Study on Oxygen Evolution Reaction Performance of Jarosite/C Composites
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of (A)Fe3(SO4)2(OH)6, (A = K+, Na+ and NH4+)
2.3. Preparation of (H2O)Fe3(SO4)2(OH)6
2.4. Preparation of Working Electrode
2.5. Characterization
2.6. Electrochemical Measurements
3. Results and Discussion
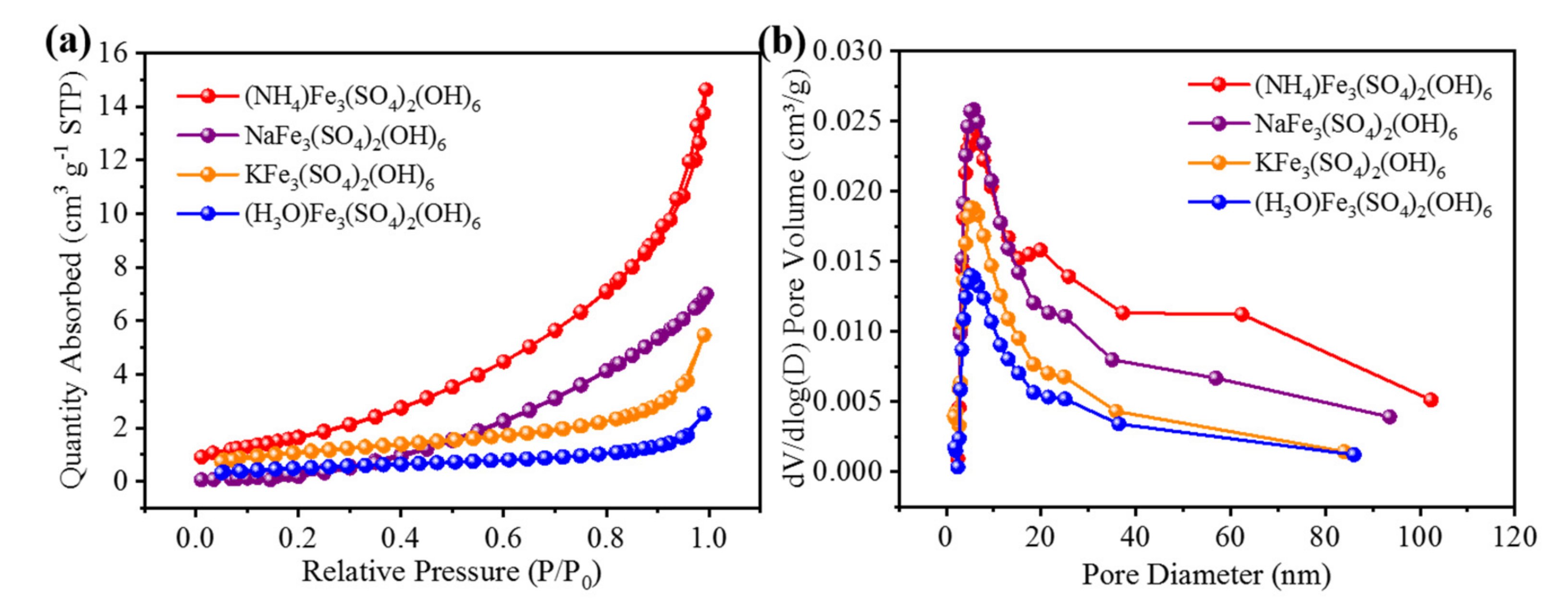
3.1. Characterization of Samples
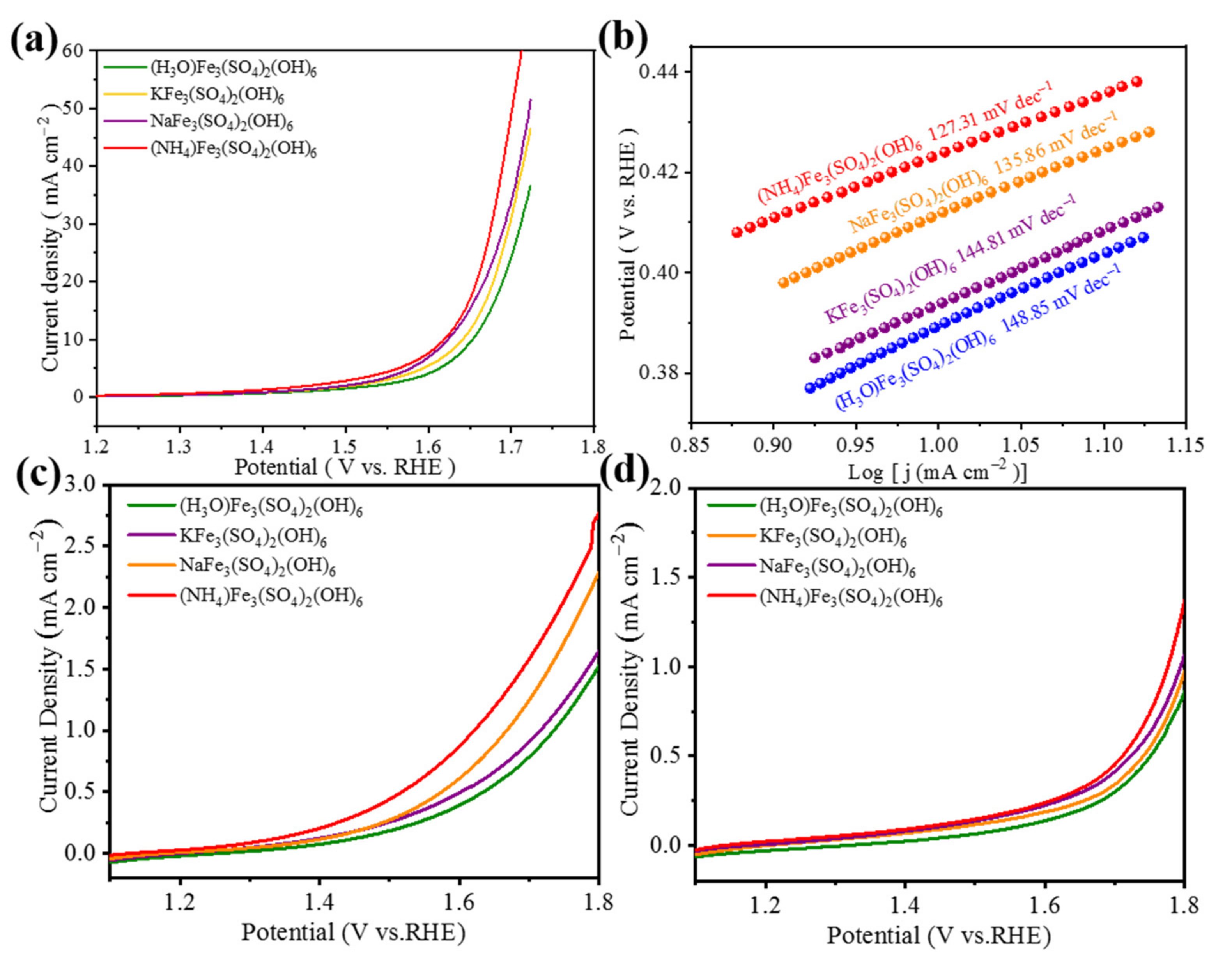
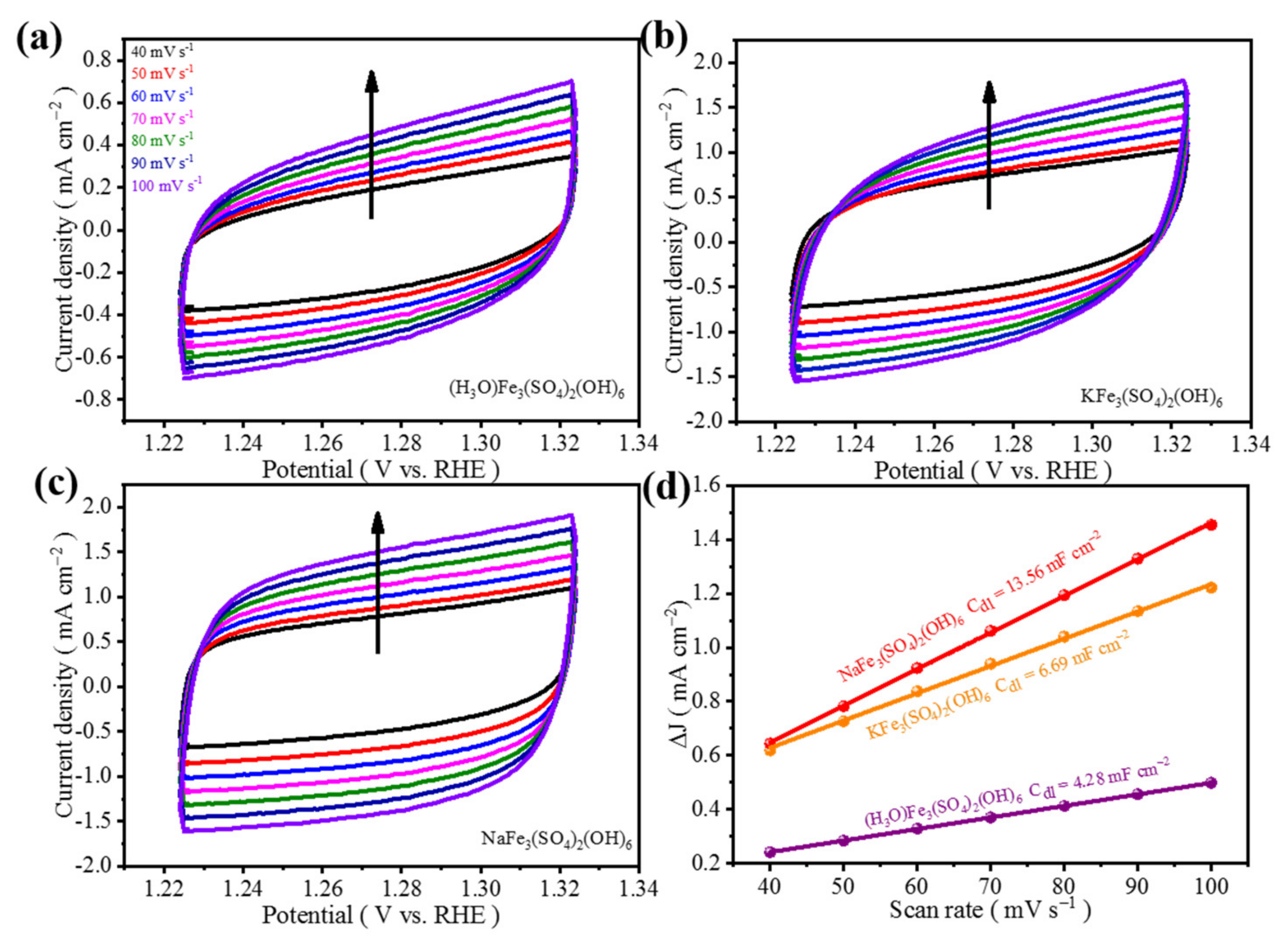
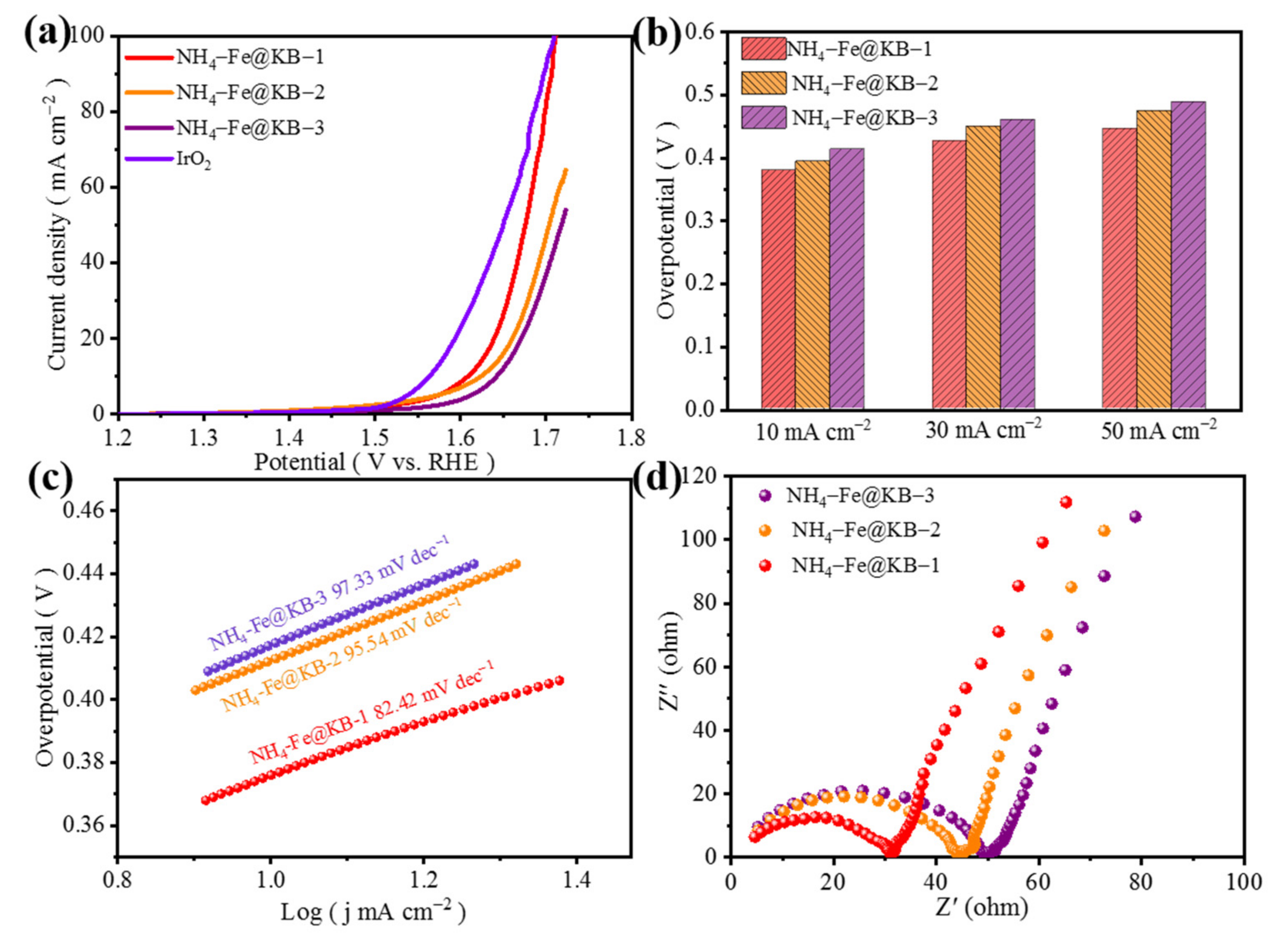
3.2. Electrochemical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, D.D.; Zhang, N.; Bu, L.Z.; Shao, Q.; Huang, X.Q. The latest development of non-noble metal electrocatalytic oxygen evolution catalysts. Electrochemistry 2018, 24, 455–465. [Google Scholar]
- Guo, Y.X.; Shang, C.S.; Li, J.; Wang, E.K. Research progress in electrocatalytic hydrogen evolution, oxygen evolution, and oxygen reduction. Sci. China Chem. 2018, 48, 926–940. [Google Scholar]
- Shao, Q.; Yang, J.; Huang, X. The Design of Water Oxidation Electrocatalysts from Nanoscale Metal-Organic Frameworks. Chem. Eur. J. 2018, 24, 15143–15155. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Park, K.S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, W.; Xu, Y.Y.; Dong, S.; Xiao, J.; Wang, F.; Liu, H.; Xia, B.Y. Hollow nitrogen-doped carbon spheres with Fe3O4 nanoparticles encapsulated as a highly active oxygen-reduction catalyst. ACS Appl. Mater. Interfaces 2017, 9, 10610–10617. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Xiao, S.; Wang, Z.; Zheng, X.; Yang, S. Co intake mediated formation of ultrathin nanosheets of transition metal LDH-an advanced electrocatalyst for oxygen evolution reaction. Chem. Commun. 2015, 51, 1120–1123. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, Y.; Wu, D.; Huang, C.; Xiao, D.; Chen, H.; Zheng, S.; Chu, P.K. NiFe-Layered Double Hydroxide synchronously activated by heterojunctions and Vacancies for the Oxygen Evolution Reaction. Appl. Mater. Interfaces 2020, 12, 42850–42858. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, X.; Xu, H.; Wu, Z.; Wang, H.; Liang, Y. Iron-Doped cobalt monophosphide Nanosheet/Carbon nanotube hybrids as active and stable electrocatalysts for water splitting. Adv. Funct. Mater. 2017, 27, 1606635. [Google Scholar] [CrossRef]
- Xu, K.; Chen, P.; Li, X.; Tong, Y.; Ding, H.; Wu, X.; Chu, W.; Peng, Z.; Wu, C.; Xie, Y. Metallic Nickel Nitride nanosheets realizing enhanced electrochemical water oxidation. J. Am. Chem. Soc. 2015, 137, 4119–4125. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Wang, Y.; Yan, L.; Xue, Y.; Li, S.; Hu, M.; Jiang, Y.; Zhai, Q.G. In situ semi-transformation from heterometallic MOFs to Fe-Ni LDH/MOF hierarchical architectures for boosted oxygen evolution reaction. Nanoscale 2020, 12, 14514. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yim, W.L.; Suryanto, B.H.; Zhao, C. Electrocatalytic oxygen evolution at Surface-Oxidized multiwall carbon nanotubes. J. Am. Chem. Soc. 2015, 137, 2901–2907. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhou, W.; Wang, L.; Jia, J.; Ke, Y.; Yang, L.; Zhou, K.; Liu, X.; Tang, Z.; Li, L.; et al. Core-shell nanocomposites based on gold nanoparticle @Zinc–iron- embedded porous carbons derived from metal-organic frameworks as efficient dual catalysts for oxygen reduction and hydrogen evolution reactions. ACS Catal. 2016, 6, 1045–1053. [Google Scholar] [CrossRef]
- Li, X.; Hao, X.; Wang, Z.; Abudula, A.; Guan, G. In-situ intercalation of NiFe LDH materials: An effificient approach to improve electrocatalytic activity and stability for water splitting. J. Power Sources 2017, 347, 193–200. [Google Scholar] [CrossRef]
- Smith, R.D.; Sporinova, B.; Fagan, R.D.; Trudel, S.; Berlinguette, C.P. Facile photochemical preparation of amorphous iridium oxide films for water oxidation catalysis. Chem. Mater. 2014, 26, 1654–1659. [Google Scholar] [CrossRef]
- Su, L.W.; Zhou, Z.; Shen, P.W. Core-shell Fe@Fe3C/C Nanocomposites as Anode Materials for Li Ion Batteries. Electrochim. Acta 2013, 87, 180–185. [Google Scholar] [CrossRef]
- Boyanov, S.; Bernardi, J.; Gillot, F.; Dupont, L.; Womes, M.; Tarascon, J.M.; Monconduit, L.; Doublet, M.L. FeP: AnotherAttractive Anode for the Li-Ion Battery Enlisting a Reversible Two-Step Insertion/Conversion Process. Chem. Mater. 2006, 18, 3531–3538. [Google Scholar] [CrossRef]
- Boyanov, S.; Womes, M.; Monconduit, L.; Zitoun, D. Mossbauer Spectroscopy and Magnetic Measurements As Comple-mentary Techniques for the Phase Analysis of FeP Electrodes Cycling in Li-Ion Batteries. Chem. Mater. 2009, 21, 3684–3692. [Google Scholar] [CrossRef]
- Ding, X.; Yin, S.; An, K.; Luo, L.; Shi, N.; Qiang, Y.; Pasupathi, S.; Pollet, B.G.; Shen, P.K. FeN stabilized FeN@Pt core-shell nanostructures for oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 4462–4469. [Google Scholar] [CrossRef]
- Cai, Z.; Zhou, D.; Wang, M.; Bak, S.M.; Wu, Y.; Wu, Z.; Tian, Y.; Xiong, X.; Li, Y.; Liu, W.; et al. Introducing Fe2+ into nickel-iron layered double hydroxide: Local structure modulated water oxidation activity. Angew. Chem.-Int. Ed. 2018, 130, 9536–9540. [Google Scholar] [CrossRef]
- Basciano, L.C.; Peterson, R.C. The crystal structure of ammoniojarosite, (NH4)Fe3(SO4)W(OH)6 and the crystal chemistry of the ammoniojarosite-hydronium jarosite solid-solution series. Mineral. Mag. 2007, 71, 427–441. [Google Scholar] [CrossRef]
- Frost, R.; Wills, R.A.; Kloprogge, J.; Martens, W. Thermal decomposition of ammonium jarosite (NH4)Fe3(SO4)2(OH)6. J. Therm. Anal. Calorim. 2006, 84, 489–496. [Google Scholar] [CrossRef] [Green Version]
- Li, H.J.; Yang, H.Y.; Chen, G.B. Catalytic performance of biological method seeds on jarosite process. Trans. Nonferrous Met. Soc. China 2016, 26, 557–564. [Google Scholar] [CrossRef]
- Liu, F.; Shi, J.; Duan, J.; Zhou, L.; Xu, J.; Hao, X.; Fan, W. Significance of jarosite dissolution from the biooxidized pyrite surface on further biooxidation of pyrite. Hydrometallurgy 2018, 176, 33–41. [Google Scholar] [CrossRef]
- Xu, W.; Xie, Z.; Cui, X.; Zhao, K.; Zhang, L.; Mai, L.; Wang, Y. Direct growth of an economic green energy storage material: A monocrystalline jarosite-KFe3(SO4)2(OH)6-nanoplates@rGO hybrid as a superior lithium-ion battery cathode. J. Mater. Chem. A 2016, 4, 3735–3742. [Google Scholar] [CrossRef]
- Wu, N.; Tian, W.; Shen, J.; Qiao, X.; Sun, T.; Wu, H.; Zhao, J.; Liu, X.; Zhang, Y. Facile fabrication of a jarosite ultrathin KFe3(SO4)2(OH)6@rGO nanosheet hybrid composite with pseudocapacitive contribution as a robust anode for lithiumion batteries. Inorg. Chem. Front. 2019, 6, 192–198. [Google Scholar] [CrossRef]
- Wang, X.L.; Dong, L.Z.; Qiao, M.; Tang, Y.J.; Liu, J.; Li, Y.; Li, S.L.; Su, J.X.; Lan, Y.Q. Exploring the performance improvement of the oxygen evolution reaction in a stable bimetal-organic framework system. Angew. Chem.-Int. Ed. 2018, 57, 1–6. [Google Scholar]
- Gao, K.; Jiang, M.; Guo, C.; Zeng, Y.; Fan, C.; Zhang, J.; Reinfelder, J.R.; Huang, W.; Lu, G.; Dang, Z. Reductive dissolution of jarosite by a sulfate reducing bacterial community: Secondary mineralization and microflflora development. Sci. Total Environ. 2019, 690, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ling, Z.; Zhang, J.; Bi, X.; Xin, Y. Laboratory Raman and VNIR spectroscopic studies of jarosite and other secondary mineral mixtures relevant to Mars. J. Raman Spectrosc. 2020, 51, 1575–1588. [Google Scholar] [CrossRef]
- Duan, Y.; Lee, J.Y.; Xi, S.; Sun, Y.; Ge, J.; Ong, S.J.H.; Chen, Y.; Dou, S.; Meng, F.; Diao, C.; et al. Anodic Oxidation Enabled Cation Leaching for Promoting Surface Reconstruction in Water Oxidation. Angew. Chem.-Int. Ed. 2021, 133, 7418–7425. [Google Scholar] [CrossRef]
- Liu, X.; Meng, J.; Ni, K.; Guo, R.; Xia, F.; Xie, J.; Li, X.; Wen, B.; Wu, P.; Li, M.; et al. Complete Reconstruction of Hydrate Pre-Catalysts for Ultrastable Water Electrolysis in Industrial-Concentration Alkali Media. Cell Rep. Phys. Sci. 2020, 1, 100241. [Google Scholar] [CrossRef]
- Hu, J.; Li, S.; Chu, J.; Niu, S.; Wang, J.; Du, Y.; Li, Z.; Han, X.; Xu, P. Understanding the Phase-Induced Electrocatalytic Oxygen Evolution Reaction Activity on FeOOH Nanostructures. ACS Catal. 2019, 9, 10705–10711. [Google Scholar] [CrossRef]
- Xiao, T.; Yang, C.; Lu, Y.; Zeng, F. One-pot hydrothermal synthesis of rod-like FeOOH/reduced graphene oxide composites for supercapacitor. Journal of Materials Science. Mater. Electron. 2014, 25, 3364–3374. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Tanaka, S. Monitoring the development of rust layers on weathering steel using in situ Raman spectroscopy under wet-and-dry cyclic conditions. J. Solid State Electrochem. 2015, 19, 3559–3566. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Li, S.; Qu, Z.; Li, Z.; Wang, D.; Shen, J.; Li, Y. Study on Oxygen Evolution Reaction Performance of Jarosite/C Composites. Materials 2022, 15, 668. https://doi.org/10.3390/ma15020668
Chen J, Li S, Qu Z, Li Z, Wang D, Shen J, Li Y. Study on Oxygen Evolution Reaction Performance of Jarosite/C Composites. Materials. 2022; 15(2):668. https://doi.org/10.3390/ma15020668
Chicago/Turabian StyleChen, Junxue, Sijia Li, Zizheng Qu, Zhonglin Li, Ding Wang, Jialong Shen, and Yibing Li. 2022. "Study on Oxygen Evolution Reaction Performance of Jarosite/C Composites" Materials 15, no. 2: 668. https://doi.org/10.3390/ma15020668
APA StyleChen, J., Li, S., Qu, Z., Li, Z., Wang, D., Shen, J., & Li, Y. (2022). Study on Oxygen Evolution Reaction Performance of Jarosite/C Composites. Materials, 15(2), 668. https://doi.org/10.3390/ma15020668




