Preliminary Study on Application and Limitation of Microbially Induced Carbonate Precipitation to Improve Unpaved Road in Lateritic Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Soils
2.1.1. Sample for Surface Spraying Method
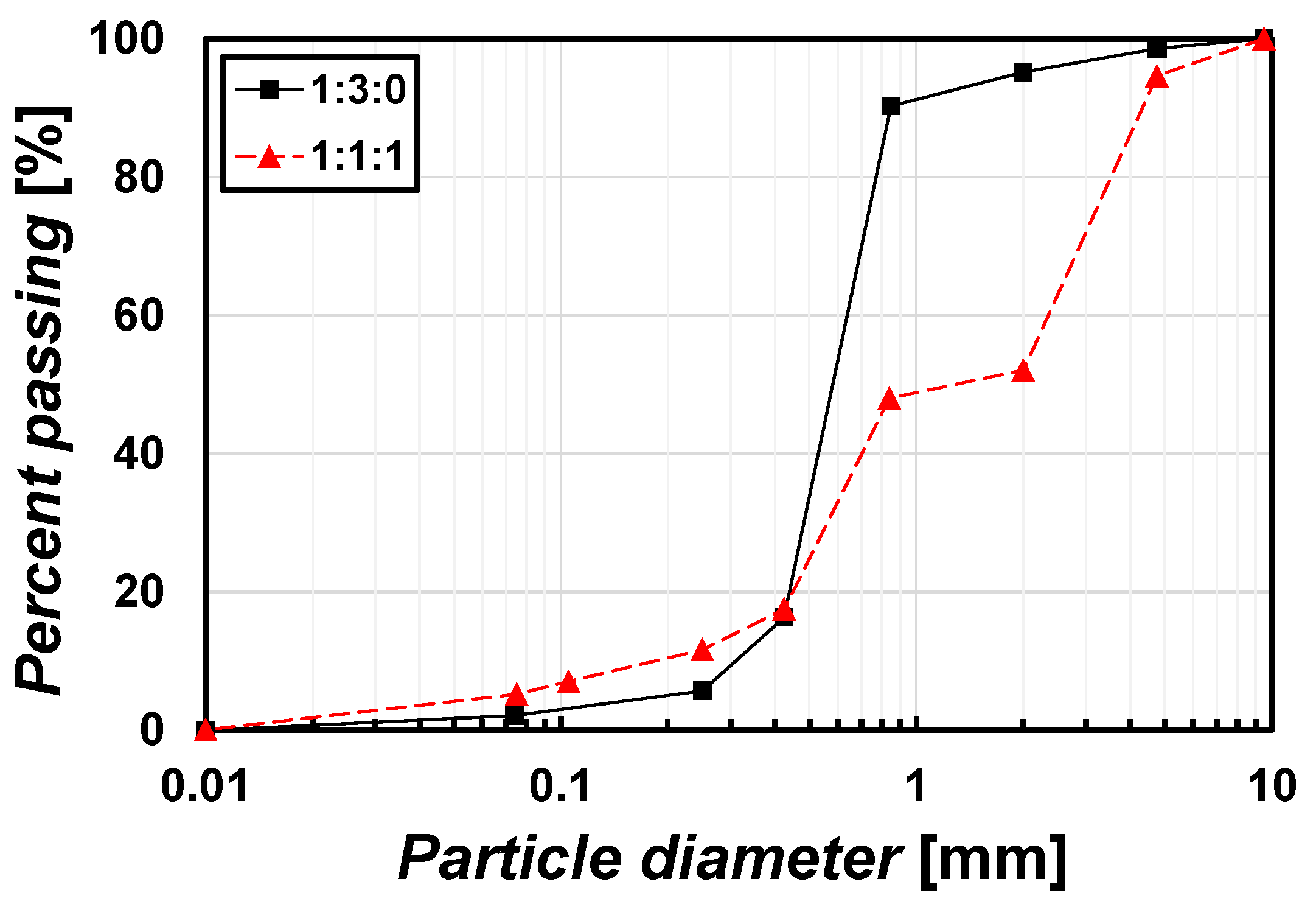
2.1.2. Sample for Mixing Method
2.2. Implementation
2.2.1. Specimen Preparation for Soil Column
2.2.2. MICP Solutions
2.2.3. MICP Treatment
2.3. Evaluation
2.3.1. Precipitation Efficiency
2.3.2. Strength
2.3.3. Microscopic Analysis
2.3.4. Quantification of Cementation Level
3. Results
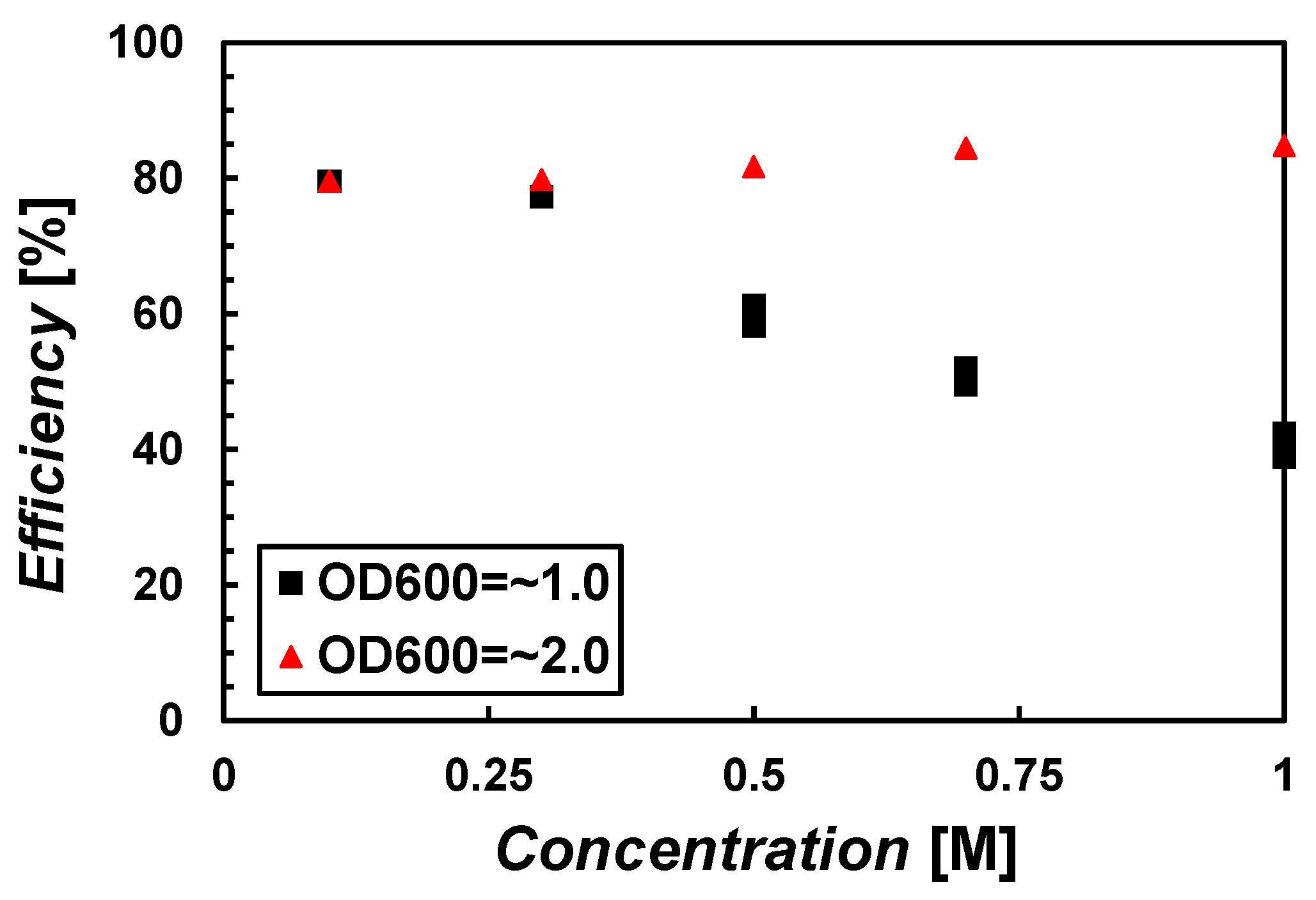
3.1. Precipitation Efficiency with Respect to Recipes
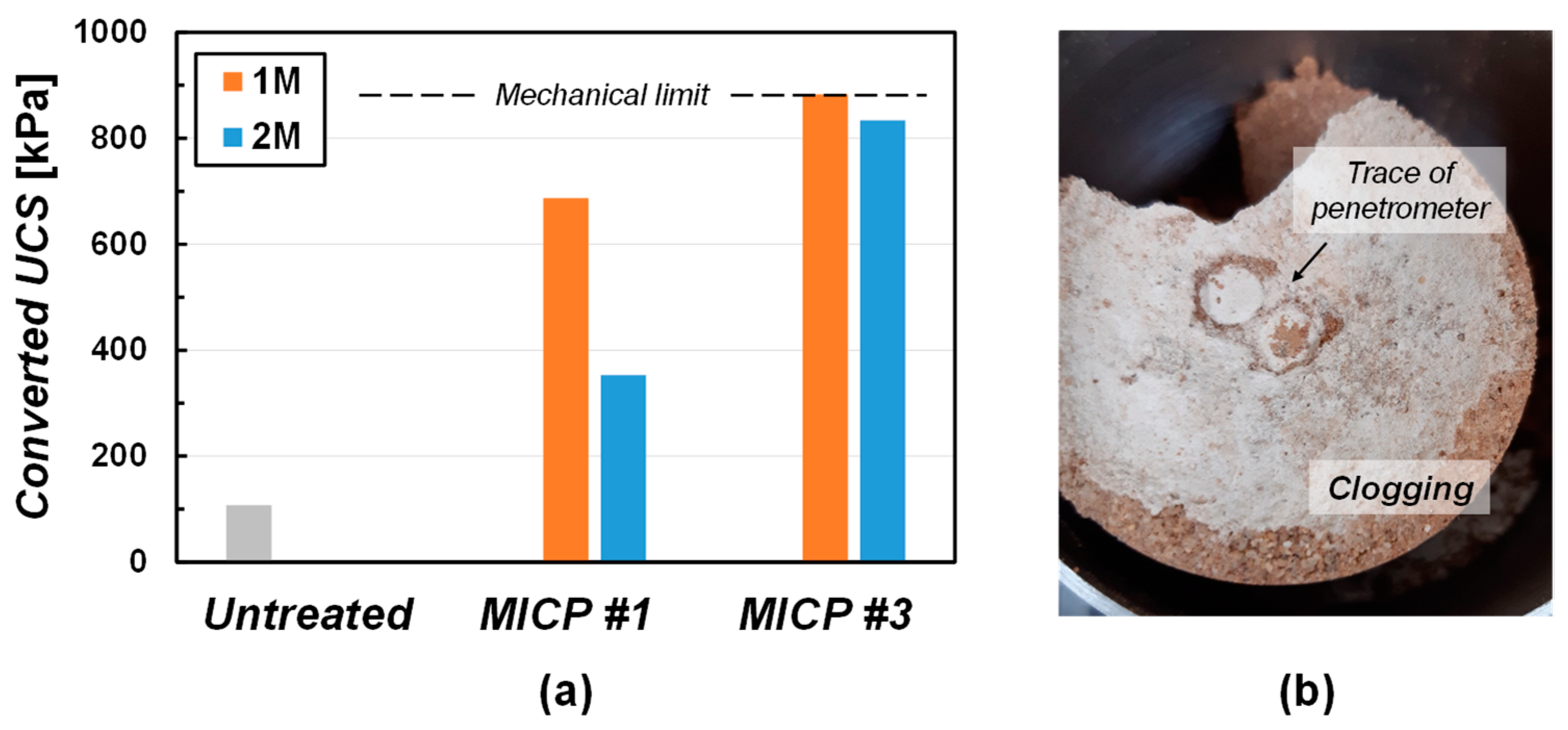
3.2. Strength of MICP-Treated Specimen
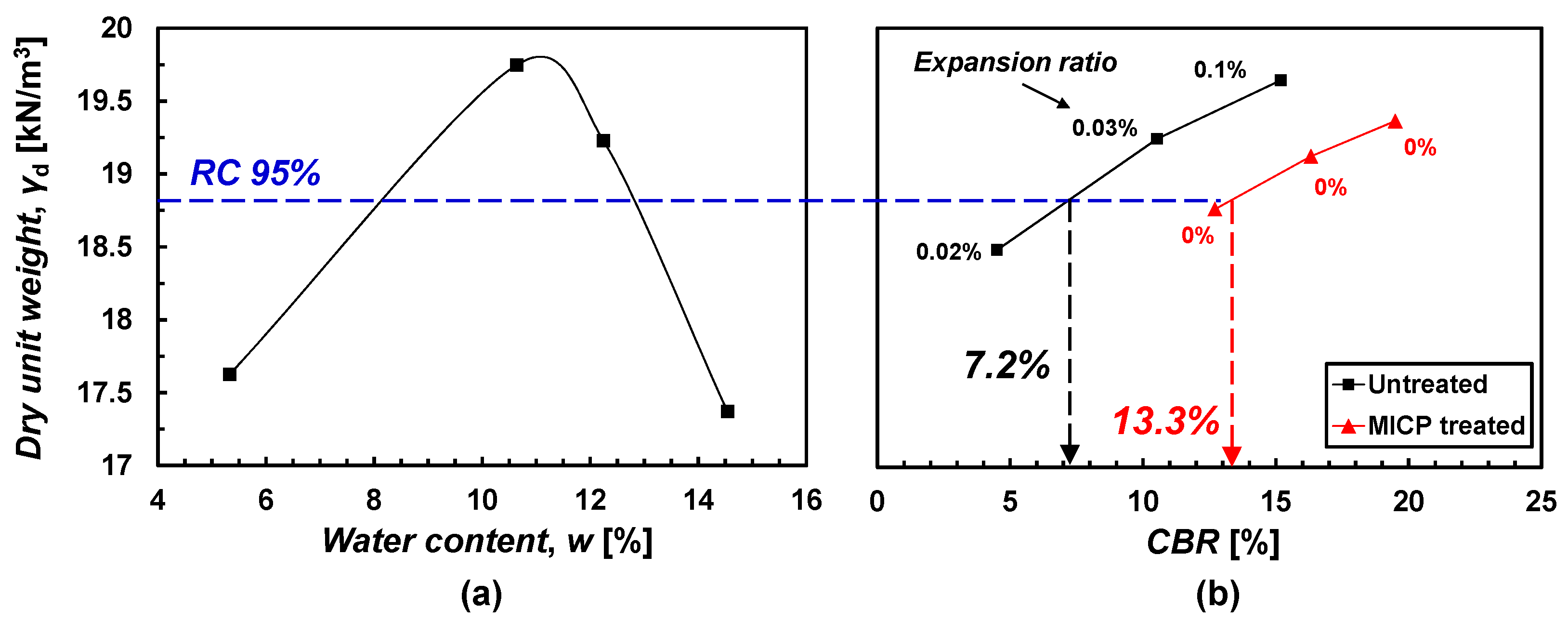
3.2.1. Specimen Treated by Surface Spraying Method
3.2.2. Specimen Treated by Mixing Method
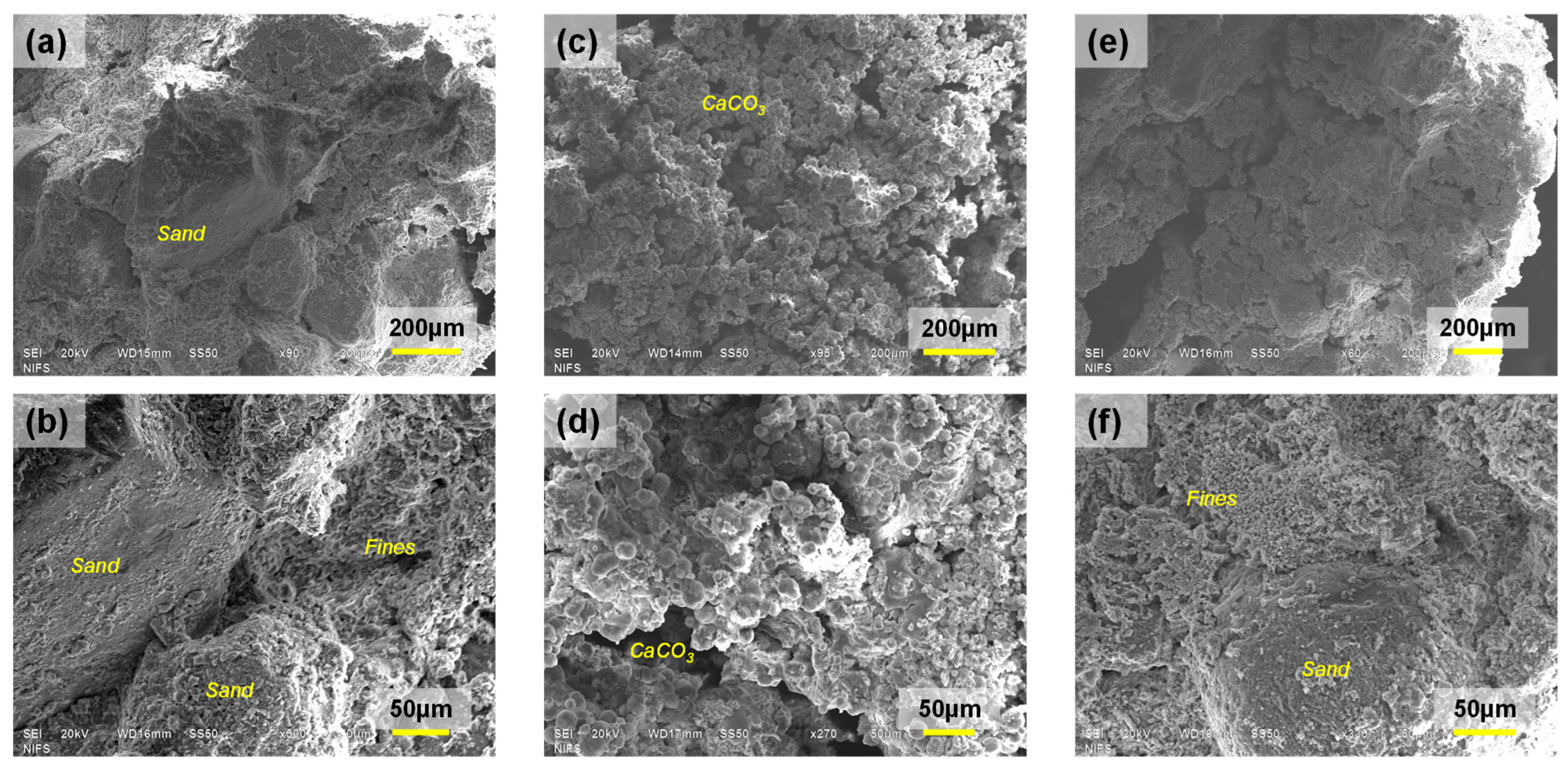
3.3. Microscopic Analysis
4. Discussion
4.1. Surface Spraying Method
4.2. Mixing Method
5. Conclusions
- The CaCO3 precipitated by the MICP process depends on the bacterial density and the solution concentrations. The higher the bacterial density and the lower the solution concentration, the higher the precipitation efficiency.
- The surface spraying method is easy to implement for MICP; however, the method requires a sufficient infiltration rate of the ground. When the infiltration rate is low, the uniformity of cementation is unfavorable due to clogging issues on the surface. If a specified surface treatment is needed, such as surficial dust control, the surface spraying method can be an attractive option to consider.
- The mixing method is a one-shot solution to cement. The MICP recipe has a great influence on the final performance of the target soil. The key is to properly optimize the recipe, such as the solution concentration and the amount of solution. It seems possible to enhance the cementation efficiency by using an additive.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korea Ministry of Land, Infrastructure and Transport. Yearbook of Road Statistics; Korea Ministry of Land, Infrastructure and Transport: Korea, 2022.
- Laos Ministry of Public Work and Transport. The Roles and Duties of Ministry of Public Works and Transport, Laos; Laos Ministry of Public Work and Transport: Laos, 2013.
- Zhan, Q.; Qian, C.; Yi, H. Microbial-induced mineralization and cementation of fugitive dust and engineering application. Constr. Build. Mater. 2016, 121, 437–444. [Google Scholar] [CrossRef]
- Parvej, S.; Naik, D.L.; Sajid, H.U.; Kiran, R.; Huang, Y.; Thanki, N. Fugitive Dust Suppression in Unpaved Roads: State of the Art Research Review. Sustainability 2021, 13, 2399. [Google Scholar] [CrossRef]
- Stocks-Fischer, S.; Galinat, J.K.; Bang, S.S. Microbiological precipitation of CaCO3. Soil Biol. Biochem. 1999, 31, 1563–1571. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hora, R.N.; Ahenkorah, I.; Beecham, S.; Karim, M.R.; Iqbal, A. State-of-the-Art Review of Microbial-Induced Calcite Precipitation and Its Sustainability in Engineering Applications. Sustainability 2020, 12, 6281. [Google Scholar] [CrossRef]
- Do, J.; Montoya, B.M.; Gabr, M.A. Debonding of Microbially Induced Carbonate Precipitation-Stabilized Sand by Shearing and Erosion. Geomech. Eng. Int. J. 2019, 17, 429–438. [Google Scholar] [CrossRef]
- Montoya, B.M.; Do, J.; Gabr, M.A. Distribution and Properties of Microbially Induced Carbonate Precipitation in Underwater Sand Bed. J. Geotech. Geoenviron. Eng. 2021, 147, 04021098. [Google Scholar] [CrossRef]
- Do, J.; Kwon, T.-H. Effect of Salt Water on the Process of Microbially Induced Carbonate Precipitation. In Proceedings of the Geo-Congress 2022, Charlotte, NC, USA, 20–23 March 2022; pp. 318–325. [Google Scholar] [CrossRef]
- Phillips, A.J.; Cunningham, A.B.; Gerlach, R.; Hiebert, R.; Hwang, C.; Lomans, B.P.; Westrich, J.; Mantilla, C.; Kirksey, J.; Esposito, R.; et al. Fracture Sealing with Microbially-Induced Calcium Carbonate Precipitation: A Field Study. Environ. Sci. Technol. 2016, 50, 4111–4117. [Google Scholar] [CrossRef]
- Chek, A.; Crowley, R.; Ellis, T.N.; Durnin, M.; Wingender, B. Evaluation of Factors Affecting Erodibility Improvement for MICP-Treated Beach Sand. J. Geotech. Geoenviron. Eng. 2021, 147, 04021001. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, C.; Tang, C.S.; Xie, Y.H.; Yin, L.Y.; Cheng, Q.; Shi, B. Bio-remediation of desiccation cracking in clayey soils through microbially induced calcite precipitation (MICP). Eng. Geol. 2020, 264, 105389. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Tang, C.S.; Pan, X.H.; Liu, B.; Xie, Y.H.; Cheng, Q.; Shi, B. Application of microbial induced carbonate precipitation for loess surface erosion control. Eng. Geol. 2021, 294, 106387. [Google Scholar] [CrossRef]
- Liu, B.; Xie, Y.H.; Tang, C.S.; Pan, X.H.; Jiang, N.J.; Singh, D.N.; Cheng, Y.J.; Shi, B. Bio-mediated method for improving surface erosion resistance of clayey soils. Eng. Geol. 2021, 293, 106295. [Google Scholar] [CrossRef]
- Do, J.; Montoya, B.M.; Gabr, M.A. Scour mitigation and erodibility improvement using microbially induced carbonate precipitation. Geotech. Test. J. 2021, 44, 1467–1483. [Google Scholar] [CrossRef]
- Gomez, M.G.; Anderson, C.M.; Graddy, C.M.R.; DeJong, J.T.; Nelson, D.C.; Ginn, T.R. Large-Scale Comparison of Bioaugmentation and Biostimulation Approaches for Biocementation of Sands. J. Geotech. Geoenviron. Eng. 2017, 143, 04016124. [Google Scholar] [CrossRef]
- van Paassen, L.A.; Harkes, M.P.; van Zwieten, G.A.; van Der Zon, W.H.; van Der Star, W.R.L.; van Loosdrecht, M.C.M. Scale up of BioGrout: A biological ground reinforcement method. In Proceedings of the 17th ICSMGE, Alexandria, Egypt, 5–9 October 2009; Volume 3, pp. 2328–2333. [Google Scholar]
- Chae, S.H.; Chung, H.; Nam, K. Evaluation of Microbially Induced Calcite Precipitation (MICP) methods on different soil types for wind erosion control. Environ. Eng. Res. 2021, 26, 190507. [Google Scholar] [CrossRef]
- Tiwari, N.; Satyam, N.; Sharma, M. Micro-mechanical performance evaluation of expansive soil biotreated with indigenous bacteria using MICP method. Sci. Rep. 2021, 11, 10324. [Google Scholar] [CrossRef] [PubMed]
- Yum, W.S.; Do, J. Use of Bacteria to Activate Ground-Granulated Blast-Furnace Slag (GGBFS) as Cementless Binder. Materials 2022, 15, 3620. [Google Scholar] [CrossRef] [PubMed]
- Gowthaman, S.; Iki, T.; Kazunori, N.; Ebina, K.; Satoru, K. Feasibility study for slope soil stabilization by microbial induced carbonate precipitation (MICP) using indigenous bacteria isolated from cold subarctic region. SN Appl. Sci. 2019, 1, 1480. [Google Scholar] [CrossRef]
- ASTM D2487-17e1; Standard Practice for Classification of Soils for Engineering Purposes (Unified Soil Classification System). ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D4318-17e1; Standard Test Methods for Liquid Limit, Plastic Limit, and Plasticity Index of Soils. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D854-14; Standard Test Methods for Specific Gravity of Soil Solids by Water Pycnometer. ASTM International: West Conshohocken, PA, USA, 2014.
- ASTM D6913-17; Standard Test Methods for Particle-Size Distribution (Gradation) of Soils Using Sieve Analysis. ASTM International: West Conshohocken, PA, USA, 2017.
- Cheng, L.; Cord-Ruwisch, R.; Shahin, M.A. Cementation of sand soil by microbially induced calcite precipitation at various degrees of saturation. Can. Geotech. J. 2013, 50, 81–90. [Google Scholar] [CrossRef]
- Cheng, L.; Cord-Ruwisch, R. Upscaling Effects of Soil Improvement by Microbially Induced Calcite Precipitation by Surface Percolation. Geomicrobiol. J. 2014, 31, 396–406. [Google Scholar] [CrossRef]
- ASTM D698-12e2; Standard Test Methods for Laboratory Compaction Characteristics of Soil Using Standard Effort. ASTM International: West Conshohocken, PA, USA, 2012.
- Wang, Y.; Soga, K.; Dejong, J.T.; Kabla, A.J. A microfluidic chip and its use in characterising the particle-scale behaviour of microbial-induced calcium carbonate precipitation (MICP). Géotechnique 2019, 69, 1086–1094. [Google Scholar] [CrossRef]
- Xiao, Y.; He, X.; Stuedlein, A.W.; Chu, J.; Matthew Evans, T.; van Paassen, L.A. Crystal Growth of MICP through Microfluidic Chip Tests. J. Geotech. Geoenviron. Eng. 2022, 148, 06022002. [Google Scholar] [CrossRef]
- Bowles, L.E. Foundation Analysis and Design; McGraw-Hill Education: New York, NY, USA, 1996. [Google Scholar]
- ASTM D1883-21; Standard Test Method for California Bearing Ratio (CBR) of Laboratory-Compacted Soils. ASTM International: West Conshohocken, PA, USA, 2021.
- Folk, R.L. The natural history of crystalline calcium carbonate; effect of magnesium content and salinity. J. Sediment. Res. 1974, 44, 40–53. [Google Scholar] [CrossRef]
- Korea Expressway Corporation. Expressway Construction Guide Specification-Civil Engineering; Korea Expressway Corporation: Korea, 2009. [Google Scholar]
- Ogino, T.; Suzuki, T.; Sawada, K. The formation and transformation mechanism of calcium carbonate in water. Geochim. Cosmochim. Acta 1987, 51, 2757–2767. [Google Scholar] [CrossRef]
- Noh, H.J.; Lee, S.K.; Yu, J.H. Identifying Effective Fugitive Dust Control Measures for Construction Projects in Korea. Sustainability 2018, 10, 1206. [Google Scholar] [CrossRef]
- National Institute of Environmental Research. 2013 National Air Pollutant Emissions; National Institute of Environmental Research: Incheon, Korea, 2015. [Google Scholar]
- Ashraf, M.S.; Azahar, S.B.; Yusof, N.Z. Soil Improvement Using MICP and Biopolymers: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2017, 226, 012058. [Google Scholar] [CrossRef]
- Yao, D.; Wu, J.; Niu, S.; Gu, Z.; Zheng, J.J.; Yan, J.; Xu, L.; Yang, M.; Yan, Y. An anionic biopolymer γ-polyglutamate enhanced the microbially induced carbonate precipitation for soil improvement: Mechanical behaviors and underlying mechanism. Acta Geotech. 2022, 17, 4485–4496. [Google Scholar] [CrossRef]
- Gowthaman, S.; Hiranya, T.; Nawarathna, K.; Ge, P.; Nayanthara, N.; Nakashima, K.; Kawasaki, S. The Amendments in Typical Microbial Induced Soil Stabilization by Low-Grade Chemicals, Biopolymers and Other Additives: A Review. In Building Materials for Sustainable and Ecological Environment; Springer: Singapore, 2021; pp. 49–72. [Google Scholar] [CrossRef]
- Cheng, L.; Shahin, M.A.; Chu, J. Soil bio-cementation using a new one-phase low-pH injection method. Acta Geotech. 2019, 14, 615–626. [Google Scholar] [CrossRef]
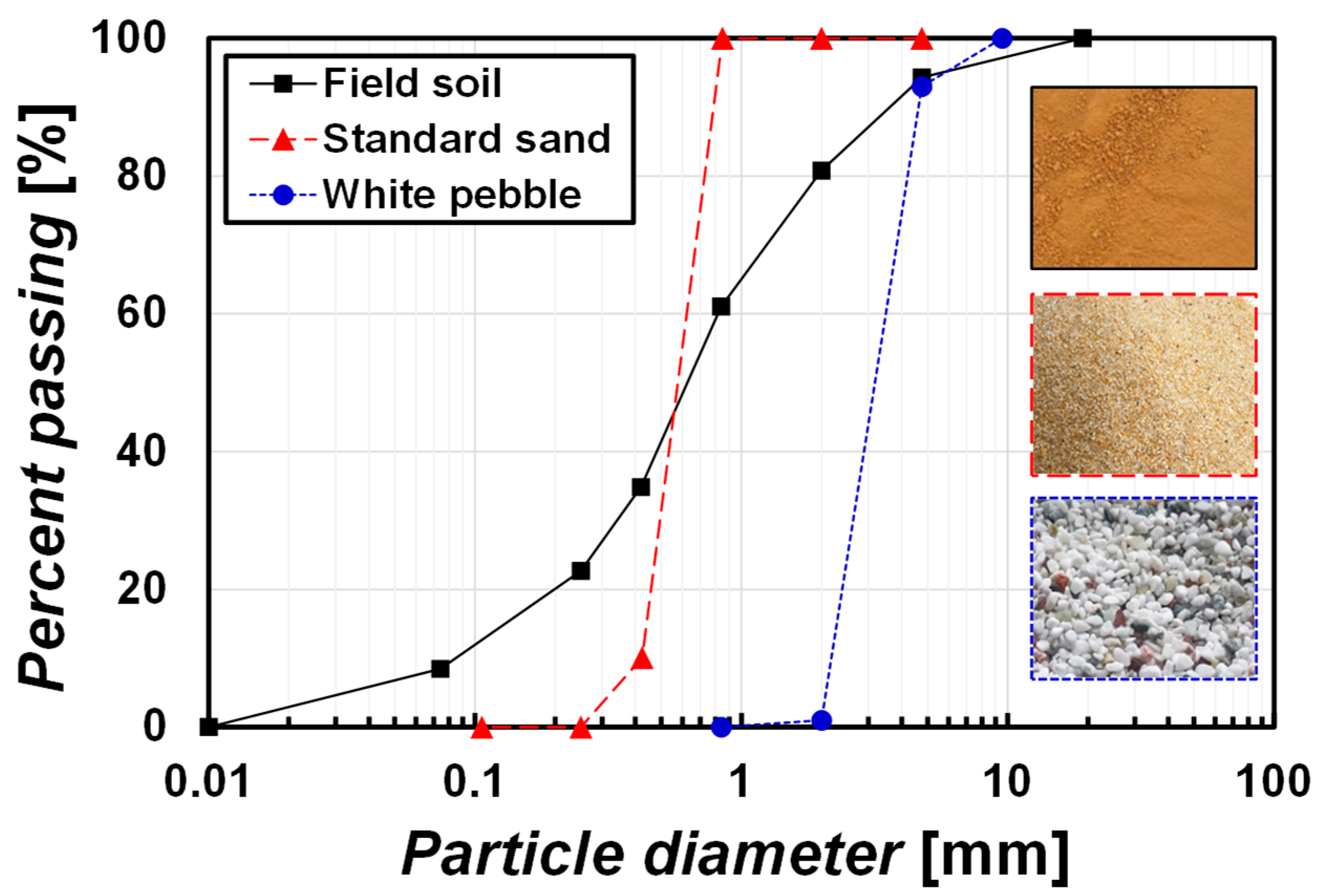



| Soil Type | D50 [mm] | Fine [%] | Gs | USCS | wL/wP [%] | emin/emax | Organic [%] |
|---|---|---|---|---|---|---|---|
| Field soil | 0.65 | 8.5 | 2.62 | SW-SM | 37.3/35.5 | N/M * | 2.7 |
| Standard sand | 0.60 | 0 | 2.63 | SP | N/A * | 0.625/0.919 | - |
| White pebble | 3.4 | 0 | 2.78 | SP | N/A | N/M | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Kim, Y.; Lee, S.; Do, J. Preliminary Study on Application and Limitation of Microbially Induced Carbonate Precipitation to Improve Unpaved Road in Lateritic Region. Materials 2022, 15, 7219. https://doi.org/10.3390/ma15207219
Kim S, Kim Y, Lee S, Do J. Preliminary Study on Application and Limitation of Microbially Induced Carbonate Precipitation to Improve Unpaved Road in Lateritic Region. Materials. 2022; 15(20):7219. https://doi.org/10.3390/ma15207219
Chicago/Turabian StyleKim, Sojeong, Yeontae Kim, Suhyung Lee, and Jinung Do. 2022. "Preliminary Study on Application and Limitation of Microbially Induced Carbonate Precipitation to Improve Unpaved Road in Lateritic Region" Materials 15, no. 20: 7219. https://doi.org/10.3390/ma15207219
APA StyleKim, S., Kim, Y., Lee, S., & Do, J. (2022). Preliminary Study on Application and Limitation of Microbially Induced Carbonate Precipitation to Improve Unpaved Road in Lateritic Region. Materials, 15(20), 7219. https://doi.org/10.3390/ma15207219






