Effect of Calcination Temperature on Mechanical Properties of Magnesium Oxychloride Cement
Abstract
:1. Introduction
2. Experimental Section
2.1. Principal Raw Materials
2.2. Preparation
2.3. Calcination Experiments
2.4. Characterisation
3. Results and Discussion
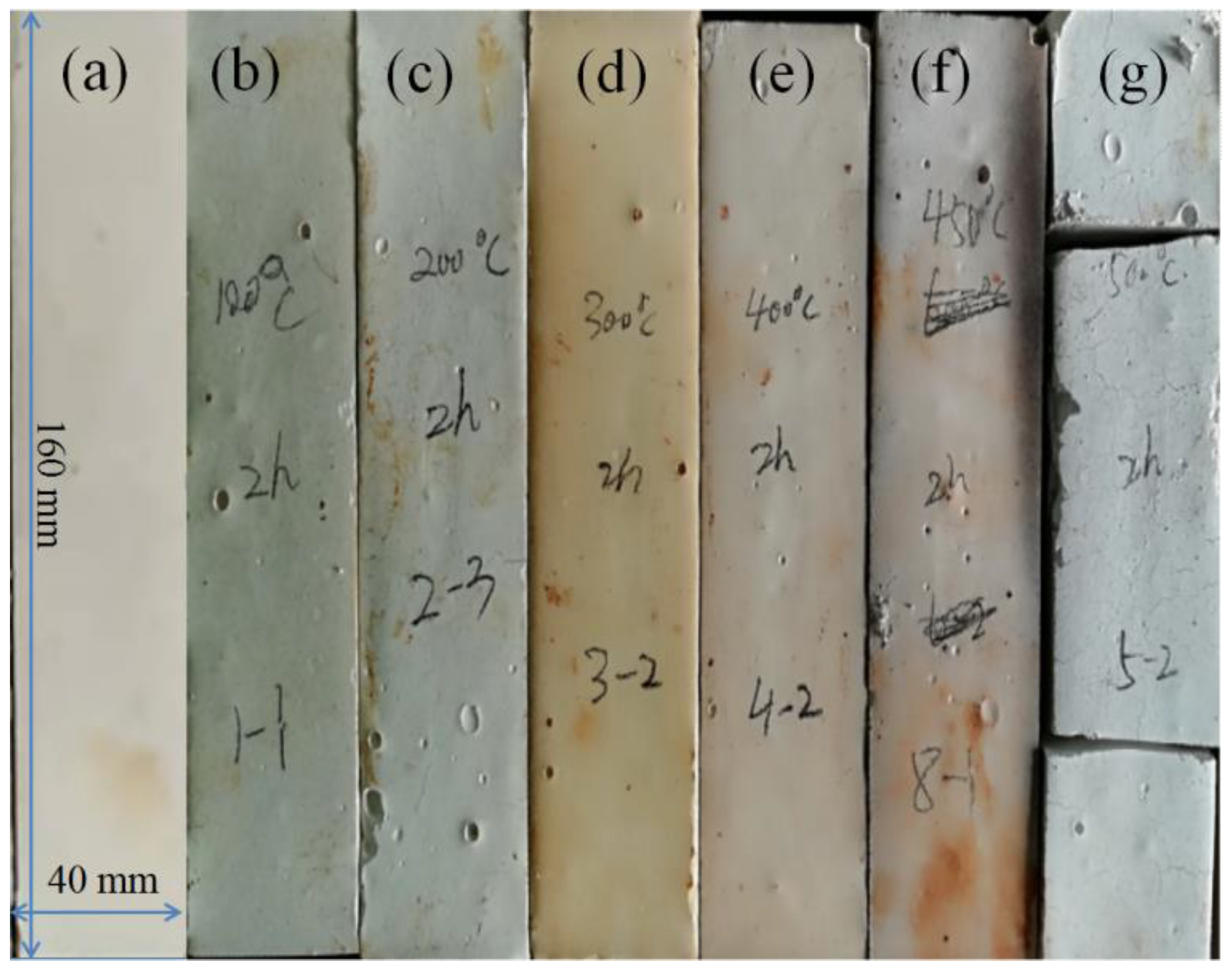
3.1. Effect of Calcination Temperature on Morphology of Magnesium Oxychloride Cement
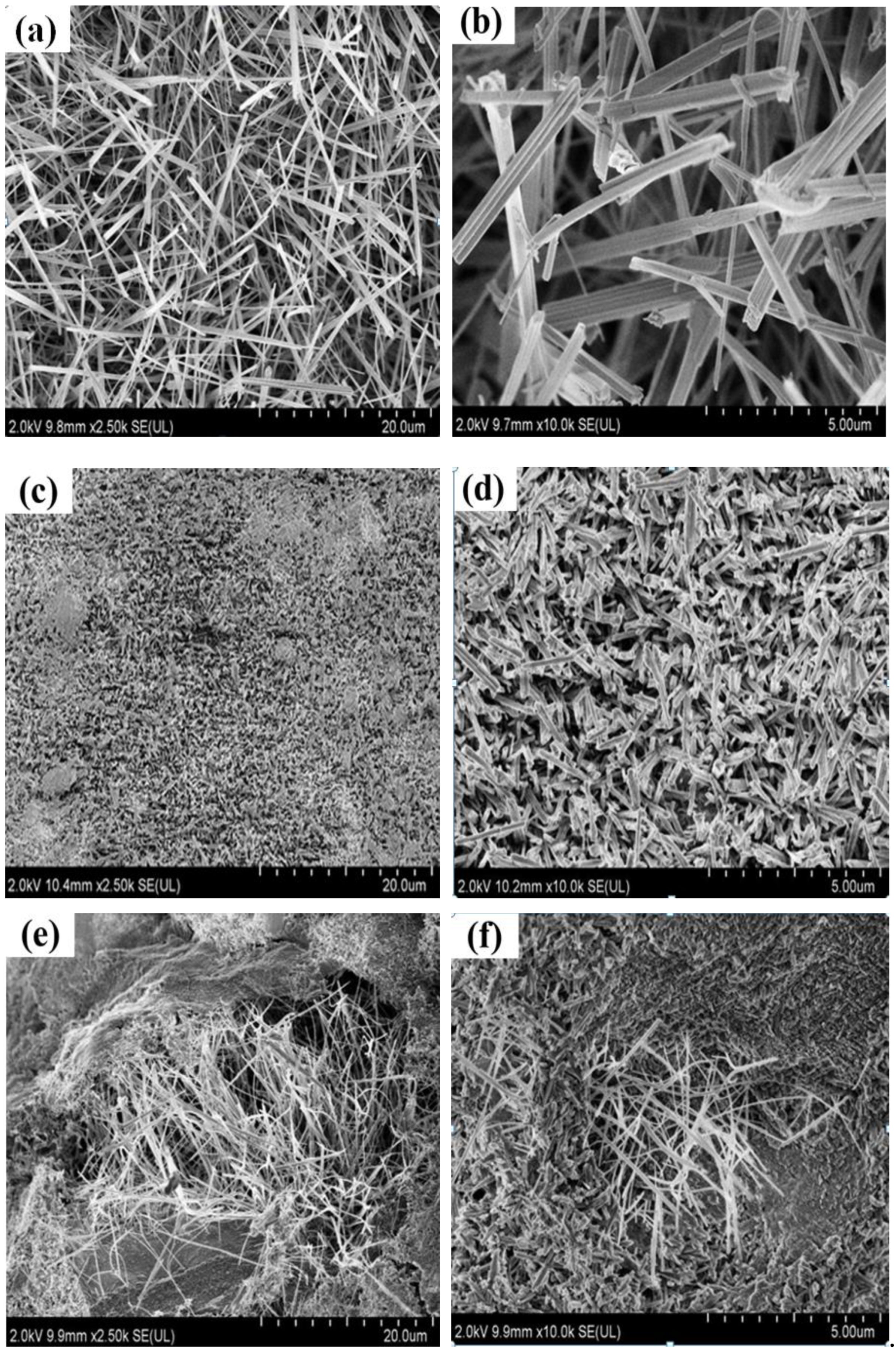
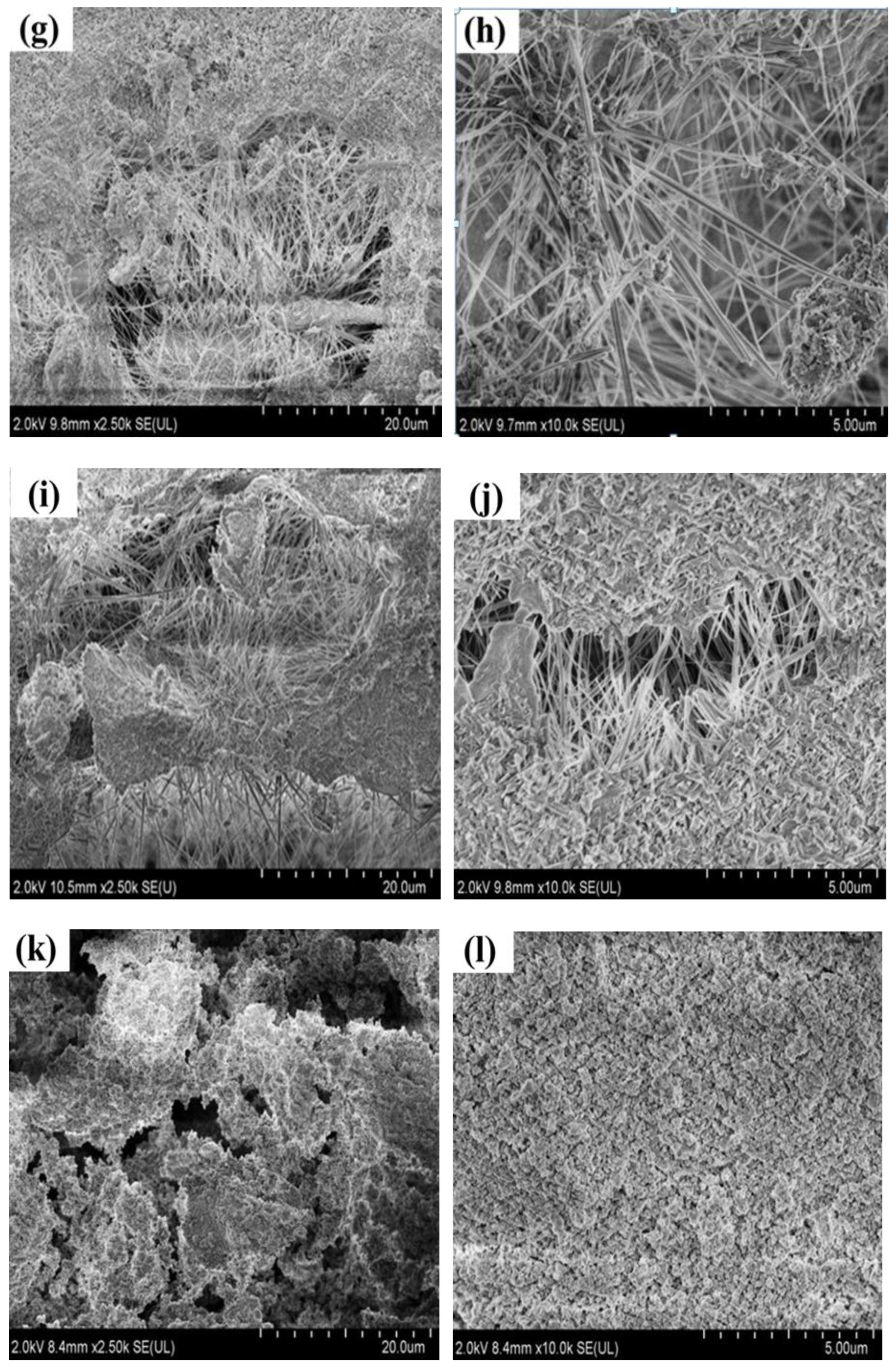
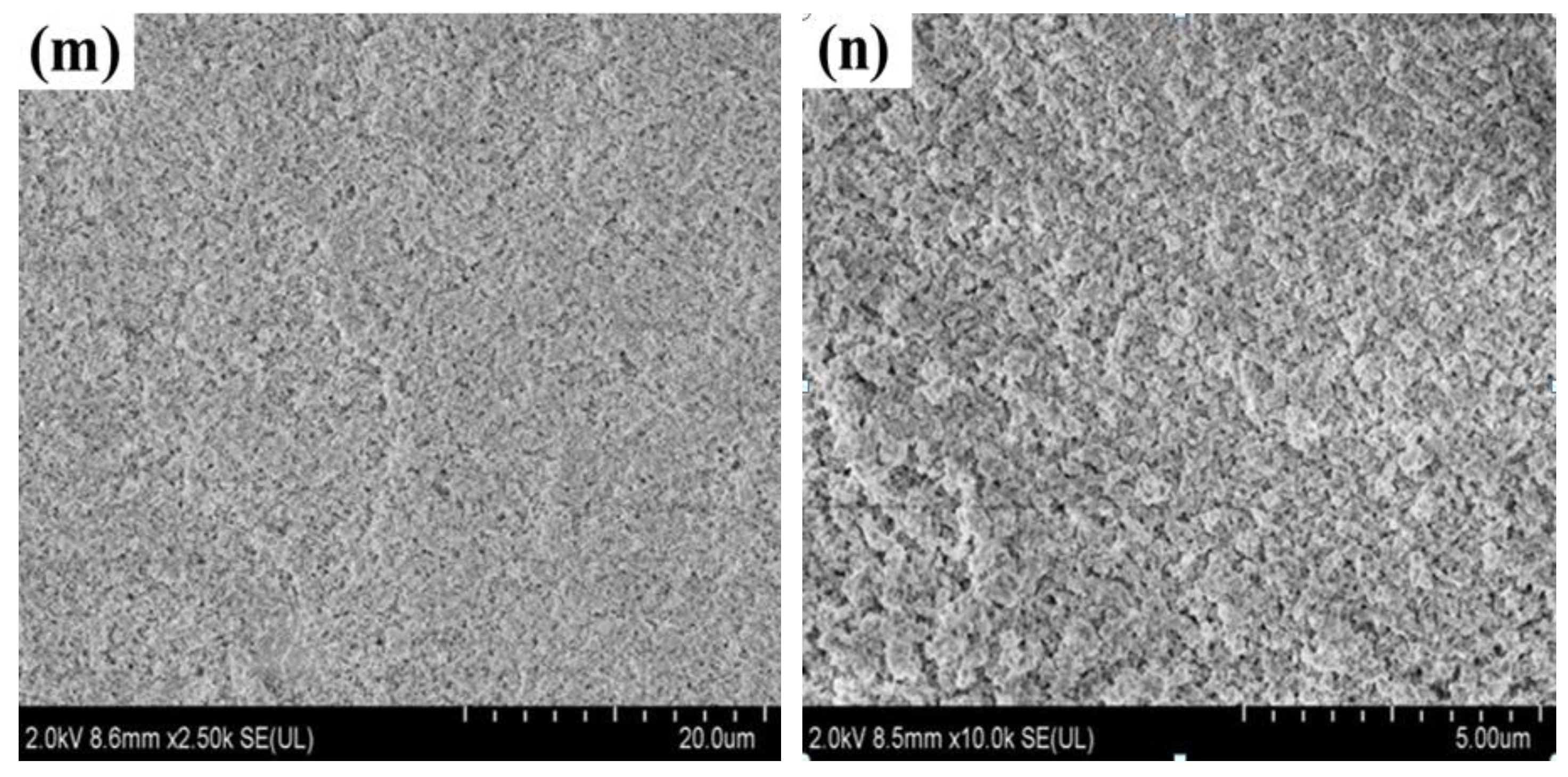
3.2. Effect of Calcination Temperature on Micromorphology of Magnesium Oxychloride Cement
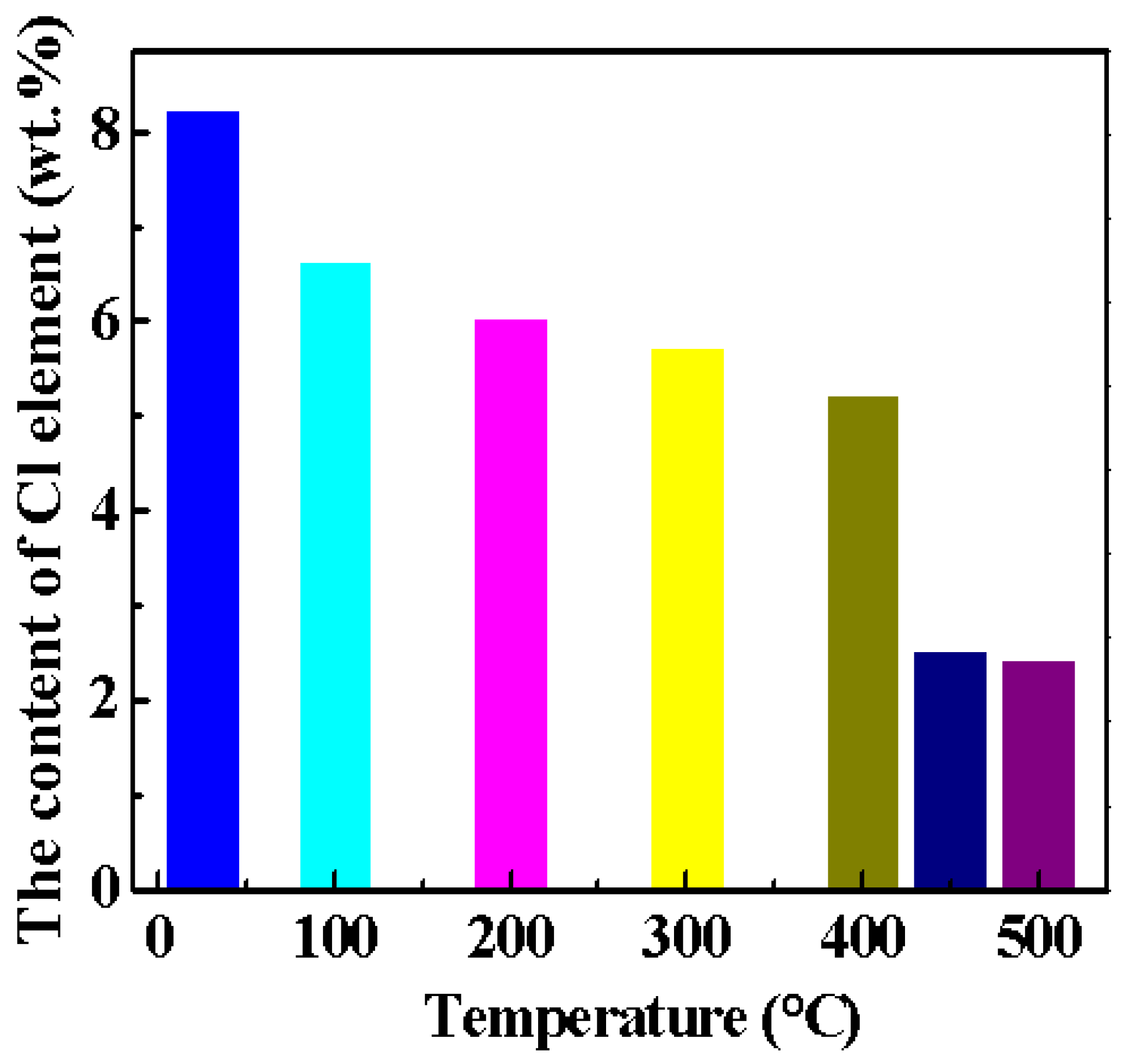
3.3. Effect of Calcination Temperature on Cl element Content in Magnesium Oxychloride Cement
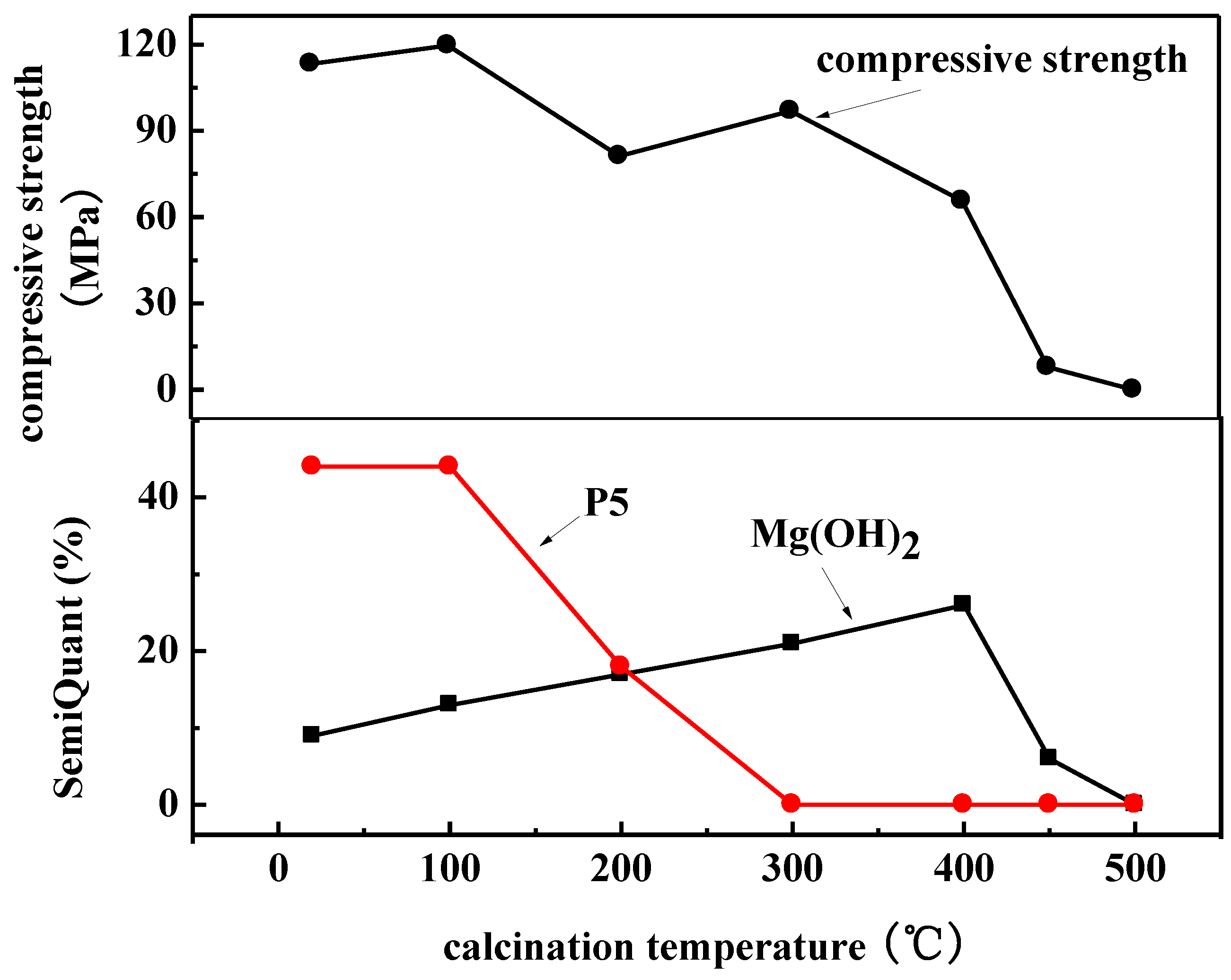
3.4. Effect of Calcination Temperature on Phase Composition of Magnesium Oxychloride Cement
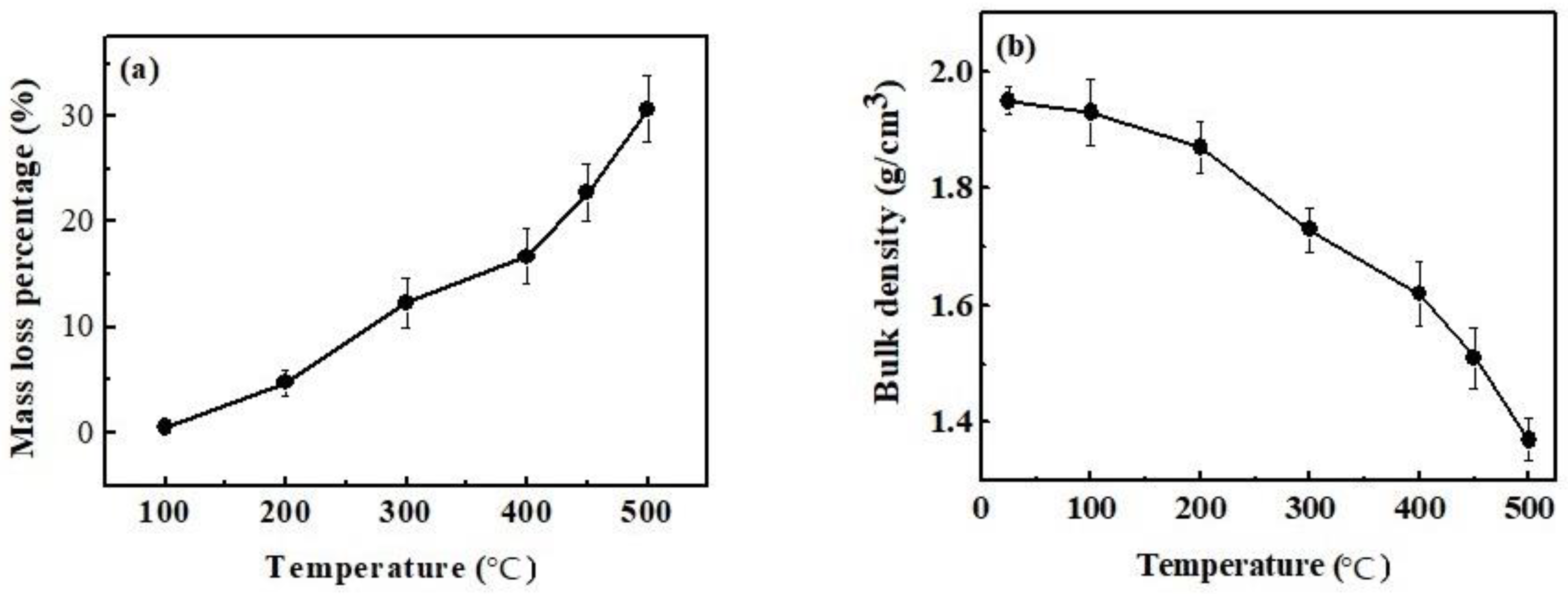
3.5. Effect of Calcination Temperature on Mass Loss Percentage and Bulk Density of Magnesium Oxychloride Cement
3.6. Effect of Calcination Temperature on Mechanical Properties of Magnesium Oxychloride Cement
4. Conclusions
- As the calcination temperature increased, the macroscopic morphology of the magnesium oxychloride cement changes significantly. At the same time, the microscopic morphology changes from being principally needle- or bar-like to fibrous and then powdery, and the phase composition changes from the 5·1·8 phase being dominant to the Mg(OH)2 gel dominating and then to MgO being dominant. The mechanical properties change significantly with these structural and compositional changes.
- At room temperature, magnesium oxychloride cement mainly consists of fine needle-like structures of the 5·1·8 phase with some Mg(OH)2 gel and a trace amount of gel water. The flexural strength is 18.4 MPa and the compressive strength is 113.3 MPa. After calcination at 100 °C, owing to the volatilisation of the gel water, the mass loss percentage is 0.52%, which causes flexural strength to decrease by 57.07%. When calcination is carried out at 400 °C, the magnesium oxychloride cement becomes fibrous. The main chemical component is Mg(OH)2 gel, which can improve the compressive strength of the materials, and hence the compressive strength remains high at 65.7 MPa. When the calcination temperature is 450 °C, a powdery microstructure is observed in the product, which is mainly composed of MgO. In this case, the flexural and compressive strengths are almost entirely lost, and the magnesium oxychloride cement fails.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| mA | Is the mean mass of cement samples after indoor natural curing; |
| mB | is the mean mass of cement samples after calcination. |
References
- Chang, C.G.; Dong, J.M.; Xiao, X.Y.; Zheng, W.X.; Wen, J.; Li, Y.; Huang, Q.; Man, Y. Long-term mechanical properties and micro mechanism of magnesium oxychloride cement concrete. Adv. Cem. Res. 2020, 32, 371–378. [Google Scholar] [CrossRef]
- Li, K.; Wang, Y.S.; Yao, N.; Zhang, A. Recent progress of magnesium oxychloride cement: Manufacture, curing, structure and performance. Constr. Build. Mater. 2020, 255, 1–16. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Zhang, Y.X.; Soe, K.; Pulham, M. Recent development in magnesium oxychloride cement. Struct. Concr. 2018, 19, 1290–1300. [Google Scholar] [CrossRef]
- Xu, B.W.; Ma, H.Y.; Hu, C.L.; Yang, S.Q.; Li, Z.J. Influence of curing regimes on mechanical properties of magnesium oxychloride cement-based composites. Constr. Build. Mater. 2016, 102, 613–619. [Google Scholar] [CrossRef]
- Zhang, X.; Ge, S.J.; Wang, H.N.; Chen, R.Y. Effect of 5-phase seed crystal on the mechanical properties and microstructure of magnesium oxychloride cement. Constr. Build. Mater. 2017, 150, 409–417. [Google Scholar] [CrossRef]
- Ye, Q.Q.; Wang, W.; Zhang, W.; Li, J.Z.; Chen, H. Tuning the phase structure and mechanical performance of magnesium oxychloride cements by curing temperature and H2O/MgCl2 ratio. Constr. Build. Mater. 2018, 179, 413–419. [Google Scholar] [CrossRef]
- Roque, G.; Wambaugh, J.; Rochner, B.; Kitchens, C.L. Kinetic study of the magnesium oxychloride cement cure reaction. J. Mater. Sci. 2017, 52, 7637–7646. [Google Scholar]
- Li, Z.; Chau, C.K. Influence of molar ratios on properties of magnesium oxychloride cement. Cem. Concr. Res. 2007, 37, 866–870. [Google Scholar] [CrossRef]
- Pavlíková, M.; Pivák, A.; Záleská, M.; Jankovský, O.; Reiterman, P.; Pavlík, Z. Magnesium oxychloride cement composites lightened with granulated scrap tires and expanded glass. Materials 2020, 13, 4828. [Google Scholar] [CrossRef]
- China Magnesite Industry Association. Magnesium Cementitious Material and Product Technology; China Building Materials Industry Press: Beijing, China, 2016. [Google Scholar]
- Wang, M.Y.; Xiao, X.Y.; Wang, J.D.; Zhang, Q.Y.; Chang, C.G.; Wang, H.P. Preliminary Research of Dolomite Magnesia Cement. J. Salt Lake Res. 2012, 20, 44–48. [Google Scholar]
- Xiao, X.Y.; Chang, C.G.; Zheng, W.X.; Fang, J.H.; Huang, S.J.; Wen, J.; Dong, J.M.; An, S.X.; Qinghai Institute of Salt Lakes; Chinese Academy of Sciences; et al. Study on Mechanical Property of Pavement Built with Magnesium Oxychloride Cement Concrete. J. Salt Lake Res. 2016, 24, 50–54. [Google Scholar]
- Chen, X.Y.; Zhang, T.T.; Bi, W.L.; Cheeseman, C. Effect of tartaric acid and phosphoric acid on the water resistance of magnesium oxychloride (MOC) cement. Constr. Build. Mater. 2019, 213, 528–536. [Google Scholar] [CrossRef]
- He, P.P.; Poon, C.S.; Tsang, D.C.W. Comparison of glass powder and pulverized fuel ash for improving the water resistance of magnesium oxychloride cement. Cem. Concr. Compos. 2018, 86, 98–109. [Google Scholar] [CrossRef]
- He, P.P.; Poon, C.S.; Tsang, D.C.W. Using incinerated sewage sludge ash to improve the water resistance of magnesium oxychloride cement(MOC). Constr. Build. Mater. 2017, 147, 519–524. [Google Scholar] [CrossRef]
- Huang, T.J.; Yuan, Q.; Deng, D.H. The role of phosphoric acid in improving the strength of magnesium oxychloride cement pastes with large molar ratios of H2O/MgCl2. Cem. Concr. Compos. 2019, 97, 379–386. [Google Scholar] [CrossRef]
- Yu, K.Q.; Guo, Y.Y.; Zhang, Y.X.; Soe, K.; Wayne, D. Magnesium oxychloride cement-based strain-hardening cement itious composite:Mechanical property and water resistance. Constr. Build. Mater. 2020, 261, 1–14. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Zhang, Y.X.; Soe, K.; Hutchison, W.D.; Timmers, H.; Poblete, M.R. Effect of fly ash on mechanical properties of magnesium oxychloride cement under water attack. Struct. Concr. 2020, 21, 1181–1199. [Google Scholar] [CrossRef]
- Wei, L.Z.; Wang, Y.C.; Yu, J.T.; Xiao, J.Z.; Xu, S.L. Feasibility study of strain hardening magnesium oxychloride cement-based composites. Constr. Build. Mater. 2018, 165, 750–760. [Google Scholar] [CrossRef]
- Zuo, Y.F.; Xiao, J.H.; Wang, J.; Liu, W.J.; Li, X.G.; Wu, Y.Q. Preparation and characterization of fire retardant straw/magnesium cement composites with an organic-inorganic network structure. Constr. Build. Mater. 2018, 171, 404–413. [Google Scholar] [CrossRef]
- Wang, Y.C.; Wei, L.Z.; Yu, J.T.; Yu, K.Q. Mechanical properties of high ductile magnesium oxychloride cement-based composites after water soaking. Cem. Concr. Compos. 2019, 97, 248–258. [Google Scholar] [CrossRef]
- Záleská, M.; Pavlíková, M.; Jankovský, O.; Lojka, M.; Pivák, A.; Pavlík, Z. Experimental Analysis of MOC Composite with a Waste-Expanded Polypropylene-Based Aggregate. Materials 2018, 11, 931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.C.; An, L.Y.; Zheng, W.X.; Wen, J.; Dong, J.M.; Yan, F.Y.; Xiao, X.X. Research and Engineering Application of Salt Erosion Resistance of Magnesium Oxychloride Cement Concrete. Materials 2021, 14, 7880. [Google Scholar] [CrossRef] [PubMed]

| Composition | MgCl2 | NaCl | MgSO4 | KCl | CaCl2 | Water Insoluble Matter | H2O |
|---|---|---|---|---|---|---|---|
| Content (wt.%) | 44.90 | 0.13 | 0.06 | 0.01 | 0.03 | 0.27 | 54.6 |
| Composition | MgO | MgCO3 | CaCO3 | f-CaO | Acid Insoluble Matter |
|---|---|---|---|---|---|
| Content (wt.%) | 69.52 | 19.80 | 1.34 | 0.38 | 8.41 |
| Calcination Temperature/°C | Time/h | Flexural Strength/MPa | Standard Deviation (σ) | Compressive Strength/MPa | Standard Deviation (σ) |
|---|---|---|---|---|---|
| 25 | – | 18.4 | 1.6892 | 113.3 | 1.9563 |
| 100 | 2 | 7.9 | 2.3160 | 119.7 | 2.0142 |
| 200 | 2 | 10.9 | 2.4562 | 81.2 | 2.3624 |
| 300 | 2 | 3.1 | 3.1156 | 96.9 | 2.8621 |
| 400 | 2 | – | – | 65.7 | 3.5610 |
| 450 | 2 | – | – | 7.9 | 3.9852 |
| 500 | 2 | – | – | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.; An, L.; Lin, R.; Wen, J.; Dong, J.; Zheng, W.; Yan, F.; Xiao, X. Effect of Calcination Temperature on Mechanical Properties of Magnesium Oxychloride Cement. Materials 2022, 15, 607. https://doi.org/10.3390/ma15020607
Chang C, An L, Lin R, Wen J, Dong J, Zheng W, Yan F, Xiao X. Effect of Calcination Temperature on Mechanical Properties of Magnesium Oxychloride Cement. Materials. 2022; 15(2):607. https://doi.org/10.3390/ma15020607
Chicago/Turabian StyleChang, Chenggong, Lingyun An, Rui Lin, Jing Wen, Jinmei Dong, Weixin Zheng, Fengyun Yan, and Xueying Xiao. 2022. "Effect of Calcination Temperature on Mechanical Properties of Magnesium Oxychloride Cement" Materials 15, no. 2: 607. https://doi.org/10.3390/ma15020607
APA StyleChang, C., An, L., Lin, R., Wen, J., Dong, J., Zheng, W., Yan, F., & Xiao, X. (2022). Effect of Calcination Temperature on Mechanical Properties of Magnesium Oxychloride Cement. Materials, 15(2), 607. https://doi.org/10.3390/ma15020607





