Eco-Friendly Approach for Graphene Oxide Synthesis by Modified Hummers Method
Abstract
1. Introduction
2. Materials and Methods
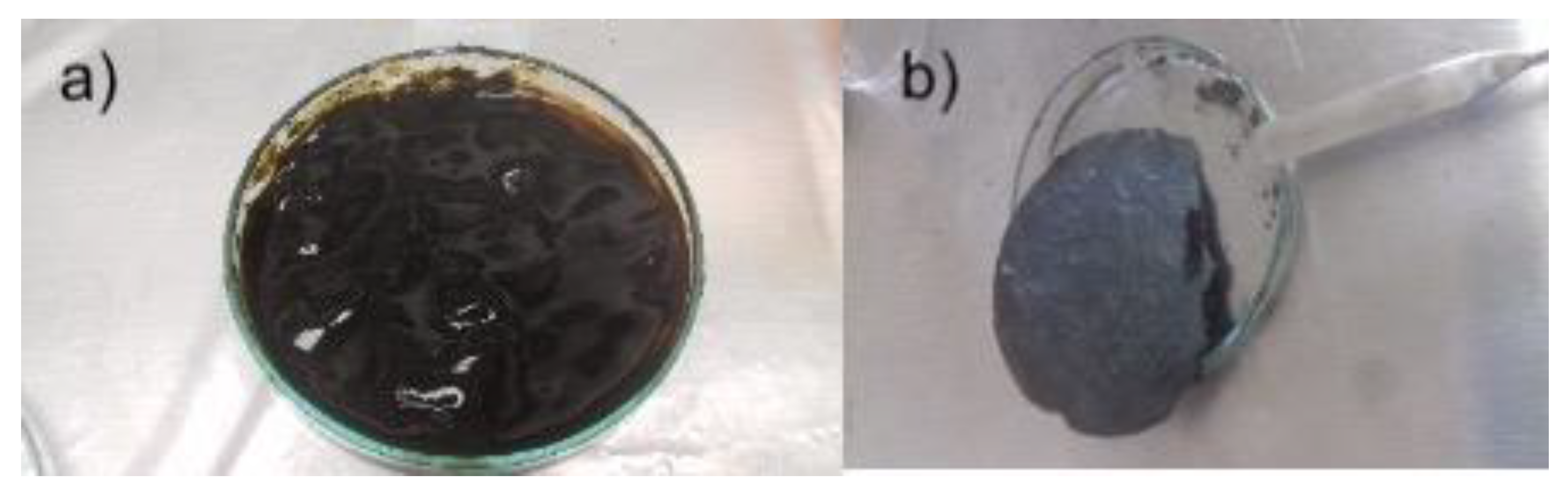
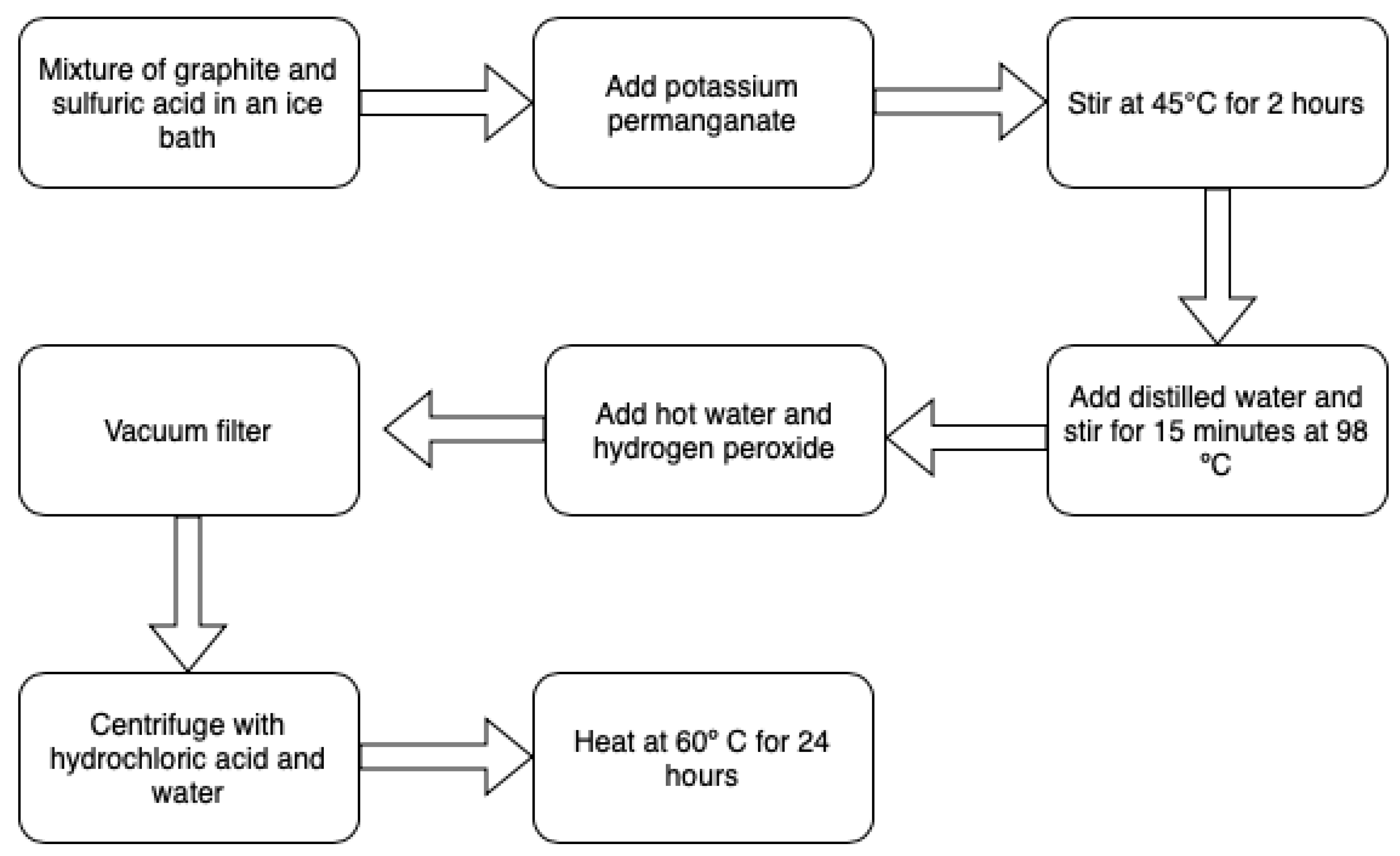
Synthesis of GO
3. Results and Discussion
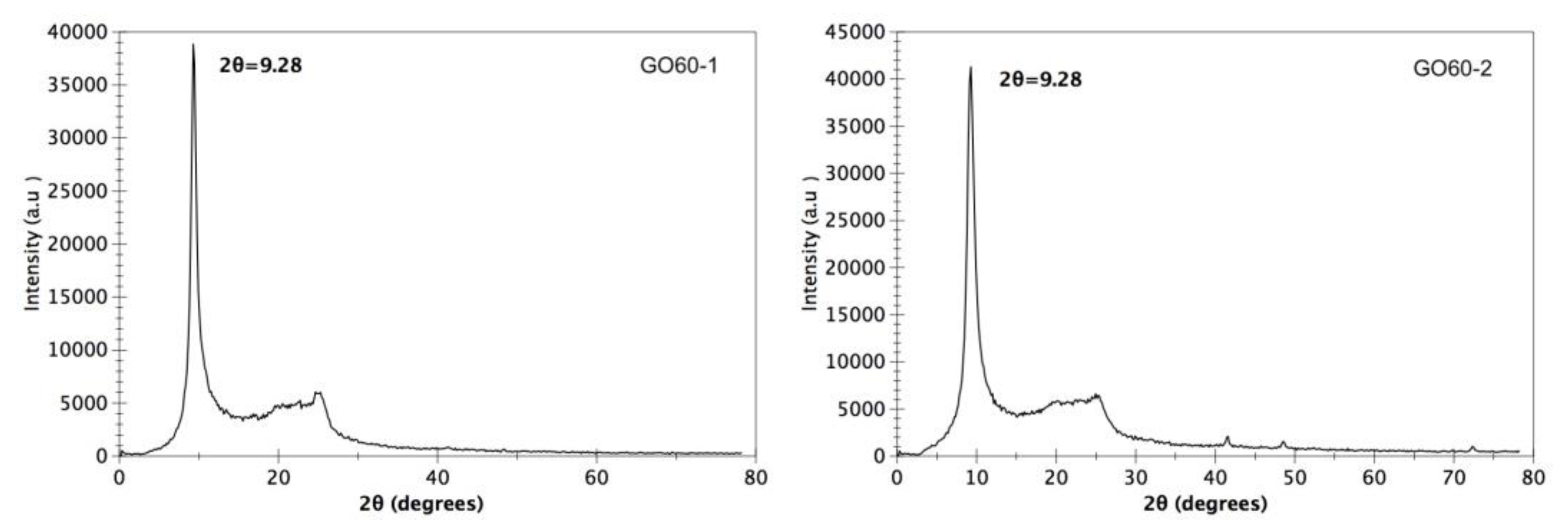
3.1. X-ray Diffraction (XRD)
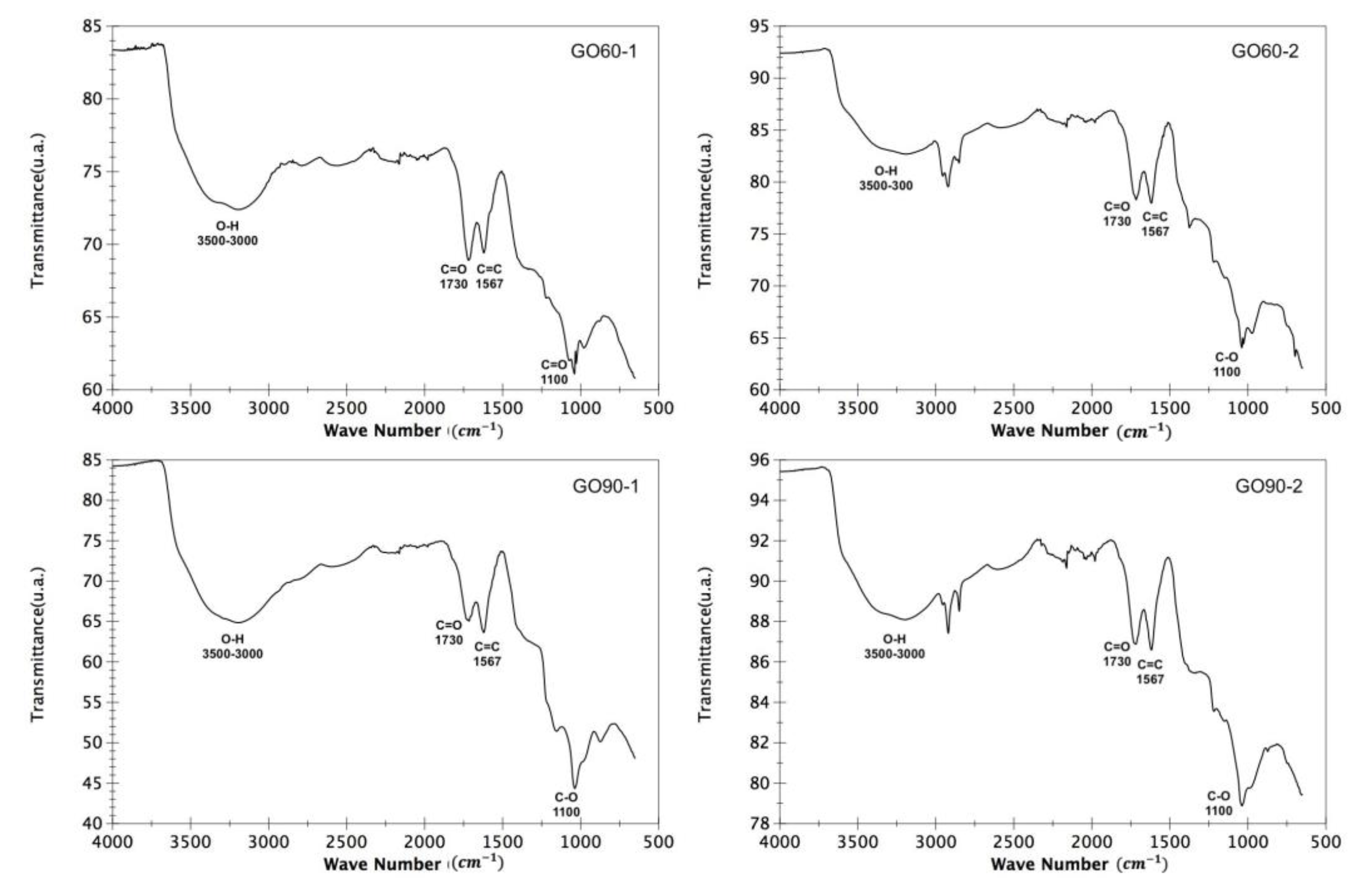
3.2. Fourier Transform Infrared Spectroscopy (FTIR)
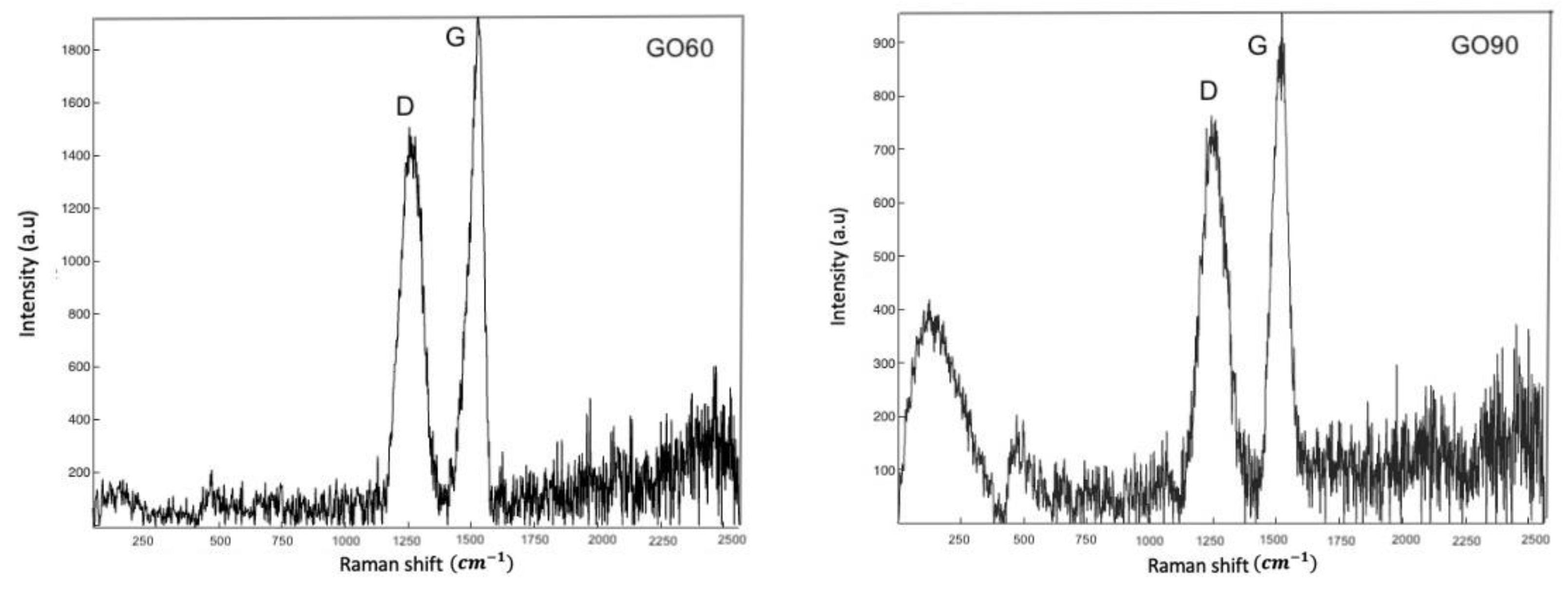
3.3. Raman Analysis
3.4. Scanning Electron Microscopy (SEM)
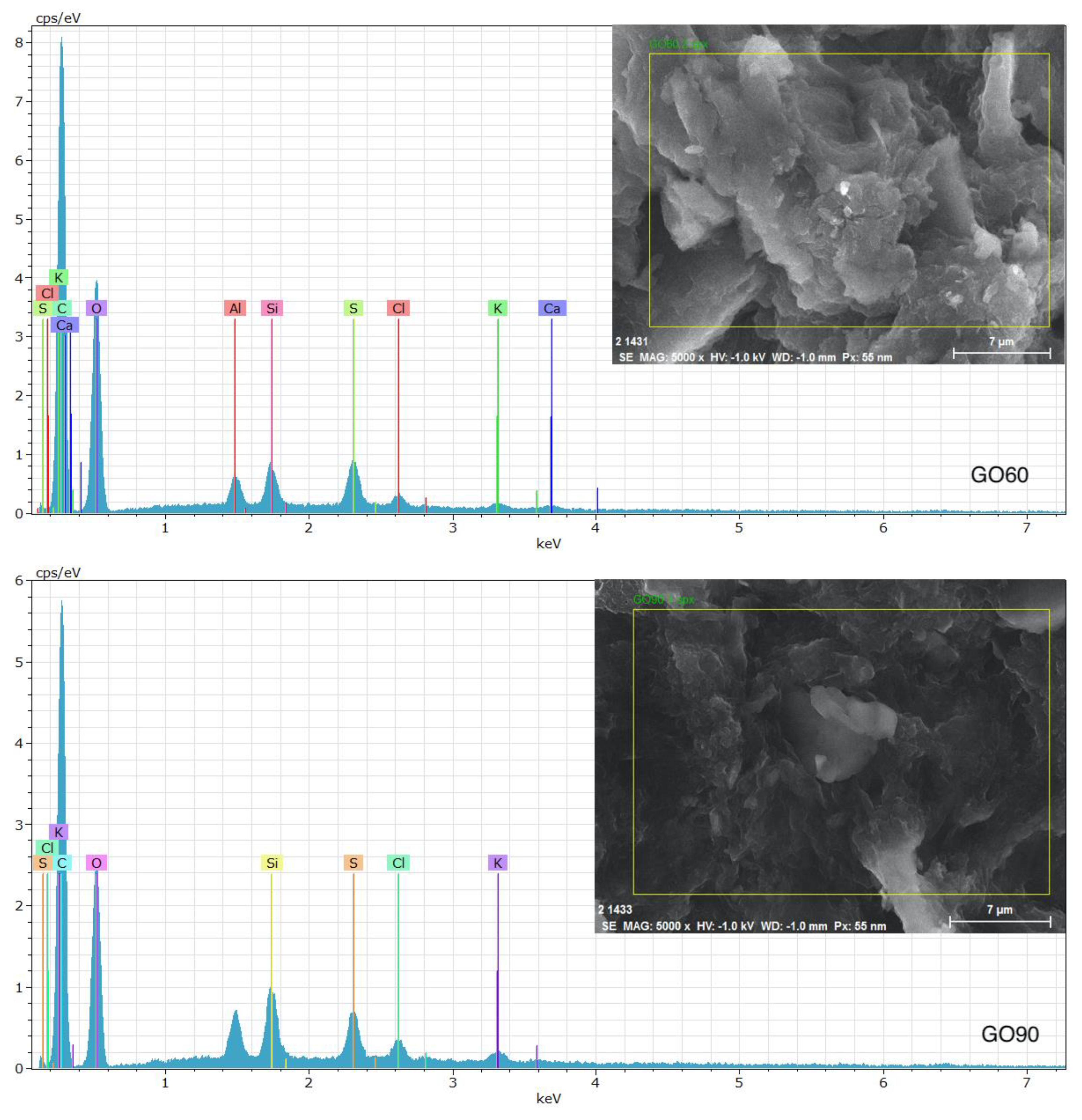
3.5. Energy-Dispersive Spectroscopy (EDS)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Zhou, X.; Cao, H.; Wang, G.; Liu, Z. Synthesis of porous graphene/activated carbon composite with high packing density and large specific surface area for supercapacitor electrode material. J. Power Sources 2014, 258, 290–296. [Google Scholar] [CrossRef]
- Bolotin, K.; Sikes, K.; Jiang, Z. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008, 146, 351–355. [Google Scholar] [CrossRef]
- Morozov, S.V.; Novoselov, K.S.; Katsnelson, M.I. Giant Intrinsic Carrier Mobilities in Graphene and Its Bilayer. Phys. Rev. Lett. 2007, 100, 016602. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Li, C.; Shi, G. Three-dimensional graphene architectures. Nanoscale 2012, 4, 5549–5563. [Google Scholar] [CrossRef] [PubMed]
- Chalmpes, N.; Patila, M.; Kouloumpis, A.; Alatzoglou, C.; Spyrou, K.; Subrati, M.; Polydera, A.C.; Bourlinos, A.B.; Stamatis, H.; Gournis, D. Graphene Oxide–Cytochrome c Multilayered Structures for Biocatalytic Applications: Decrypting the Role of Surfactant in Langmuir–Schaefer Layer Deposition. ACS Appl. Mater. Interfaces 2022, 14, 26204–26215. [Google Scholar] [CrossRef]
- Kouloumpis, A.; Vourdas, N.; Zygouri, P.; Chalmpes, N.; Potsi, G.; Kostas, V.; Spyrou, K.; Stathopoulos, V.N.; Gournis, D.; Rudolf, P. Controlled deposition of fullerene derivatives within a graphene template by means of a modified Langmuir-Schaefer method. J. Colloid Interface Sci. 2018, 524, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Tsirka, K.; Katsiki, A.; Chalmpes, N.; Gournis, D.; Paipetis, A.S. Mapping of graphene oxide and single layer graphene flakes—Defects annealing and healing. Front. Mater. 2018, 5, 37. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Shin, Y.R.; Jung, S.M.; Jeon, I.Y.; Baek, J.B. The oxidation mechanism of highly ordered pyrolytic graphite in a nitric acid/sulfuric acid mixture. Carbon 2013, 52, 493–498. [Google Scholar] [CrossRef]
- Avdeev, V.V.; Monyakina, L.A.; Nikolskaya, I.V.; Sorokina, N.E.; Semenenko, K.N. The choice of oxidizers for graphite hydrogenosulfate chemical synthesis. Carbon 1992, 30, 819–823. [Google Scholar] [CrossRef]
- Sorokina, N.E.; Khaskov, M.A.; Avdeev, V.V.; Nikol’skaya, I.V. Reaction of graphite with sulfuric acid in the presence of KMnO4. Russ. J. Gen. Chem. 2005, 75, 162–168. [Google Scholar] [CrossRef]
- Zhang, X.; Li, K.; Li, H.; Lu, J.; Fu, Q.; Chu, Y. Graphene nanosheets synthesis via chemical reduction of graphene oxide using sodium acetate trihydrate solution. Synth. Met. 2014, 193, 132–138. [Google Scholar] [CrossRef]
- Saleem, H.; Haneef, M.; Abbasi, H.Y. Synthesis route of reduced graphene oxide via thermal reduction of chemically exfoliated graphene oxide. Mater. Chem. Phys. 2018, 204, 1–7. [Google Scholar] [CrossRef]
- Escalona-Villalpando, R.A.; Gurrola, M.P.; Trejo, G.; Guerra-Balcázar, M.; Ledesma-García, J.; Arriaga, L.G. Electrodeposition of gold on oxidized and reduced graphite surfaces and its influence on glucose oxidation. J. Electroanal. Chem. 2018, 816, 92–98. [Google Scholar] [CrossRef]
- Habte, A.T.; Ayele, D.W. Synthesis and characterization of reduced graphene oxide (rGO) started from graphene oxide (GO) using the tour method with different parameters. Adv. Mater. Sci. Eng. 2019, 2019, 5058163. [Google Scholar] [CrossRef]
- Zeng, F.; Sun, Z.; Sang, X.; Diamond, D.; Lau, K.T.; Liu, X.; Su, D.S. In situ one-step electrochemical preparation of graphene oxide nanosheet-modified electrodes for biosensors. ChemSusChem 2011, 4, 1587–1591. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.E.; Wang, C.S.; Sanderson, R.N.; McDevitt, K.M.; Zhang, Y.; Valdevit, L.; Ragan, R. Scalable synthesis of gyroid-inspired freestanding three-dimensional graphene architectures. Nanoscale Adv. 2019, 1, 3870–3882. [Google Scholar] [CrossRef]
- He, D.; Peng, Z.; Gong, W.; Luo, Y.; Zhao, P.; Kong, L. Mechanism of a green graphene oxide reduction with reusable potassium carbonate. RSC Adv. 2015, 5, 11966–11972. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, B.; Dai, Y.; Liu, Y.; Zhang, X.; Qin, X.; Wang, J.; Zheng, Z.; Cheng, H. Crystal facets-controlled synthesis of graphene@TiO2 nanocomposites by a one-pot hydrothermal process. CrystEngComm 2012, 14, 1687–1692. [Google Scholar] [CrossRef]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud’homme, R.K.; Aksay, I.A.; Car, R. Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- King, A.A.; Davies, B.R.; Noorbehesht, N.; Newman, P.; Church, T.L.; Harris, A.T.; Razal, J.M.; Minett, A.I. A New Raman Metric for the Characterisation of Graphene oxide and its Derivatives. Sci. Rep. 2016, 6, 19491. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. The Raman fingerprint of graphene. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, A.; Lee, D.J.; Park, S.S. Estimation of number of graphene layers using different methods: A focused review. Materials 2021, 14, 4590. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.L.; Campos, J.L.E.; Fernandes, T.F.; Rocha, J.N.; Machado, L.R.; Soares, E.M.; Miquita, D.R.; Miranda, H.; Rabelo, C.; Neto, O.P.V.; et al. Raman spectroscopy analysis of number of layers in mass-produced graphene flakes. Carbon 2020, 161, 181–189. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]



| Washed | Cycles (rpm) | Time (min) |
|---|---|---|
| 1 to 10 | 300 | 5 |
| 11 | 300 | 10 |
| 12 to 14 | 300 | 5 |
| 15 | 300 | 10 |
| 16 to 19 | 300 | 5 |
| 20 | 300 | 10 |
| Method | Oxidants | Advantages | Disadvantages |
|---|---|---|---|
| Traditional | KCIO3 (NaCIO3) ,HNO3, H2SO4 | - | Time-consuming and dangerous method. The risk of explosions are a constant danger. |
| Hummers modified | KMnO4, H2SO4 ,NaNO3 | Improved level of oxidation and, therefore, product performance. | Separation and purification processes are tedious. Highly time- consuming. |
| Band Shift (cm−1) | Functional Group |
|---|---|
| 3500–3000 | O-H |
| 1730 | C=O |
| 1567 | C=C |
| 1100 | C-O |
| Sample | Element | wt % |
|---|---|---|
| GO60 | C | 49.29 |
| O | 42.09 | |
| Si | 6.73 | |
| S | 1.13 | |
| Cl | 0.25 | |
| K | 0.46 | |
| GO90 | C | 51.14 |
| O | 43.23 | |
| Si | 2.60 | |
| S | 1.42 | |
| Cl | 0.53 | |
| K | 0.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Méndez-Lozano, N.; Pérez-Reynoso, F.; González-Gutiérrez, C. Eco-Friendly Approach for Graphene Oxide Synthesis by Modified Hummers Method. Materials 2022, 15, 7228. https://doi.org/10.3390/ma15207228
Méndez-Lozano N, Pérez-Reynoso F, González-Gutiérrez C. Eco-Friendly Approach for Graphene Oxide Synthesis by Modified Hummers Method. Materials. 2022; 15(20):7228. https://doi.org/10.3390/ma15207228
Chicago/Turabian StyleMéndez-Lozano, Néstor, Francisco Pérez-Reynoso, and Carlos González-Gutiérrez. 2022. "Eco-Friendly Approach for Graphene Oxide Synthesis by Modified Hummers Method" Materials 15, no. 20: 7228. https://doi.org/10.3390/ma15207228
APA StyleMéndez-Lozano, N., Pérez-Reynoso, F., & González-Gutiérrez, C. (2022). Eco-Friendly Approach for Graphene Oxide Synthesis by Modified Hummers Method. Materials, 15(20), 7228. https://doi.org/10.3390/ma15207228






