Magnetically Actuated Shape Memory Polymers for On-Demand Drug Delivery
Abstract
1. Introduction
2. Materials and Methods
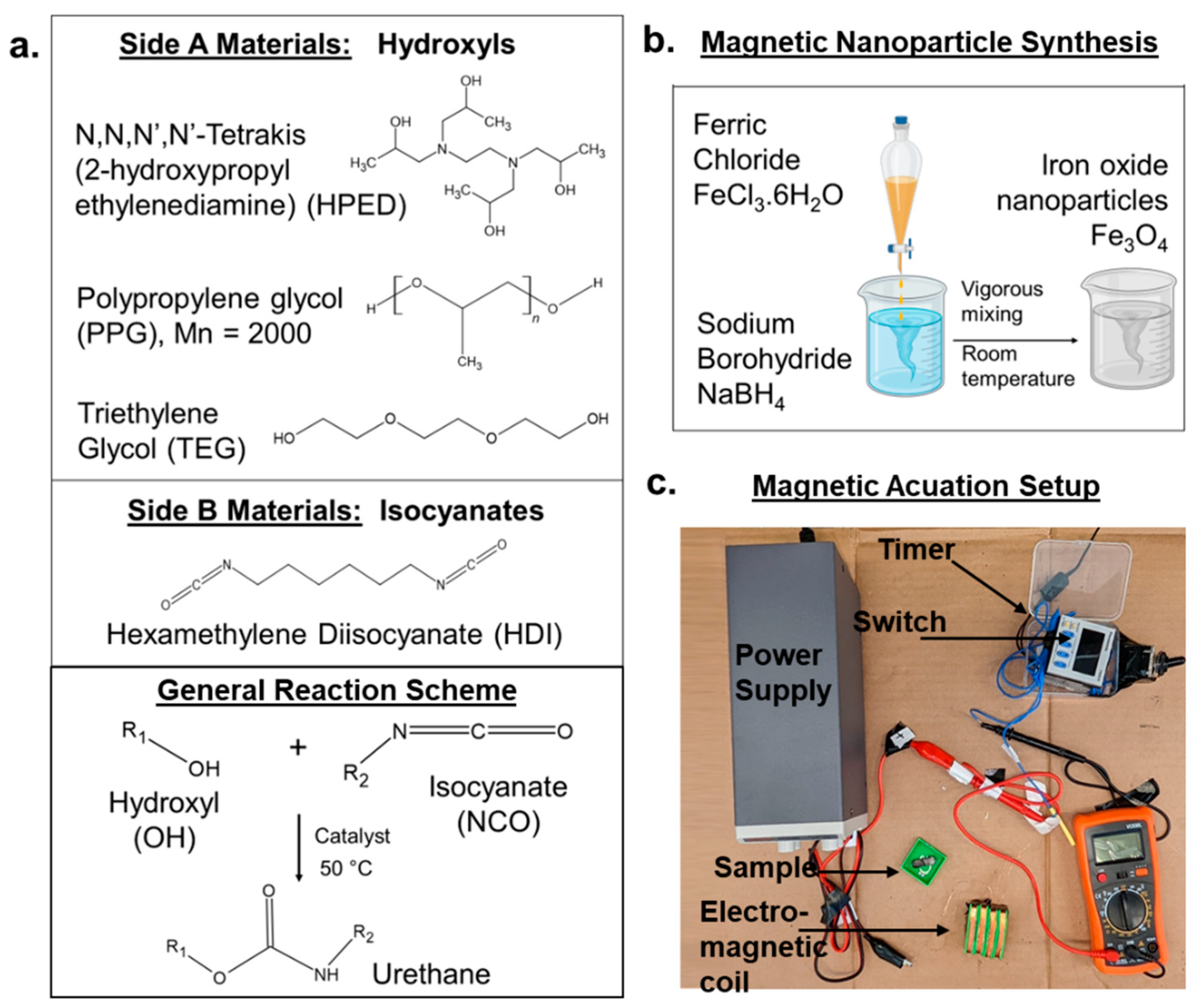
2.1. Materials
2.2. Synthesis
2.2.1. Magnetic Nanoparticles
2.2.2. Polymers
2.3. Hydrophobicity
2.4. Mechanical Properties
2.5. Thermal Characterization
2.6. Shape Memory Properties
2.7. Cytocompatibility
2.8. Drug Release
2.8.1. Magnetic Circuit
2.8.2. Drug Release Measurements
2.9. Statistics
3. Results and Discussion
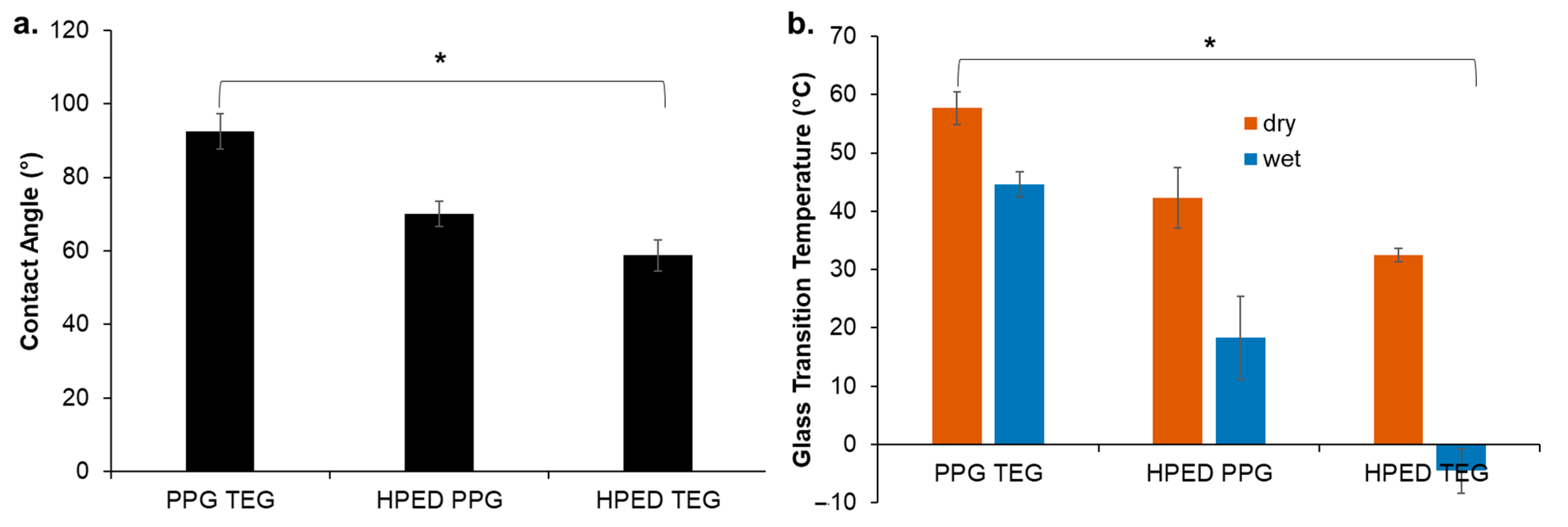
3.1. Hydrophobicity
3.2. Thermal Characterization
3.3. Mechanical Properties
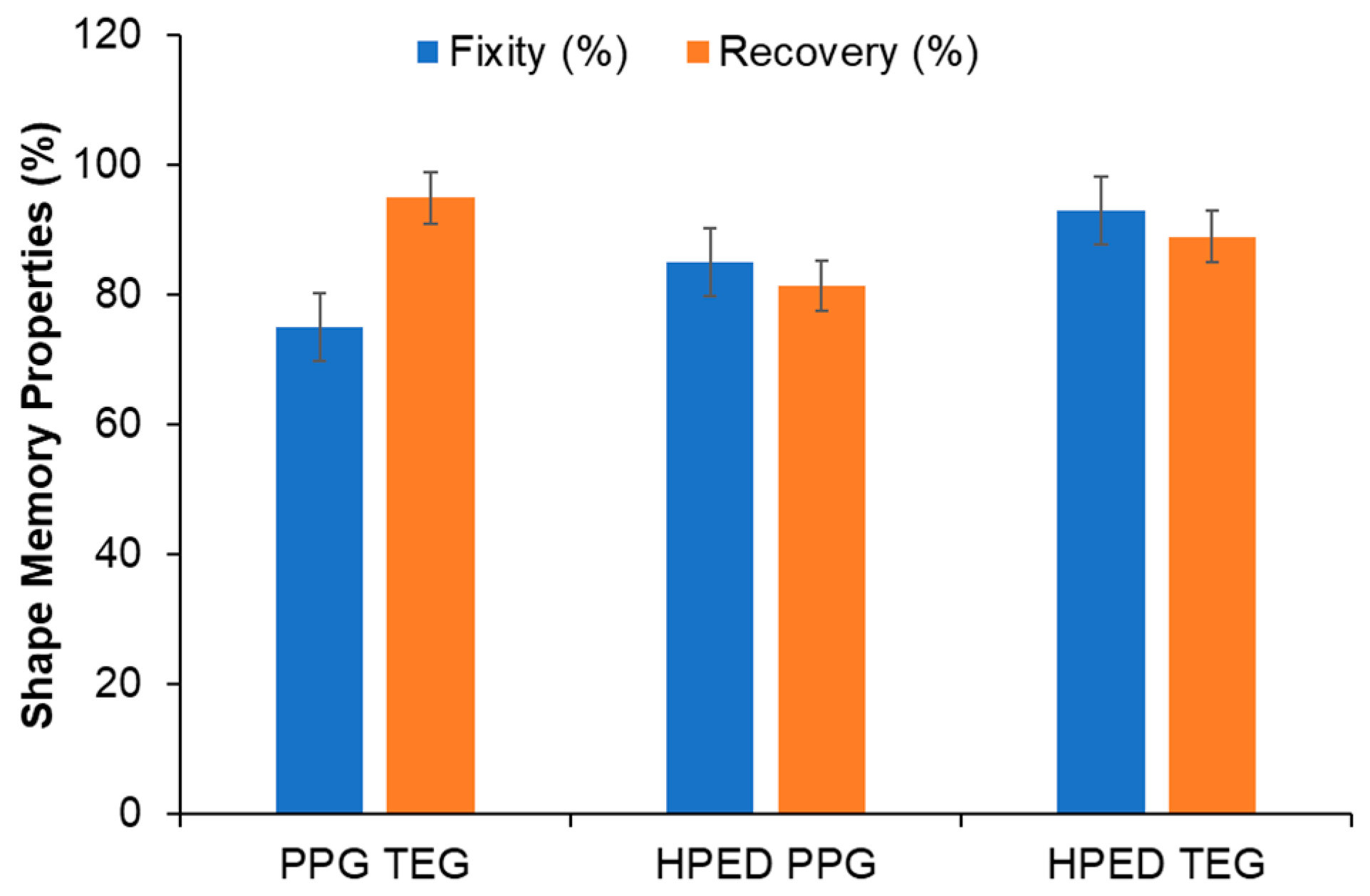
3.4. Shape Memory Properties
3.5. Cytocompatibility
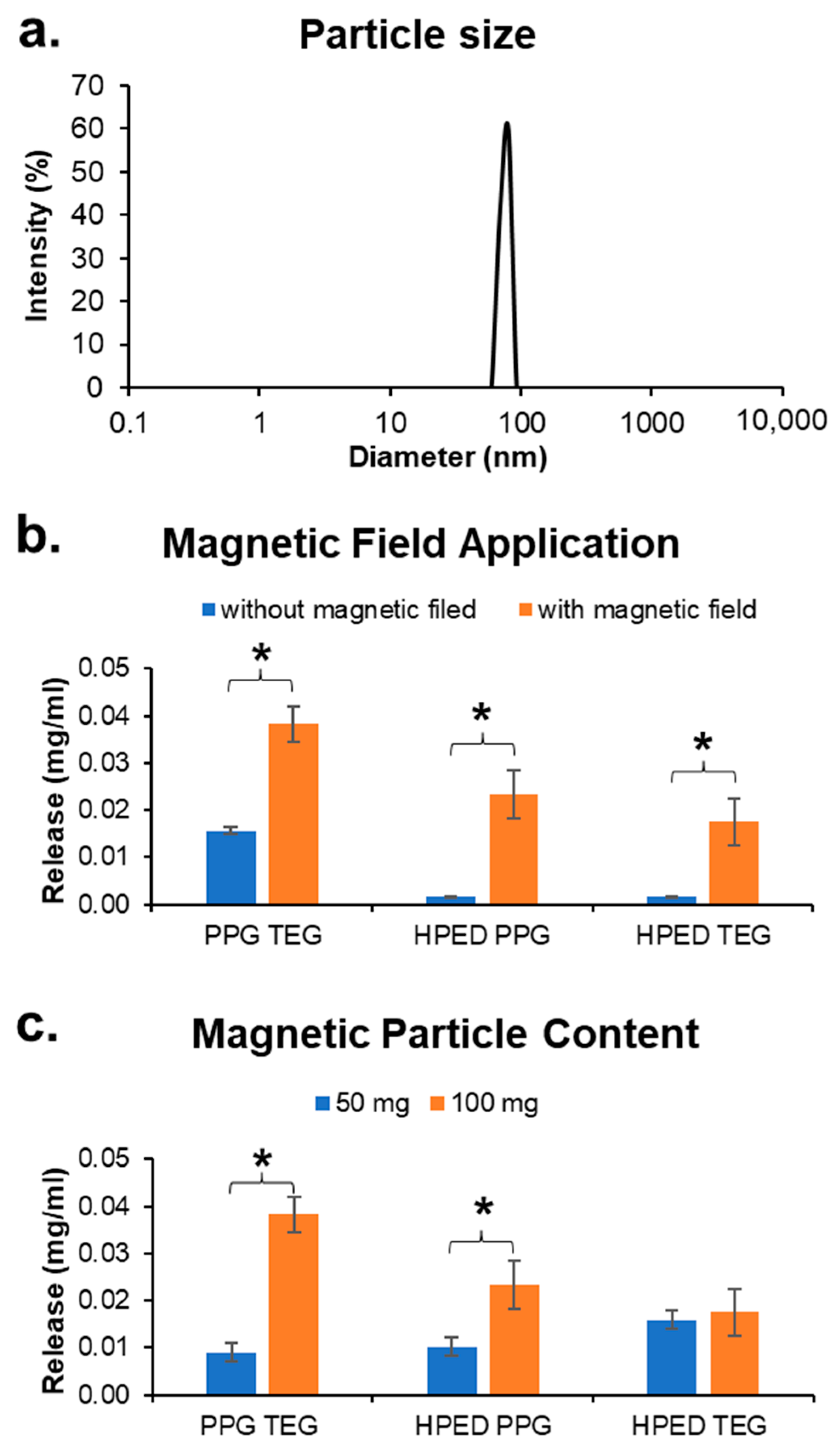
3.6. Magnetic Particles
3.7. Drug Release
3.7.1. Effects of Polymer Formulation
3.7.2. Effects of Applied Strain/Shape Fixing
3.7.3. Effects of Drug Hydrophobicity
3.7.4. Effect of a Magnetic Field
3.7.5. Effect of Mnp Concentration
3.8. Dual Drug Release
3.9. Design Principles for SMP-Based Drug Delivery Devices
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tewabe, A.; Abate, A.; Tamrie, M.; Seyfu, A.; Siraj, E.A. Targeted drug delivery—From magic bullet to nanomedicine: Principles, challenges, and future perspectives. J. Multidiscip. Healthc. 2021, 14, 1711–1724. [Google Scholar] [CrossRef] [PubMed]
- Tekade, R.K.; Maheshwari, R.; Soni, N.; Tekade, M.; Chougule, M.B. Nanotechnology for the Development of Nanomedicine. In Nanotechnology-Based Approaches for Targeting and Delivery of Drugs and Genes; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–61. [Google Scholar]
- Perrie, Y.; Rades, T. Controlling Drug Delivery. In Pharmaceutics—Drug Delivery and Targeting, 2nd ed.; Pharmaceutical Press: London, UK, 2012; pp. 1–24. [Google Scholar]
- Hillery, A.; Park, K. (Eds.) Drug Delivery: Fundamentals and Application, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Husch, M.; Sullivan, C.; Rooney, D.; Barnard, C.; Fotis, M.; Clarke, J.; Noskin, G. Insights from the sharp end of intravenous medication errors: Implications for infusion pump technology. Qual. Saf. Health Care 2005, 14, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, A.; Sharon, E.; Teena, J.; Nobil, S.; Nazeer, I. A clinical study on drug-related problems associated with intravenous drug administration. J. Basic Clin. Pharm. 2014, 5, 49–53. [Google Scholar] [CrossRef] [PubMed]
- King, B.R. Alternative Routes of Drug Administration. Emerg. Med. News 2005, 27, 23–24. [Google Scholar] [CrossRef]
- Yun, Y.H.; Lee, B.K.; Park, K. Controlled Drug Delivery: Historical perspective for the next generation. J. Control. Release 2015, 219, 2–7. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Mitragotri, S.; Langer, R. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug Discov. 2004, 3, 115–124. [Google Scholar] [CrossRef]
- Prausnitz, M.R. Microneedles for transdermal drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 581–587. [Google Scholar] [CrossRef]
- Bariya, S.H.; Gohel, M.C.; Mehta, T.A.; Sharma, O.P. Microneedles: An emerging transdermal drug delivery system. J. Pharm. Pharmacol. 2012, 64, 11–29. [Google Scholar] [CrossRef]
- Pflegel, P. Pulsatile Drug Delivery—Current Applications and Future Trends. Herausgegeben von Robert Gruny, Genf, Hans E. Junginger, Leiden, und Nikolaos A. Peppas, West Lafayette (Indiana). Stuttgart 1993, 186 Seiten, Zahlreiche Abbildungen und Tabellen. ISBN 3-804. Pharm. Unserer Zeit 1994, 23, 265–266. [Google Scholar]
- Chunder, A.; Sarkar, S.; Yu, Y.; Zhai, L. Fabrication of ultrathin polyelectrolyte fibers and their controlled release properties. Colloids Surf. B Biointerfaces 2007, 58, 172–179. [Google Scholar] [CrossRef]
- Carling, C.-J.; Viger, M.L.; Huu, V.A.N.; Garcia, A.V.; Almutairi, A. In vivo visible light-triggered drug release from an implanted depot. Chem. Sci. 2014, 6, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Sharifianjazi, F.; Irani, M.; Esmaeilkhanian, A.; Bazli, L.; Asl, M.S.; Jang, H.W.; Kim, S.Y.; Ramakrishna, S.; Shokouhimehr, M.; Varma, R.S. Polymer incorporated magnetic nanoparticles: Applications for magnetoresponsive targeted drug delivery. Mater. Sci. Eng. B 2021, 272, 115358. [Google Scholar] [CrossRef]
- Gholami, A.; Mousavi, S.M.; Hashemi, S.A.; Ghasemi, Y.; Chiang, W.-H.; Parvin, N. Current trends in chemical modifications of magnetic nanoparticles for targeted drug delivery in cancer chemotherapy. Drug Metab. Rev. 2020, 52, 205–224. [Google Scholar] [PubMed]
- Satarkar, N.S.; Hilt, J.Z. Magnetic hydrogel nanocomposites for remote controlled pulsatile drug release. J. Control. Release 2008, 130, 246–251. [Google Scholar] [CrossRef]
- Zhou, L.; He, B.; Zhang, F. Facile One-Pot Synthesis of Iron Oxide Nanoparticles Cross-linked Magnetic Poly(vinyl alcohol) Gel Beads for Drug Delivery. ACS Appl. Mater. Interfaces 2012, 4, 192–199. [Google Scholar] [CrossRef]
- Bardajee, G.R.; Hooshyar, Z. One-pot synthesis of biocompatible superparamagnetic iron oxide nanoparticles/hydrogel based on salep: Characterization and drug delivery. Carbohydr. Polym. 2014, 101, 741–751. [Google Scholar] [CrossRef]
- Zhao, Q.; Behl, M.; Lendlein, A. Shape-memory polymers with multiple transitions: Complex actively moving polymers. Soft Matter 2013, 9, 1744–1755. [Google Scholar] [CrossRef]
- Ze, Q.; Kuang, X.; Wu, S.; Wong, J.; Montgomery, S.M.; Zhang, R.; Kovitz, J.M.; Yang, F.; Qi, H.J.; Zhao, R. Magnetic Shape Memory Polymers with Integrated Multifunctional Shape Manipulation. Adv. Mater. 2020, 32, e1906657. [Google Scholar] [CrossRef]
- Sun, Y.; Chu, M.; Huang, M.; Hegazi, O.; Naguib, H.E. Hybrid Electroactive Shape Memory Polymer Composites with Room Temperature Deformability. Macromol. Mater. Eng. 2019, 304, 1900196. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Liu, D.; Wang, W.; Liu, Y.; Zhou, S. pH-Responsive Shape Memory Poly(ethylene glycol)–Poly(ε-caprolactone)-based Polyurethane/Cellulose Nanocrystals Nanocomposite. ACS Appl. Mater. Interfaces 2015, 7, 12988–12999. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Wang, S.; Yang, Y.; Qin, B.; Wang, K.; Xie, T.; Wei, Y.; Ji, Y. Polydopamine coated shape memory polymer: Enabling light triggered shape recovery, light controlled shape reprogramming and surface functionalization. Chem. Sci. 2016, 7, 4741–4747. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.C.; Carbajal, L.; Ameer, G.A. Novel Biodegradable Shape-Memory Elastomers with Drug-Releasing Capabilities. Adv. Mater. 2011, 23, 2211–2215. [Google Scholar] [CrossRef] [PubMed]
- Vakil, A.U.; Petryk, N.M.; Shepherd, E.; Monroe, M.B.B. Biostable Shape Memory Polymer Foams for Smart Biomaterial Applications. Polymers 2021, 13, 4084. [Google Scholar] [CrossRef]
- Ramezani, M.; Monroe, M.B.B. Biostable Segmented Thermoplastic Polyurethane Shape Memory Polymers for Smart Biomedical Applications. ACS Appl. Polym. Mater. 2022, 4, 1956–1965. [Google Scholar] [CrossRef]
- Chen, W.; Karde, V.; Cheng, T.N.H.; Ramli, S.S.; Heng, J.Y.Y. Surface hydrophobicity: Effect of alkyl chain length and network homogeneity. Front. Chem. Sci. Eng. 2020, 15, 90–98. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009; p. 34.
- Physical Constants of Organic Compounds. In CRC Handbook of Chemistry and Physics, Internet Version 2005; Lide, D.R., Ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Sivak, W.N.; Zhang, J.; Petoud, S.; Beckman, E.J. Simultaneous drug release at different rates from biodegradable polyurethane foams. Acta Biomater. 2009, 5, 2398–2408. [Google Scholar] [CrossRef]
- Chen, M.-C.; Ling, M.-H.; Wang, K.-W.; Lin, Z.-W.; Lai, B.-H.; Chen, D.-H. Near-Infrared Light-Responsive Composite Microneedles for On-Demand Transdermal Drug Delivery. Biomacromolecules 2015, 16, 1598–1607. [Google Scholar] [CrossRef]



| Sample | HDI | PPG | TEG | HPED | DBTDL | Fe3O4 Particles | Drug (5 mg/10 g) | Mixing Time (s) |
|---|---|---|---|---|---|---|---|---|
| PPG TEG | 29.4 | 50.8 | 19.8 | 0 | 0.8 | 50 mg | Dox 6-MP Rhod | 15 |
| 100 mg | Dox 6-MP Rhod | |||||||
| HPED PPG | 29.6 | 51.0 | 0 | 19.4 | 0.8 | 50 mg | Dox 6-MP Rhod | 30 |
| 100 mg | Dox 6-MP Rhod | |||||||
| HPED TEG | 55.8 | 0 | 22.4 | 21.8 | 0 | 50 mg | Dox 6-MP Rhod | 60 |
| 100 mg | Dox 6-MP Rhod | |||||||
| Dual-R Dual-L | 29.4 55.8 | 50.8 0 | 19.8 22.4 | 0 21.8 | 0.8 0 | 100 mg 50 mg | Rhod 6-MP | 15 60 |
| Sample | Elastic Modulus (kPa) | Elongation at Break (%) | Ultimate Tensile Strength (kPa) | |||
|---|---|---|---|---|---|---|
| Dry | Wet | Dry | Wet | Dry | Wet | |
| PPG TEG | 80 ± 35 | 4 ± 2 | 680 ± 210 | 1050 ± 450 | 8300 ± 3200 | 1770 ± 120 |
| HPED PPG | 46 ± 4 | 21 ± 2 | 85 ± 9 | 94 ± 14 | 2660 ± 330 | 1570 ± 70 |
| HPED TEG | 595 ± 75 | 25 ± 2 | 170 ± 50 | 40 ± 14 | 29,200 ± 4000 | 730 ± 340 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vakil, A.U.; Ramezani, M.; Monroe, M.B.B. Magnetically Actuated Shape Memory Polymers for On-Demand Drug Delivery. Materials 2022, 15, 7279. https://doi.org/10.3390/ma15207279
Vakil AU, Ramezani M, Monroe MBB. Magnetically Actuated Shape Memory Polymers for On-Demand Drug Delivery. Materials. 2022; 15(20):7279. https://doi.org/10.3390/ma15207279
Chicago/Turabian StyleVakil, Anand Utpal, Maryam Ramezani, and Mary Beth B. Monroe. 2022. "Magnetically Actuated Shape Memory Polymers for On-Demand Drug Delivery" Materials 15, no. 20: 7279. https://doi.org/10.3390/ma15207279
APA StyleVakil, A. U., Ramezani, M., & Monroe, M. B. B. (2022). Magnetically Actuated Shape Memory Polymers for On-Demand Drug Delivery. Materials, 15(20), 7279. https://doi.org/10.3390/ma15207279







