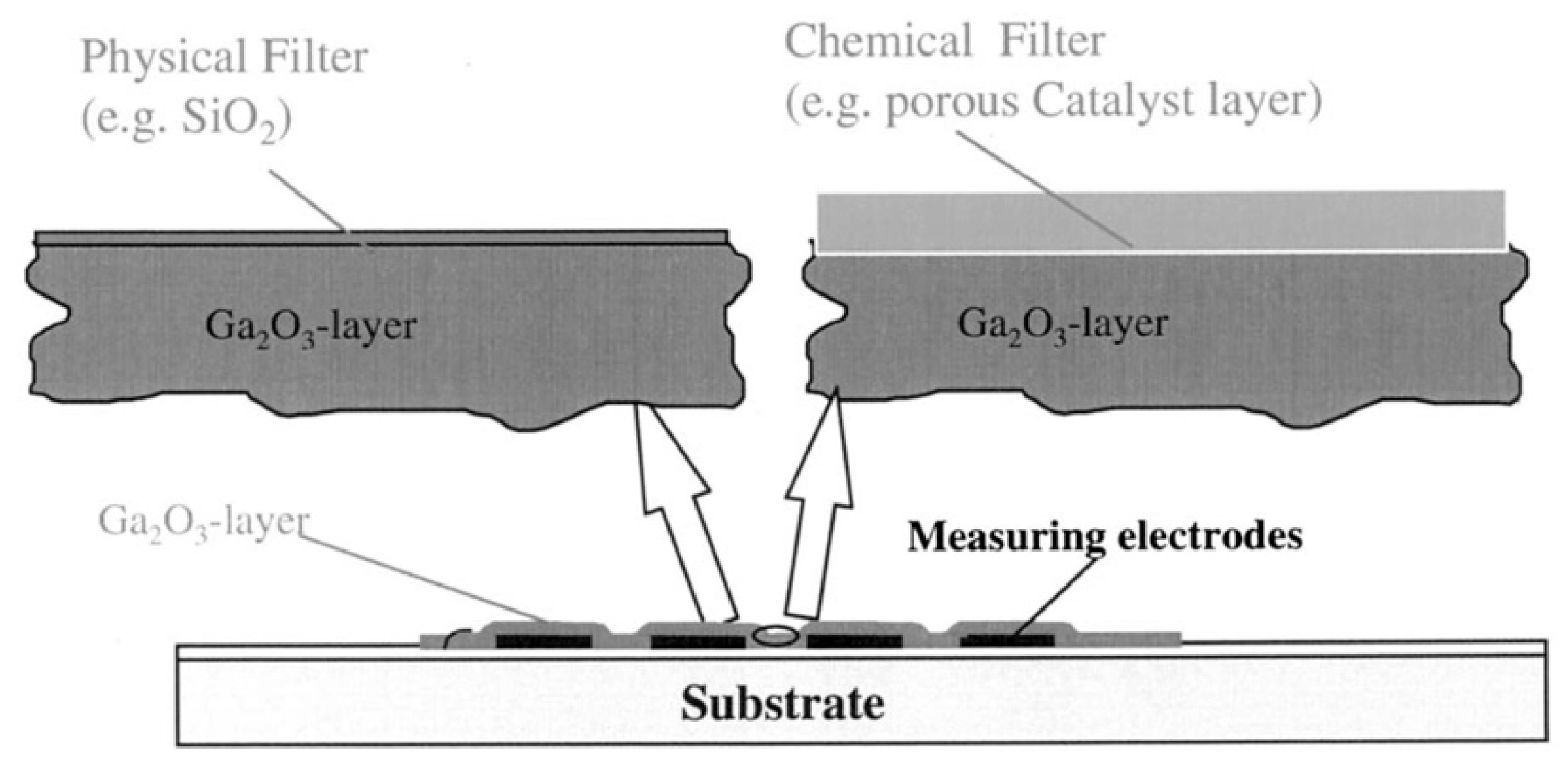
- Weh, T.; Fleischer, M.; Meixner, H. Optimization of physical filtering for selective high temperature H2 sensors. Sens. Actuators B Chem. 2000, 68, 146–150. [Google Scholar] [CrossRef]
- Weh, T.; Frank, J.; Fleischer, M.; Meixner, H. On the mechanism of hydrogen sensing with SiO2 modificated high temperature Ga2O3 sensors. Sens. Actuators B Chem. 2001, 78, 202–207. [Google Scholar] [CrossRef]
- Wiesner, K.; Knozinger, H.; Fleischer, M.; Meixner, H. Working mechanism of an ethanol filter for selective high-temperature methane gas sensors. IEEE Sens. J. 2002, 2, 354–359. [Google Scholar] [CrossRef]
- Biskupski, D.; Geupel, A.; Wiesner, K.; Fleischer, M.; Moos, R. Platform for a hydrocarbon exhaust gas sensor utilizing a pumping cell and a conductometric sensor. Sensors 2009, 9, 7498–7508. [Google Scholar] [CrossRef] [PubMed]
- Giber, J.; Perczel, I.V.; Gerblinger, J.; Lampe, U.; Fleischer, M. Coadsorption and cross sensitivity on high temperature semiconducting metal oxides: Water effect on the coadsorption process. Sens. Actuators B Chem. 1994, 18–19, 113–118. [Google Scholar] [CrossRef]
- Reti, F.; Fleischer, M.; Meixner, H.; Giber, J. Effect of coadsorption of reducing gases on the conductivity of β-Ga2O3 thin films in the presence of O2. Sens. Actuators B Chem. 1995, 18–19, 573–577. [Google Scholar] [CrossRef]
- Reti, F.; Fleischer, M.; Meixner, H.; Giber, J. Influence of water on the coadsorption of oxidizing and reducing gases on the β-Ga2O3 surface. Sens. Actuators B Chem. 1994, 18, 138–142. [Google Scholar] [CrossRef]
- Reti, F.; Fleischer, M.; Gerblinger, J.; Lampe, U.; Varhegyi, E.B.; Perczel, V.; Meixner, H.; Giber, J. Comparison of the water effect on the resistance of different semiconducting metal oxides. Sens. Actuators B Chem. 1995, 26–27, 103–107. [Google Scholar] [CrossRef]
- Pohle, R.; Fleischer, M.; Meixner, H. In situ infrared emission spectroscopic study of the adsorption of H2O and hydrogen-containing gases on Ga2O3 gas sensors. Sens. Actuators B Chem. 2000, 68, 151–156. [Google Scholar] [CrossRef]
- Varhegyi, E.B.; Perczel, I.V.; Gerblinger, J.; Fleischer, M.; Meixner, H.; Giber, J. Auger and SIMS study of segregation and corrosion some semiconducting oxide gas-sensor materials. Sens. Actuators B Chem. 1994, 18–19, 569–572. [Google Scholar] [CrossRef]
- Hovhannisyan, R.V.; Khondkaryan, H.D.; Aleksanyan, M.S.; Arakelyan, V.M.; Semerjyan, B.O.; Gasparyan, F.V.; Aroutiounian, V.M. Static and noise characteristics of nanocomposite gas sensors. J. Contemp. Phys. 2014, 49, 151–157. [Google Scholar] [CrossRef]
- Dyndal, K.; Zarzycki, A.; Andrysiewicz, W.; Grochala, D.; Marszalek, K.; Rydosz, A. CuO-Ga2O3 thin films as a gas-sensitive material for acetone detection. Sensors 2020, 20, 3142. [Google Scholar] [CrossRef]
- Lundstrom, I.; Shivaraman, S.; Svensson, C.; Lundkvist, L. A hydrogen-sensitive MOS field-effect transistor. Appl. Phys. Lett. 1975, 26, 55–57. [Google Scholar] [CrossRef]
- Leu, M.; Doll, T.; Flietner, B.; Lechner, J.; Eisele, I. Evaluation of gas mixtures with different sensitive layers incorporated in hybrid FET structures. Sens. Actuators B Chem. 1994, 18–19, 678–681. [Google Scholar] [CrossRef]
- Geistlinger, H. Accumulation layer model for Ga2O3 thin-film gas sensors based on the Volkenstein theory of catalysis. Sens. Actuators B Chem. 1994, 18, 125–131. [Google Scholar] [CrossRef]
- Geistlinger, H.; Eisele, I.; Flietner, B.; Winter, R. Dipole- and charge transfer contributions to the work function change of semiconducting thin films: Experiment and theory. Sens. Actuators B Chem. 1996, 34, 499–505. [Google Scholar] [CrossRef]
- Shin, W.; Hong, S.; Jeong, Y.; Jung, G.; Park, J.; Kim, D.; Park, B.-G.; Lee, J.-H. Effects of channel length scaling on the signal-to-noise ratio in FET-type gas sensor with horizontal floating-gate. IEEE Electron Device Lett. 2022, 43, 442–445. [Google Scholar] [CrossRef]
- Imanaka, N.; Tamura, S.; Adachi, G. Ammonia sensor based on ionically exchanged NH4+-gallate solid electrolytes. Electrochem. Solid State Lett. 1999, 1, 282. [Google Scholar] [CrossRef]
- Westphal, D.; Jakobs, S.; Guth, U. Gold-composite electrodes for hydrocarbon sensors based on YSZ solid electrolyte. Ionics 2001, 7, 182–186. [Google Scholar] [CrossRef]
- Zosel, J.; Westphal, D.; Jakobs, S.; Muller, R.; Guth, U. Au-oxide composites as HC-sensitive electrode material for mixed potential gas sensors. Solid State Ion. 2002, 152–153, 525. [Google Scholar] [CrossRef]
- Zosel, J.; Ahlborn, K.; Muller, R.; Westphal, D.; Vashook, V.; Guth, U. Selectivity of HC-sensitive electrode materials for mixed potential gas sensors. Solid State Ion. 2002, 169, 115–119. [Google Scholar] [CrossRef]
- Zhang, W.-F.; Schmidt-Zhang, P.; Guth, U. Electrochemical studies on cells M/YSZ/Pt (M = Pt, Pt-Ga2O3) in NO, O2, N2 gas mixtures. Solid State Ion. 2004, 169, 121–128. [Google Scholar] [CrossRef]
- Shuk, P.; Bailey, E.; Zosel, J.; Guth, U. New advanced in situ carbon monoxide sensor for the process application. Ionics 2009, 15, 131–138. [Google Scholar] [CrossRef]
- Wu, N.Q.; Chen, Z.; Xu, J.H.; Chyu, M.; Mao, S.X. Impedance-metric Pt/YSZ/Au-Ga2O3 sensor for CO detection at high temperature. Sens. Actuators B Chem. 2005, 110, 49–53. [Google Scholar] [CrossRef]
- Yan, S.C.; Wan, L.J.; Li, Z.S.; Zhou, Y.; Zou, Z.G. Synthesis of a mesoporous single crystal Ga2O3 nanoplate with improved photoluminescence and high sensitivity in detecting CO. Chem. Commun. 2010, 46, 6388. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Han, S.H.; Cho, B.K. Material design for metal oxide chemiresistive gas sensors. J. Sens. Sci. Technol. 2013, 22, 1–17. [Google Scholar] [CrossRef]
- Bartic, M.; Ogita, M.; Isai, M.; Baban, C.; Suzuki, H. Oxygen sensing properties at high temperatures of β-Ga2O3 thin films deposited by the chemical solution deposition method. J. Appl. Phys. 2007, 102, 023709. [Google Scholar] [CrossRef]
- Wang, D.; Lou, Y.L.; Wang, R.; Wang, P.P.; Zheng, X.J.; Zhang, Y.; Jiang, N. Humidity sensor based on Ga2O3 nanorods doped with Na+ and K+ from GaN powder. Ceram. Int. 2015, 41, 14790–14797. [Google Scholar] [CrossRef]
- Potje-Kamloth, K. Semiconductor junction gas sensors. Chem. Rev. 2008, 108, 367–399. [Google Scholar] [CrossRef]
- Ratko, A.; Babushkin, O.; Baran, A.; Baran, S. Sorption and gas sensitive properties of In2O3 based ceramics doped with Ga2O3. J. Eur. Ceram. Soc. 1998, 18, 2227–2232. [Google Scholar] [CrossRef]
- Silver, A.T.; Juarez, A.S. SnO2: Ga thin films as oxygen gas sensor. Mater. Sci. Eng. B 2004, 110, 268–271. [Google Scholar] [CrossRef]
- Bagheri, M.; Khodadadi, A.A.; Mahjoub, A.R.; Mortazavi, Y. Highly sensitive gallia-SnO2 nanocomposite sensors to CO and ethanol in presence of methane. Sens. Actuators B Chem. 2013, 188, 45–52. [Google Scholar] [CrossRef]
- Du, L.T.; Li, H.Y.; Li, S.; Liu, L.; Li, Y.; Xu, S.Y.; Gong, Y.M.; Cheng, Y.L.; Zeng, X.G.; Liang, Q.C. A gas sensor based on Ga-doped SnO2 porous microflowers for detecting formaldehyde at low temperature. Chem. Phys. Lett. 2018, 713, 235–241. [Google Scholar] [CrossRef]
- Kevin, M.; Tho, W.H.; Ho, G.W. Transferability of solution processed epitaxial Ga:ZnO films; tailored for gas sensor and transparent conducting oxide applications. J. Mater. Chem. 2012, 22, 16442. [Google Scholar] [CrossRef]
- Vorobyeva, N.; Rumyantseva, M.; Filatova, D.; Konstantinova, E.; Grishina, D.; Abakumov, A.; Turner, S.; Gaskov, A. Nanocrystalline ZnO(Ga): Paramagnetic centers, surface acidity and gas sensor properties. Sens. Actuators B Chem. 2013, 182, 555–564. [Google Scholar] [CrossRef]
- Rashid, T.-R.; Phan, D.-T.; Chung, G.-S. Effect of Ga-modified layer on flexible hydrogen sensor using ZnO nanorods decorated by Pd catalysts. Sens. Actuators B Chem. 2014, 193, 869–876. [Google Scholar] [CrossRef]
- Girija, K.G.; Somasundaram, K.; Debnath, A.K.; Topkar, A.; Vatsa, R.K. Enhanced H2S sensing properties of gallium doped ZnO nanocrystalline films as investigated by DC conductivity and impedance spectroscopy. Mater. Chem. Phys. 2018, 214, 297–305. [Google Scholar] [CrossRef]
- Fleischer, M.; Meixner, H. Selectivity in high-temperature operated semiconductor gas-sensors. Sens. Actuators B Chem. 1998, 52, 179–187. [Google Scholar] [CrossRef]
- Anichini, C.; Czepa, W.; Pakulski, D.; Aliprandi, A.; Ciesielski, A.; Samori, P. Chemical sensing with 2D materials. Chem. Soc. Rev. 2018, 47, 4860–4908. [Google Scholar] [CrossRef]
- Zhao, J.L.; Huang, X.R.; Yin, Y.H.; Liao, Y.K.; Mo, H.W.; Qian, Q.K.; Guo, Y.Z.; Chen, X.L.; Zhang, Z.F.; Hua, M.Y. Two-dimensional gallium oxide monolayer for gas-sensing application. J. Phys. Chem. Lett. 2021, 12, 5813–5820. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Huang, H.; Cao, X.; Chen, X.; Cao, D. Porous organic polymers as a platform for sensing applications. Chem. Soc. Rev. 2022, 51, 2031–2080. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, P. Dissociation of H2 molecule on the β-Ga2O3 (100)B surface: The critical role of oxygen vacancy. Phys. Lett. A 2010, 374, 4169–4173. [Google Scholar] [CrossRef]
- Nagarajan, V.; Chandiramouli, R. Methane adsorption characteristics on β-Ga2O3 nanostructures: DFT investigation. Appl. Surf. Sci. 2015, 344, 65–78. [Google Scholar] [CrossRef]
- Yaqoob, U.; Younis, M.I. Chemical gas Sensors: Recent developments, challenges, and the potential of machine Learning—A review. Sensors 2021, 21, 2877. [Google Scholar] [CrossRef] [PubMed]
- Mohmed, M.; Attia, N.; Elashery, S. Greener and facile synthesis of hybrid nanocomposite for ultrasensitive iron (II) detection using carbon sensor. Microporous Mesoporous Mater. 2021, 313, 110832. [Google Scholar] [CrossRef]
- Elashery, S.; Attia, N.; Oh, H. Design and fabrication of novel flexible sensor based on 2D Ni-MOF nanosheets as a preliminary step toward wearable sensor for onsite Ni (II) ions detection in biological and environmental samples. Anal. Chim. Acta 2022, 1197, 339518. [Google Scholar] [CrossRef] [PubMed]
- Rahman, T.; Masui, T.; Ichiki, T. Single-crystal gallium oxide-based biomolecular modified diode for nucleic acid sensing. Jpn. J. Appl. Phys. 2015, 54, 04DL08. [Google Scholar] [CrossRef]
- Das, M.; Chakraborty, T.; Lin, C.H.; Lin, R.; Kao, C.H. Screen-printed Ga2O3 thin film derived from liquid metal employed in highly sensitive pH and non-enzymatic glucose recognition. Mater. Chem. Phys. 2022, 278, 125652. [Google Scholar] [CrossRef]

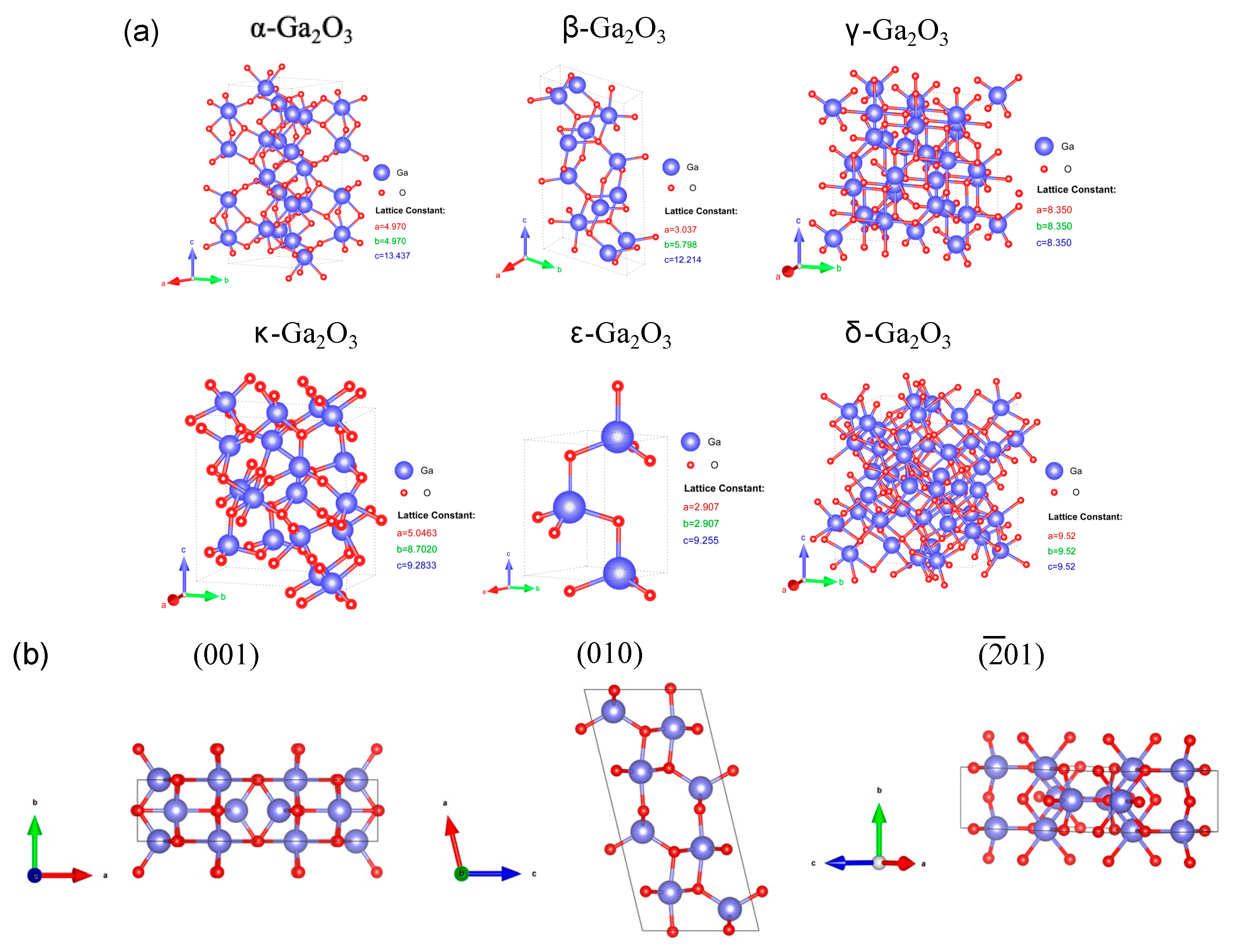

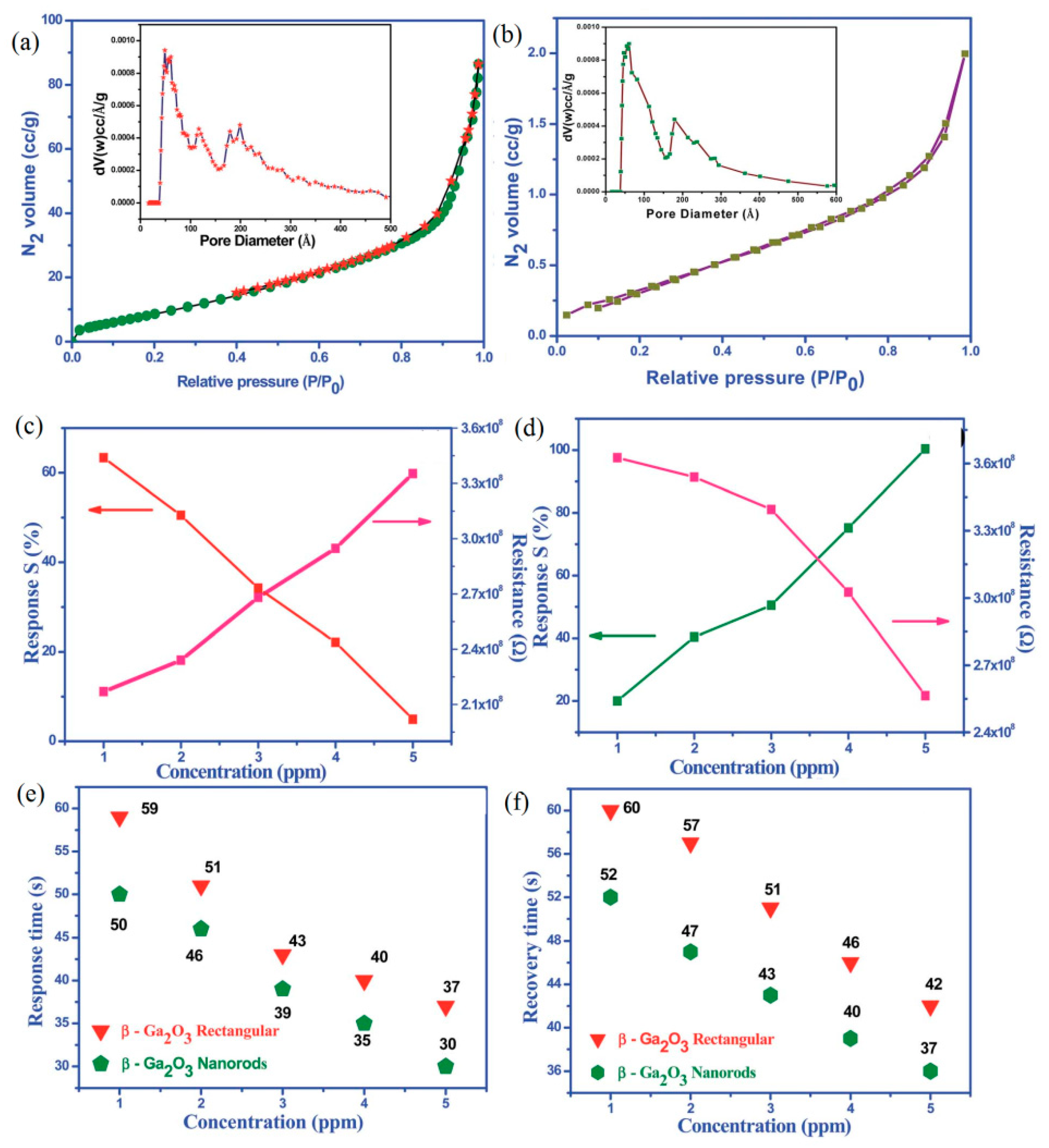

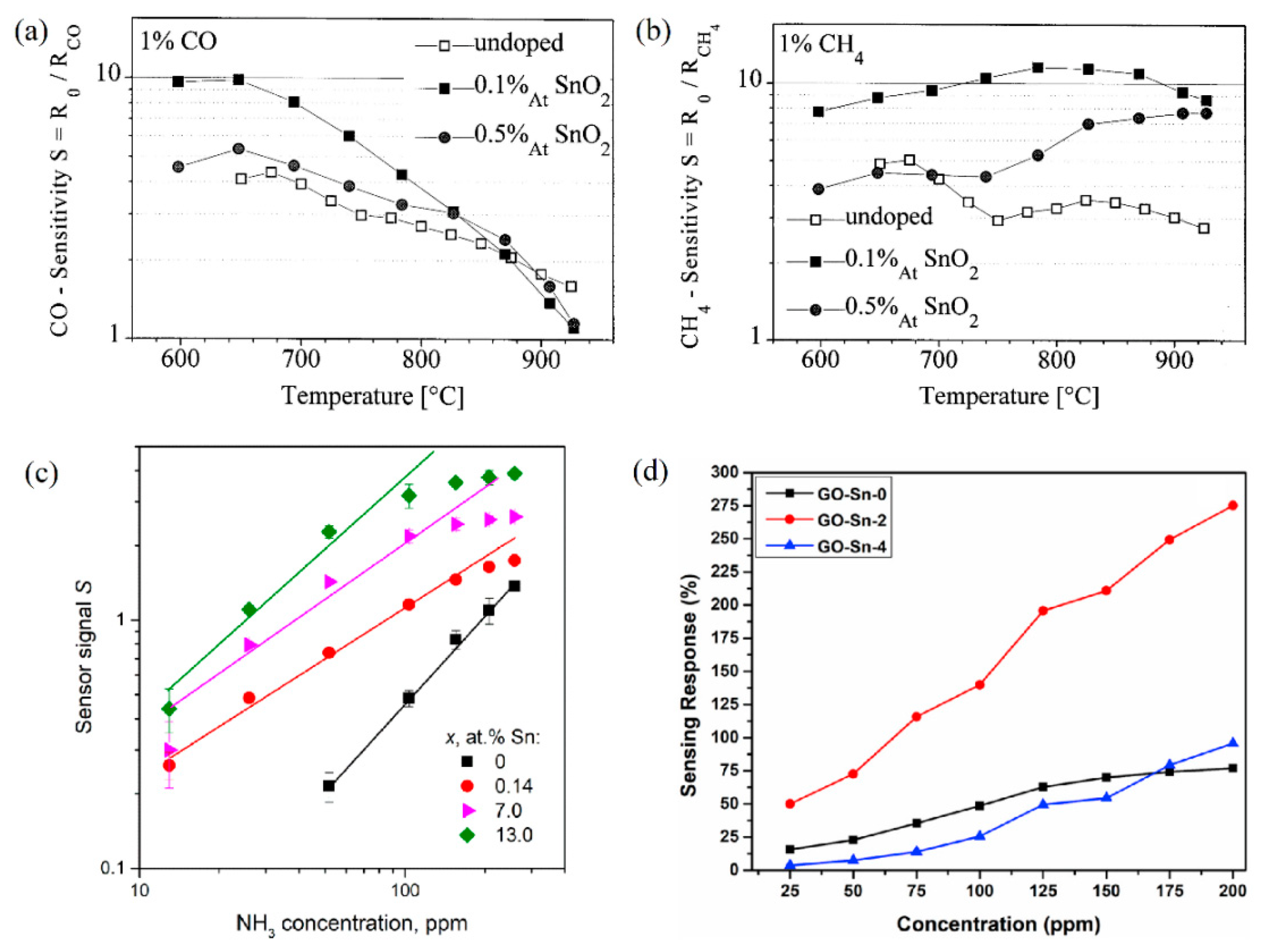
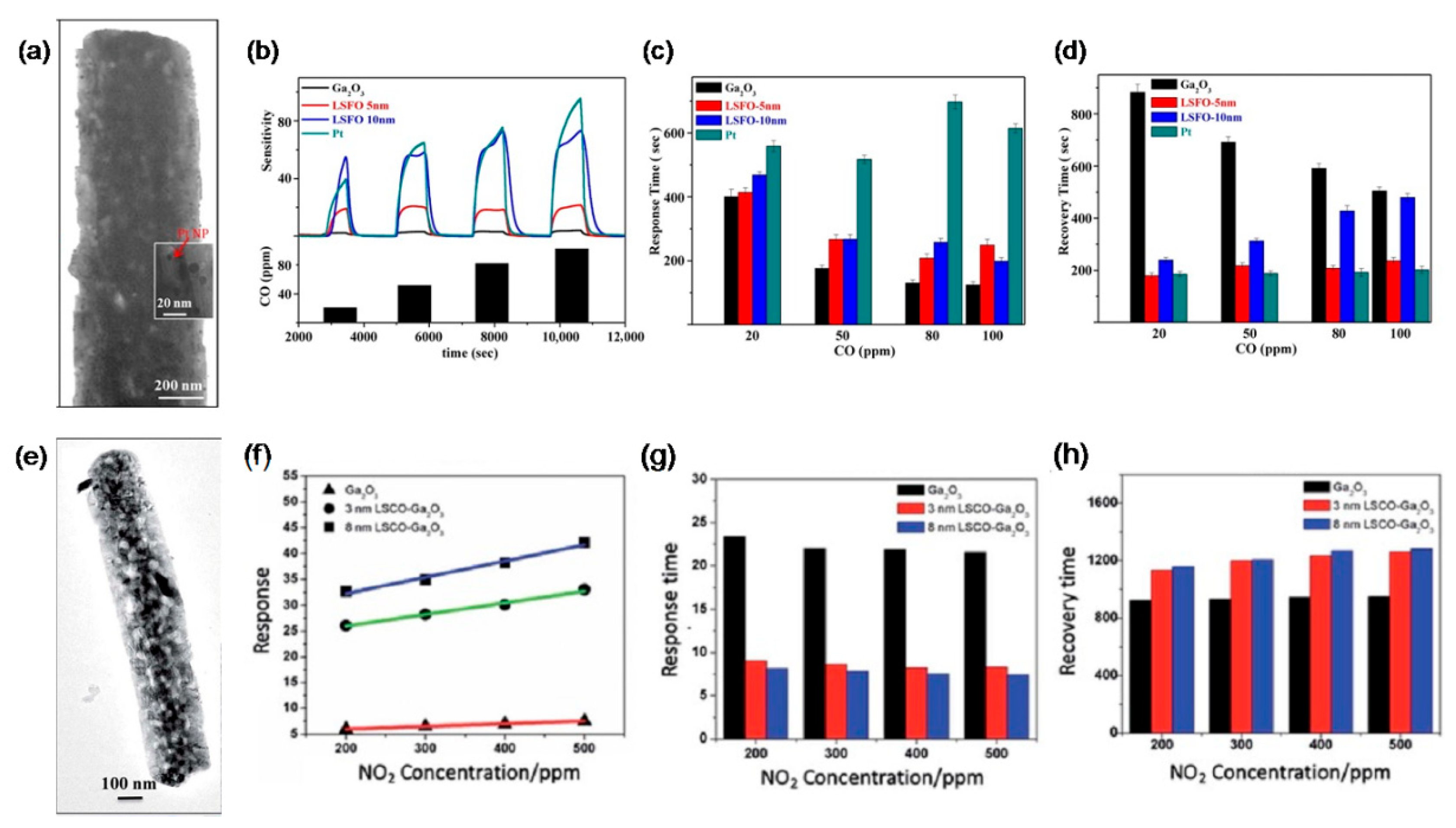

| Polymorph | System | Space Group | Lattice Parameters |
|---|---|---|---|
| α | hexagonal | a = b = 4.98–5.04 Å, c = 13.4–13.6 Å, α = β = 90°, γ = 120° | |
| β | monoclinic | C2/m | a = 12.12–12.34 Å, b = 3.03–3.04 Å, c = 5.80–5.87 Å, α = β = 90°, γ = 103.8° |
| γ | cubic | a = b = c = 8.24–8.30 Å, α = β = γ = 90° | |
| δ | cubic | Ia3 | a = b = c = 9.40–10.1 Å, α = β = γ = 90° |
| ε | hexagonal | P63mc | a = 5.06–5.12 Å, b = 8.69–8.79 Å, c = 9.3–9.4 Å, α = β = 90°, γ = 120° |
| κ | orthorhombic | Pna21 | a = 5.05 Å, b = 8.69 Å, c = 9.27 Å, α = β = γ = 90° |
| Electric Properties | Value |
|---|---|
| Electronic effective mass (m0) | 0.28 |
| Static dielectric constant | 10 |
| High frequency dielectric constant | 3.9 |
| Electron mobility (cm2·V−1·s−1) | 200 |
| Range of free electron concentration (cm−3) | 1016~1020 |
| Range of doping concentration (cm−3) | 1017~1020 |
| Electron affinity (eV) | 4.0 |
| Break down field (eV/cm) | 8.0 |
| Typical types of shallow donors | Sn, Ge, Si |
| Electrical Gas Sensors | Electrochemical Gas Sensor | Optical Gas Sensor | ||||
|---|---|---|---|---|---|---|
| Resistor | SBD | FET | Capacitor | |||
| Environmental gases | O2, CO2, O3, NH3, SO2 | O2, NH3 | NH3 | NH3 | ||
| Highly toxic gases | CO, H2S, NO, NO2 | CO, NO | CO, NO2 | CO | ||
| Combustible gases | H2, CH4, C4H10, C3H8, C4H8, C7H8 | H2, CH4, C3H6 | H2 | C7H8 | C3H6 | |
| VOCs | C2H6O, C3H6O, C3H8O | C2H6O, C3H6O, C3H8O | C2H6O, C3H6O, C3H8O | |||
| Humidity | H2O | |||||
| Other gases | C2H6S | CH3NO2, C6H15N | ||||
| Sensing Material | Preparation | Sensitivity @Gas Concentration | Operating Temperature | Response/Recover Time (s) | Other Observations | Reference |
|---|---|---|---|---|---|---|
| Ga-doped In2O3 nanowire | CVD | △1.05 @ 80 ppm C2H6O | 200 | 40/800 | [141] | |
| △2.2 @ 4 ppm NO2 | 200 | 1980/2780 | ||||
| Ga-doped In2O3 nanofiber | Hydrothermal synthesis | ◊52.5 @ 100 ppm CH2O | 150 | 1/70 | The low limit of detection is 0.2 ppm | [156] |
| Ga-doped In2O3 film | PLD | □2.15 @ 25 ppm NH3 | 623 | [164] | ||
| □20.5 @ 25 ppm C2H6O | 504 | |||||
| □24.4 @ 25 ppm C3H6O | 504 | |||||
| □7.47 @ 25 ppm CH4 | 500 | [167] | ||||
| Ga-doped In2O3 ceramics | Coprecipitation | △0.85 @ 0.5% CH4 | 380 | [222] | ||
| Ga-doped SnO2 film | Spray pyrolysis | ◊3.1 @ 1 Torr O2 | 350 | [223] | ||
| Ga-doped SnO2 nanocomposites | Coprecipitation | ◊315 @ 300 ppm CO | 300 | 36 | [224] | |
| Ga-doped SnO2 nanocomposites | ◊119 @ 300 ppm C2H6O | 250 | 93 | |||
| Ga-doped SnO2 microflowers | Hydrothermal synthesis | ◊95.8 @ 50 ppm CH2O | 230 | 3 | The low limit of detection is 0.1 ppm | [225] |
| Ga-doped ZnO film | Sol–gel synthesis | △0.5 @ 500 ppm H2 | 130 | 475 | [145] | |
| Ga-doped ZnO Nanorod | Hydrothermal synthesis | △1.01% @ 250 ppm C2H6O | RT | [226] | ||
| Ga-doped ZnO nanoparticle | Spray pyrolysis | △56 @ 2 ppm NO2 | 250 | [227] | ||
| △8 @ 1 ppm H2S | 250 | |||||
| Ga-doped ZnO nanorod | Sol–gel synthesis | △0.91 @ 100 ppm H2 | RT | 20 | [228] | |
| Ga-doped ZnO film | Magnetron sputtering | △2.4 @ 5 ppm H2S | 300 | [229] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Xu, Z.; Ha, S.; Li, D.; Zhang, K.; Zhang, H.; Feng, J. Gallium Oxide for Gas Sensor Applications: A Comprehensive Review. Materials 2022, 15, 7339. https://doi.org/10.3390/ma15207339
Zhu J, Xu Z, Ha S, Li D, Zhang K, Zhang H, Feng J. Gallium Oxide for Gas Sensor Applications: A Comprehensive Review. Materials. 2022; 15(20):7339. https://doi.org/10.3390/ma15207339
Chicago/Turabian StyleZhu, Jun, Zhihao Xu, Sihua Ha, Dongke Li, Kexiong Zhang, Hai Zhang, and Jijun Feng. 2022. "Gallium Oxide for Gas Sensor Applications: A Comprehensive Review" Materials 15, no. 20: 7339. https://doi.org/10.3390/ma15207339
APA StyleZhu, J., Xu, Z., Ha, S., Li, D., Zhang, K., Zhang, H., & Feng, J. (2022). Gallium Oxide for Gas Sensor Applications: A Comprehensive Review. Materials, 15(20), 7339. https://doi.org/10.3390/ma15207339







