Study Progress on Inorganic Fibers from Industry Solid Wastes and the Key Factors Determining Their Characteristics
Abstract
1. Introduction
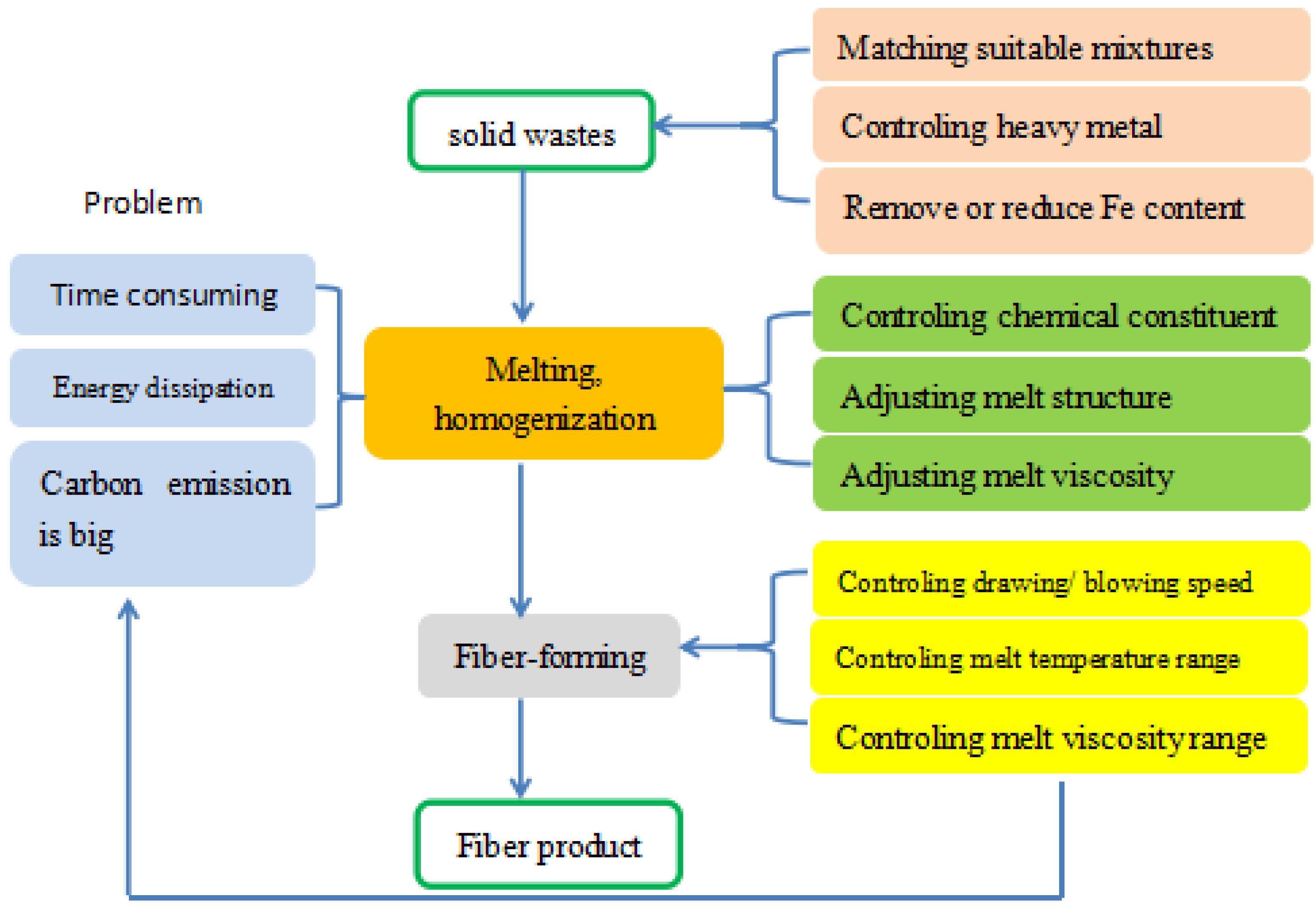
2. The Production Process of Inorganic Fibers from Solid Wastes
2.1. Selection of Solid Wastes for the Preparation of the Inorganic Fibers
2.2. Melting–Homogenization
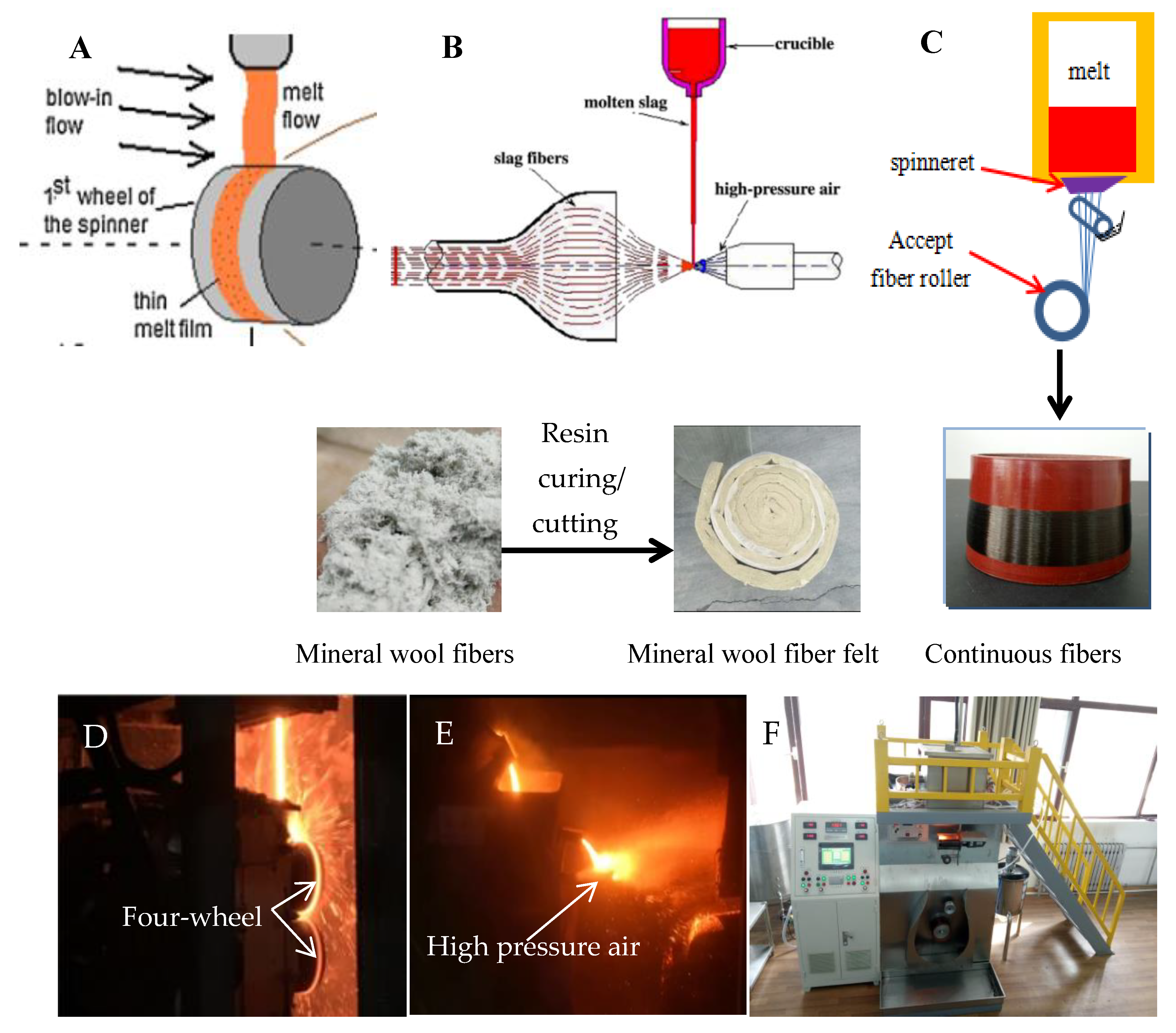
2.3. Fiber-Forming Process
3. The Key Factors Determining the Performances of the Fibers
3.1. Composition of Vitreous Melt
3.2. Chemical Composition of Fibers
3.2.1. Influence of Chemical Composition on the Properties of BCFs
3.2.2. Difference in Chemical Composition between Mineral Fibers and Continuous Fibers Produced from Solid Wastes
3.3. Fiber and Melt Structure
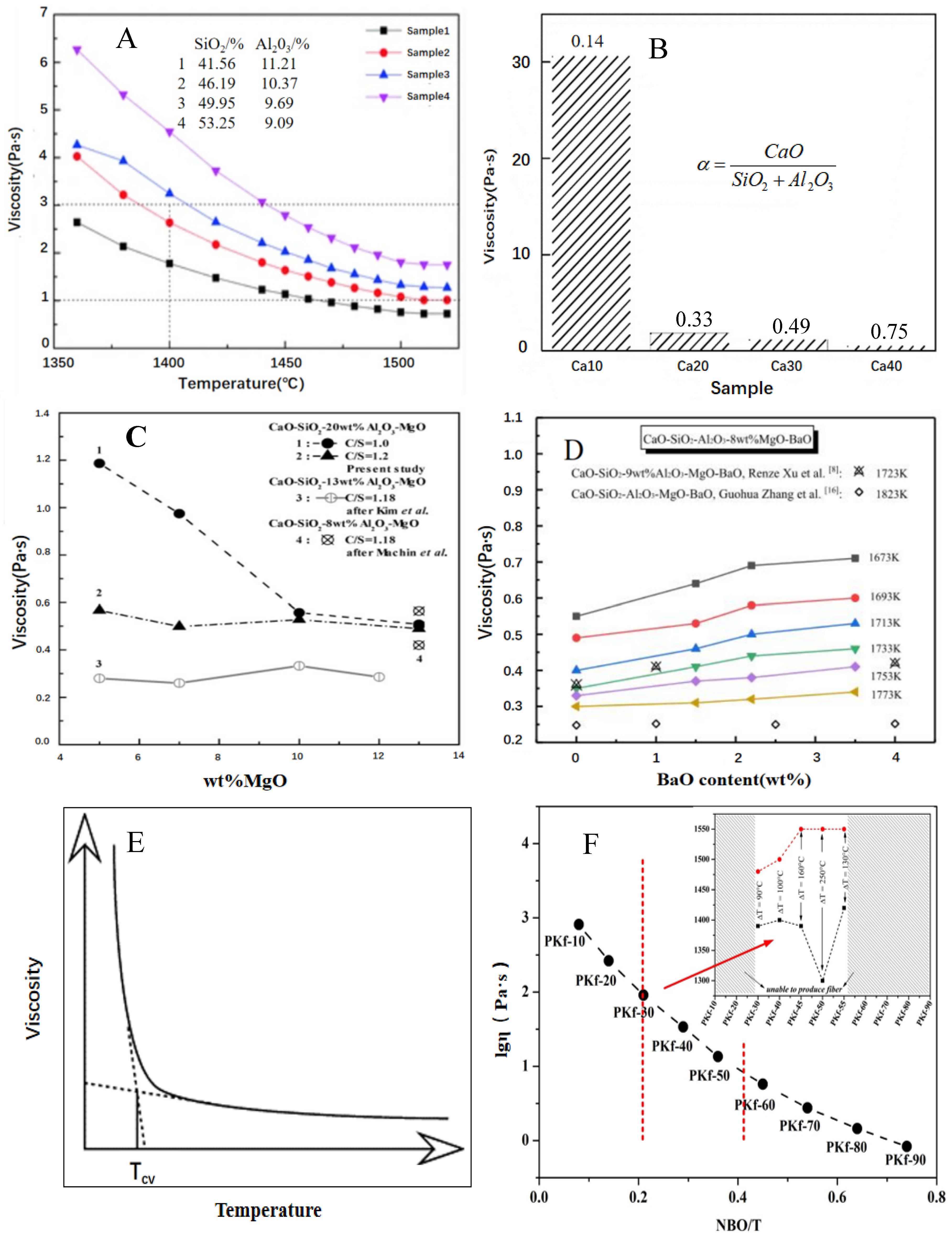
3.4. Viscosity of Melts
4. The Relationship between Winding Speed, Diameter, Mk, and Tensile Strength of the Fibers Produced from Solid Wastes
5. Conclusions and Outlook
- (1)
- For fiber production, matching of solid wastes containing enough total content of SiO2 and Al2O3, and a suitable amount of MgO and CaO was beneficial to the structure control of the melt.
- (2)
- The study found that the melt consisted of Q2 and Q3, and Q3 content more than Q2, and was more suitable for the production of fibers and production performance improvement. Thus, melt structure can be obtained by controlling the degree of depolymerization and suitable temperature range.
- (3)
- Further study showed that the viscosity of the melt could be effectively controlled by regulating its chemical composition, especially the content of the network formers.
- (4)
- The optimum technology parameters for fiber production also were found. The new ultrasonic technology could rapidly shorten the homogenization time, save energy costs and reduce carbon emissions. The practical application of these findings in production will promote the development of the solid waste fiber industry while reducing energy consumption and carbon emissions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, H.-D.; Feng, X.-Y.; Liu, J.-X.; Yang, Z.-M.; He, C.; Li, S.-K. An investigation on anti-impact and penetration performance of basalt fiber composites with different weave and lay-up modes. Def. Technol. 2019, 16, 787–801. [Google Scholar] [CrossRef]
- Supian, A.; Sapuan, S.; Zuhri, M.; Zainudin, E.; Ya, H. Crashworthiness performance of hybrid kenaf/glass fiber reinforced epoxy tube on winding orientation effect under quasi-static compression load. Def. Technol. 2020, 16, 1051–1061. [Google Scholar] [CrossRef]
- Gorlov, Y.; Ustenko, A.; Sardarov, B.; Kharitonova, L. Materrial based on heat resistant fiber made from powder station fly ash. Glass Ceram. 1979, 36, 539–542. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Cheng, F. Process on the study of solid waste based inorganic fibers. Mater. Rep. 2021, 35, 07019–07026. [Google Scholar]
- Peng, S.; Liu, Q.; Dai, H.; Wang, G. New building materials with fire-prevention and energy-saving made from fly ash. Coal. Process. Compr. Util. 1999, 1, 49–52. [Google Scholar]
- Zhao, D.; Zhang, Z.; Tang, X.; Liu, L.; Wang, X. Preparation of Slag Wool by Integrated Waste-Heat Recovery and Resource Recycling of Molten Blast Furnace Slags: From Fundamental to Industrial Application. Energies 2014, 7, 3121–3135. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, F.; Wen, X.; Cheng, F. Melting process and melt structure of fly ash/ magnesium slag blends for the production of inorganic fibers. Ceram. Int. 2021, 47, 21013–21023. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, X.; Cheng, F. Preparation, thermal stability and mechanical properties of inorganic continuous fibers produced from fly ash and magnesium slag. Waste Manag. 2020, 120, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Tian, X.; Liao, H.; Guo, Y.; Cheng, F. Improvement of fly ash fusion characteristics by adding metallurgical slag at high temperature for production of continuous fiber. J. Clean. Prod. 2018, 171, 464–481. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, M.; Sohn, J.; Park, H. Applicability of gold tailings, waste limestone, red mud, and ferronickel slag for producing glass fibers. J. Clean. Prod. 2018, 203, 957–965. [Google Scholar] [CrossRef]
- Wu, Z.S.; Wang, X.; Liu, J.X.; Chen, X.F. Mineral Fibres: Basalt. In Handbook of Natrural Fibres, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Kim, M.; Ko, H.; Kwon, T.; Bae, H.C.; Jang, C.H.; Heo, B.U.; Park, S.M. Development of novel refractory cermic continuous fibers of fly ash and comparision of mechanical properties with those of E-glass fibers using the Weibull disribution. Ceram. Int. 2020, 46, 13255–13262. [Google Scholar] [CrossRef]
- Ko, H.; Kim, M.; Park, S.-M.; Lim, H.M. Correlation between viscoelasticity of aluminosilicate melts and elastic properties of melt-spun fibers. J. Non-Cryst. Solids 2021, 572, 121110. [Google Scholar] [CrossRef]
- Bloise, A.; Barca, D.; Gualtieri, A.F.; Pollastri, S.; Belluso, E. Trace elements in hazardous mineral fibres. Environ. Pollut. 2016, 216, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Yang, L.; Zhu, O.; Yu, J.; Jia, X.; Dong, T.; Lu, R. Multivariate Analysis of Trace Elements Distribution in Hair of Pleural Plaques Patients and Health Group in a Rural Area from China. Hair Ther. Transplant. 2014, 4, 1–7. [Google Scholar] [CrossRef]
- Bargagli, E.; Monaci, F.; Bianchi, N.; Bucci, C.; Rottoli, P. Analysis of Trace Elements in Bronchoalveolar Lavage of Patients with Diffuse Lung Diseases. Biol. Trace Elem. Res. 2008, 124, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-M.; Hou, K.-H.; Chang, Y.T.; Lee, W.-C.; Ger, M.-D. The preparation of slag fiber and its application in heat resistant friction composites. Mater. Des. 2010, 31, 4296–4301. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.L.; Zhao, G.Z.; Cang, D.Q. Pilot Trial of Direct Modification of Molten Blast Furnance Slag and Production of High Acidity Coefficient Slag Wool Fibers. In TMS Annual Meeting & Exhibition; The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2018; pp. 113–120. [Google Scholar]
- Zhao, G.Z.; Zhang, L.L.; Cang, D.Q. Pilot trial of detoxification of chromium slag in cyclone furnace and production of slag wool fibers. J. Hazard. Mater. 2018, 358, 122–128. [Google Scholar] [CrossRef]
- Wang, W.; Dai, S.; Zhou, L.; Zhang, T.; Tian, W.; Xu, J. Effect of B2O3 on the properties of ferronickel melt and mineral wool. Ceram. Int. 2020, 46, 13460–13465. [Google Scholar] [CrossRef]
- Wang, X.D.; Zhao, D.; Lu, K.; Wang, C. A Method of Preparing Continuous Basalt Fiber from Industrial Solid Waste. China Patent ZL201410498584X, 25 September 2014. [Google Scholar]
- Chan, W.H.; Mazlee, M.N.; Ahmad, Z.A.A.; Ishak, M.A.M.; Shamsul, J.V. Effects of fly ash addition on physical properties of porous clay-fly ash composites via polymeric replica technique. J. Mater. Cycles. Waste Manag. 2017, 17, 794–803. [Google Scholar] [CrossRef]
- Liu, X.L. Investigation on the Heat-Insulation Conveying and Component Modulating for Molten Blast Furnace Slag in its Direct Wool Producing Technology. Master’s Dissertation, Anhui University of Technology, Maanshan, China, 2013; pp. 30–42. [Google Scholar]
- Vargas, S.; Frandsen, F.J.; Dam-Johansen, K. Rheological properties of high-temperature melts of coal ashes and other silicates. Prog. Energ. Combust. 2001, 27, 237–429. [Google Scholar] [CrossRef]
- Hsieh, P.Y.; Kwong, K.-S.; Bennett, J. Correlation between the critical viscosity and ash fusion temperatures of coal gasifier ashes. Fuel Process. Technol. 2016, 142, 13–26. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J. Facile preparation of slag or fly ash geopolymer composite coatings with flame resistance. Constr. Build. Mater. 2019, 203, 655–661. [Google Scholar] [CrossRef]
- Sheldon, G.L. Forming fibres from basalt rock. Platin. Met. Rev. 1977, 21, 18–24. [Google Scholar]
- Gutnikov, S.I.; Malakho, A.P.; Lazoryak, B.I.; Loginov, V. Influence of alumina on the properties of continuous basalt fibers. Russ. J. Inorg. Chem. 2009, 54, 191–196. [Google Scholar] [CrossRef]
- Bauer, F.; Kempf, M.; Weiland, F.; Middendorf, P. Structure-property relationships of basalt fibers for high performance applications. Compos. Part B Eng. 2018, 145, 121–128. [Google Scholar] [CrossRef]
- Deák, T.; Czigány, T. Chemical Composition and Mechanical Properties of Basalt and Glass Fibers: A Comparison. Text. Res. J. 2009, 79, 645–651. [Google Scholar] [CrossRef]
- Liu, J.; Yang, J.; Chen, M.; Lei, L.; Wu, Z. Effect of SiO2, Al2O3 on heat resistance of basalt fiber. Thermochim. Acta 2017, 660, 56–60. [Google Scholar] [CrossRef]
- Xing, D.; Xi, X.-Y.; Ma, P.-C. Factors governing the tensile strength of basalt fibre. Compos. Part A Appl. Sci. Manuf. 2019, 119, 127–133. [Google Scholar] [CrossRef]
- Yilmaz, S.; Özkan, O.T.; Günay, V. Crystallization kinetics of basalt glass. Ceram. Int. 1996, 22, 477–481. [Google Scholar] [CrossRef]
- Karamanov, A.; Pisciella, P.; Pelino, M. The crystallisation kinetics of iron rich glass in different atmospheres. J. Eur. Ceram. Soc. 2000, 20, 2233–2237. [Google Scholar] [CrossRef]
- Manylov, M.S.; Gutnikov, S.I.; Lipatov, Y.V.; Malakho, A.P.; Lazorya, B.I. Effect of deferrization on continuous basalt fiber properties. Mendeleev Commun. 2015, 25, 386–388. [Google Scholar] [CrossRef]
- Liu, C.; Tong, X.; Liu, Z.; Guo, L.; Yang, C.; Li, H.; Jiang, L. Preparation of continuous silicate fiber from pyroxene and K-feldspar mixture. J. Non-Cryst. Solids 2021, 575, 121173. [Google Scholar] [CrossRef]
- Moiseev, E.A.; Gutnikov, S.I.; Malakho, A.P.; Lazoryak, B.I. Effect of iron oxides on the fabrication and properties of continuous glass fibers. Inorg. Mater. 2008, 44, 1026–1030. [Google Scholar] [CrossRef]
- Sørensen, P.; Pind, M.; Yue, Y.; Rawlings, R.; Boccaccini, A.; Nielsen, E. Effect of the redox state and concentration of iron on the crystallization behavior of iron-rich aluminosilicate glasses. J. Non-Cryst. Solids 2005, 351, 1246–1253. [Google Scholar] [CrossRef]
- Raskina, É.M.; Zemtsov, A.N. Gabbro-Basaltic Raw Material for the Production of Basalt Fiber. Consruction Materials Industry. VNIIESM Perm 2003, 1–2. [Google Scholar]
- Zemtsov, A.N.; Ogarysheva, S.I. Basalt Wool: History and Present. IIET RAN Perm 2003. Available online: https://www.c-o-k.ru/articles/bazal-tovye-tehnologii-istoriya-i-perspektivy (accessed on 26 September 2022). (In Russian).
- Kochergin, A.V.; Granovskaya, N.V.; Kochergin, D.V.; Savchenko, V.A.; Galimov, N.R. Ways to supply gabbro-basalt raw materials to mineral fiber producers. Glass Ceram. 2013, 69, 11–12. [Google Scholar] [CrossRef]
- Liu, Z.J.; Tu, Z.Y. Experiment of using manganese ferroalloy slag to produce mineral wool. Chinas Manganese Ind. 2014, 32, 40–41. [Google Scholar]
- Zhao, D.W.; Zhang, Z.T.; Liu, L.; Wang, X.D. Investigation on slag fiber characteristics: Mechanical property and anti-corrosion performance. Ceram. Int. 2015, 41, 5677–5687. [Google Scholar] [CrossRef]
- Zhu, H.G. Study on Producing Fiber with gangue and copper slag. Non-Ferr. Min. Metall. 2016, 32, 53–55. [Google Scholar]
- Ma, Q.; Ding, L.; Wang, Q.; Yu, Y.; Luo, L.; Li, H. Preparation and characterization of continuous fly ash derived glass fibers with improved tensile trength. Mater. Lett. 2018, 231, 119–121. [Google Scholar] [CrossRef]
- Bottinga, Y.; Weill, D.F. The viscosity of magmatic silicate liquids; a model calculation. Am. J. Sci. 1972, 272, 438–475. [Google Scholar] [CrossRef]
- Goto, A.; Oshima, H.; Nishida, Y. Empirical method of calculating the viscosity of peraluminous silicate melts at high temperatures. J. Volcanol. Geotherm. Res. 1997, 76, 319–327. [Google Scholar] [CrossRef]
- Nowok, J.W.; Hurley, J.P.; Stanley, D.C. Local structure of a lignitic coal ash slag and its effect on viscosity. Energy Fuels 1993, 7, 1135–1140. [Google Scholar] [CrossRef]
- Sato, R.K.; McMillan, P.F.; Dennison, P.; Dupree, R.A. Structral Investigation of High Alumina Glasses in the CaO-Al2O3-SiO2 System via Raman and Magic Angle Spinning Nulcear Magnetic Resonance Spectroscopy. Phys. Chem. Glasses 1991, 32, 149–156. [Google Scholar]
- Virgo, D.; Mysen, B.O. The structural state of iron in oxidized vs. reduced glasses at 1 atom: A 57Fe Mössbauer study. Phys. Chem. Miner. 1985, 2, 65–76. [Google Scholar] [CrossRef]
- Burkhard, D.J. Iron-bearing silicate glasses at ambient conditions. J. Non-Cryst. Solids 2000, 275, 175–188. [Google Scholar] [CrossRef]
- Polyakov, V.B.; Ariskin, A.A.; Shildt, A.V. Analysis of Disproportionation of Qn Structons in the Simulation of the Structure of Melts in the Na2O-SiO2 System. Glass. Phys. Chem. 2010, 36, 579–588. [Google Scholar] [CrossRef]
- Min, Y.; Luo, J.; Liu, C.J. Viscosity and related structure transformation of fluorine bearing silicate melt under ultrasonic field. Ultrason. Sonochem. 2019, 55, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.L.; Sun, S.B. New Glass Technology, 1st ed.; China Light Industry Press: Beijing, China, 2016. [Google Scholar]
- Mysen, B.; Richet, P. Silicate Glass and Melts, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 978-0-444-63708-6. [Google Scholar]
- He, C.; Bai, J.; Ilyushechkin, A.; Zhao, H.; Kong, L.X.; Li, H.Z.; Bai, Z.Q.; Guo, Z.X.; Li, W. Effect of chemical composition on the fusion behavior of synthetic high-iron coal ash. Fuel 2019, 253, 1465–1472. [Google Scholar] [CrossRef]
- Kim, H.; Kim, W.H.; Sohn, I.; Min, D.J. The effect of MgO on the viscosity of the CaO-SiO2-20 wt% Al2O3-MgO slag system. Steel. Res. Int. 2010, 81, 261–264. [Google Scholar] [CrossRef]
- Zhang, G.-H.; Chou, K.-C.; Mills, K. A Structurally Based Viscosity Model for Oxide Melts. Met. Mater. Trans. A 2013, 45, 698–706. [Google Scholar] [CrossRef]
- Xing, X.D.; Pang, Z.G.; Mo, C.; Wang, S.; Ju, J.T. Effect of MgO and BaO on viscosity and structure of blast furnace slag. J. Non-Cryst. Solids. 2020, 530, 119801. [Google Scholar] [CrossRef]
- He, C.; Ilyushechkin, A.; Bai, J.; Hla, S.S.; Kong, L.X.; Li, W. Viscosity and crystallization behavior of coal ash slag from the primary phase of anorthite. Fuel. Process. Technol. 2021, 213, 106680. [Google Scholar] [CrossRef]
- He, C.; Bai, J.; Kong, L.; Xu, J.; Guhl, S.; Li, X.; Ge, Z.; Cao, X.; Bai, Z.; Li, W. Effects of atmosphere on the oxidation state of iron and viscosity behavior of coal ash slag. Fuel 2019, 243, 41–51. [Google Scholar] [CrossRef]
- He, C.; Bai, J.; Li, W.; Kong, L.; Xu, J.; Guhl, S.; Li, X.; Bai, Z.; Li, W. Iron transformation behavior in coal ash slag in the entrained flow gasifier and the application for Yanzhou coal. Fuel 2018, 237, 851–859. [Google Scholar] [CrossRef]
- Karamanov, A.; Ergul, S.; Akyildiz, M.; Pelino, M. Sinter-crystallization of a glass obtained from basaltic tuffs. J. Non-Cryst. Solids 2008, 354, 290–295. [Google Scholar] [CrossRef]
- Nowok, J.W. Viscosity and Phase Transformation in Coal Ash Slags near and below the Temperature of Critical Viscosity. Energy Fuels 1994, 8, 1324–1336. [Google Scholar] [CrossRef]
- Hurley, J.P.; Watne, T.M.; Nowok, J.W. The effects of atmosphere and additives on coal slag viscosity. ACS Div. Fuel. Chem. 1996, 41, 691–694. [Google Scholar]
- Ilyushechkin, A.Y.; Roberts, D. Slagging behaviour of Australian brown coals and implications for their use in gasification technologies. Fuel Process. Technol. 2016, 147, 47–56. [Google Scholar] [CrossRef]
- Kondratiev, A.; Ilyushechkin, A. Flow behaviour of crystallising coal ash slags:Shear viscosity, non-Newtonian flow and temperature of critical viscosity. Fuel 2018, 224, 783–800. [Google Scholar] [CrossRef]
- Yliniemi, J.; Ramaswamy, R.; Luukkonen, T.; Laitinen, O.; de Sousa, N.; Huuhtanen, M.; Illikainen, M. Characterization of mineral wool waste chemical composition, organic resin content and fiber dimensions: Aspects for valorization. Waste Manag. 2021, 131, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Tatarintseva, O.S.; Khodakova, N.N. Effect of production conditions of basalt glasses on their physicochemical properties and drawing temperature range of continuous fibers. Glas. Phys. Chem. 2012, 38, 89–95. [Google Scholar] [CrossRef]
- Wallenberger, F.T.; Bingham, P.A. Fiberglass and Glass Technology, Energy-Friendly Compositions and Applications; Springer: Cham, Switzerland, 2010; ISBN 978-1-4419-0735-6. [Google Scholar]
- Park, H.-J.; Park, S.-M.; Lee, J.-W.; Roh, G.-C.; Kim, J.-K. Studies on the Melting Characterization of Basalt and its Continuous Fiber Spinning. J. Korean Soc. Compos. Mater. 2010, 23, 43–49. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, M.; Lim, T.Y.; Lee, Y.; Jeon, D.W.; Hyun, S.K.; Kim, J.H. Physical properties of AR-glass fibers in continuous fiber spinning conditions. Kor. J. Met. Mater. 2017, 55, 290–295. [Google Scholar]
- Jamshaid, H.; Mishara, R. A green material from rock: Basalt fiber—A review. J. Text. Inst. 2016, 107, 923–937. [Google Scholar] [CrossRef]



| Fiber Raw | SiO2 | Al2O3 | CaO | MgO | K2O | Na2O | FexOy | TiO2 | B2O3 | Method | Tff | Diameter* | Strength | Mk | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wt% | °C | μm | MPa | ||||||||||||
| Fa + Bo | 35.60 | 11.90 | 24.61 | 1.84 | 0.73 | 4.27 | 9.73 | -- | -- | Blowing | 1600 | 0.5–5.5 | -- | 1.80 | [17] |
| BQFB | 35.39 | 15.74 | 34.93 | 7.44 | -- | -- | 1.77 | -- | -- | Centrifugal | 1430 | 4.5 | -- | 1.21 | [23] |
| MSS | 32.74 | 10.0 | 24.86 | 3.80 | -- | -- | -- | -- | -- | Centrifugal | 1350 | 5.1 | -- | 1.49 | [42] |
| BFS | 36.10 | 26.70 | 25.30 | 6.20 | 0.60 | 0.40 | 1.50 | 1.40 | -- | Centrifugal | -- | 7.0 | 2579 | 1.99 | [43] |
| CSCD | 54.32 | 13.58 | 14.01 | 8.13 | -- | -- | 6.05 | -- | -- | Centrifugal | 1600 | 11.43 | 1806 | 3.07 | [44] |
| CHCS | 47.68 | 13.96 | 33.68 | 4.86 | -- | -- | -- | -- | -- | Centrifugal | 1450 | ≤5.0 | -- | 1.60 | [19] |
| BFS + quartz 1 | 41.56 | 11.21 | 38.99 | 4.86 | -- | -- | -- | -- | -- | Centrifugal | 1450–1550 | 4.5 | -- | 1.20 | [18] |
| BFS + quartz 2 | 46.19 | 10.37 | 35.75 | 4.46 | -- | -- | -- | -- | -- | Centrifugal | 1450–1550 | 4.9 | -- | 1.41 | [18] |
| BFS + quartz 3 | 49.95 | 9.69 | 33.10 | 4.13 | -- | -- | -- | -- | -- | Centrifugal | 1450–1550 | 5.6 | -- | 1.60 | [18] |
| BFS + quartz 4 | 53.25 | 9.09 | 30.79 | 3.84 | -- | -- | -- | -- | -- | Centrifugal | 1500–1600 | 6.4 | -- | 1.80 | [18] |
| Fes + B2O3 1 | 49.28 | 5.35 | 3.51 | 29.36 | 0.64 | 0.31 | 6.68 | 0.13 | 2.93 | Blowing | 1500 | 5.6 | 1724 | 1.66 | [20] |
| Fes + B2O3 2 | 48.49 | 5.23 | 3.26 | 28.94 | 0.62 | 0.30 | 6.53 | 0.13 | 4.67 | Blowing | 1500 | 4.7 | 1775 | 1.67 | [20] |
| Fes + B2O33 | 47.41 | 5.06 | 3.14 | 28.38 | 0.60 | 0.29 | 6.65 | 0.12 | 6.58 | Blowing | 1500 | 4.3 | 1810 | 1.66 | [20] |
| Fes + B2O3 4 | 46.60 | 4.98 | 3.17 | 27.79 | 0.60 | 0.29 | 6.51 | 0.12 | 8.17 | Blowing | 1500 | 3.4 | 2114 | 1.67 | [20] |
| Fiber | SiO2 | Al2O3 | CaO | MgO | K2O | Na2O | FexOy | TiO2 | Tff | Diameter | Strength | Speed | Mk | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wt% | °C | μm | MPa | |||||||||||
| F45 | 53.4 | 12.58 | 21.17 | -- | -- | -- | 9.70 | -- | 1330 | 35.0 | 420 | 50 m/s | 3.12 | [45] |
| GWRF | 45.4 | 12.40 | 10.20 | 11.2 | 1.00 | 1.90 | 15.4 | 2.40 | 1230 | 61.1 | 639 | 1.3 m/s | 2.70 | [10] |
| Faf1 | 55.43 | 19.59 | 6.57 | 2.76 | 1.92 | 2.34 | 5.89 | 0.74 | 1380 | 17.0 | 704 | 300 rpm | 8.04 | [12] |
| Faf2 | 55.84 | 13.67 | 17.31 | 6.36 | 1.54 | 2.75 | 5.15 | 0.65 | 1260 | 13.0 | 1753 | 1000 rpm | 2.94 | [12] |
| Faf3 | 38.75 | 13.39 | 15.90 | 6.02 | 1.29 | 1.30 | 4.57 | 0.62 | 1320 | 11.58 | 1650 | 1400 rpm | 2.38 | [12] |
| FMPM | 47.7 | 18.8 | 15.0 | 4.60 | 2.47 | 1.32 | 2.49 | -- | 1410 | 14.04 | 903 | 5 m/s | 3.39 | [8] |
| FMM | 38.6 | 16.1 | 27.1 | 7.15 | 0.85 | 0.37 | 3.15 | -- | 1320 | 25.75 | 539 | 5 m/s | 1.60 | [8] |
| VF1 | 52.5 | 14.3 | 21.1 | 3.40 | 0.30 | 1.40 | 0.40 | -- | 1200 | 10.20 | 1268 | 1400 rpm | 2.73 | [13] |
| VF3 | 48.4 | 19.1 | 11.3 | 3.40 | 2.30 | 1.80 | 2.50 | 0.60 | 1280 | 9.11 | 1823 | 1400 rpm | 4.59 | [13] |
| VF6 | 38.9 | 13.4 | 25.4 | 9.90 | 1.30 | 1.30 | 4.60 | 0.60 | 1320 | 11.37 | 1571 | 1400 rpm | 1.48 | [13] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Xu, X.; Cheng, F.; Ramakrishna, S. Study Progress on Inorganic Fibers from Industry Solid Wastes and the Key Factors Determining Their Characteristics. Materials 2022, 15, 7256. https://doi.org/10.3390/ma15207256
Zhang J, Xu X, Cheng F, Ramakrishna S. Study Progress on Inorganic Fibers from Industry Solid Wastes and the Key Factors Determining Their Characteristics. Materials. 2022; 15(20):7256. https://doi.org/10.3390/ma15207256
Chicago/Turabian StyleZhang, Jincai, Xing Xu, Fangqin Cheng, and Seeram Ramakrishna. 2022. "Study Progress on Inorganic Fibers from Industry Solid Wastes and the Key Factors Determining Their Characteristics" Materials 15, no. 20: 7256. https://doi.org/10.3390/ma15207256
APA StyleZhang, J., Xu, X., Cheng, F., & Ramakrishna, S. (2022). Study Progress on Inorganic Fibers from Industry Solid Wastes and the Key Factors Determining Their Characteristics. Materials, 15(20), 7256. https://doi.org/10.3390/ma15207256









