Treatment of Wastewater Containing Nonsteroidal Anti-Inflammatory Drugs Using Activated Carbon Material
Abstract
:1. Introduction
2. Materials and Methods
Chemicals and Reagents
3. Results and Discussion
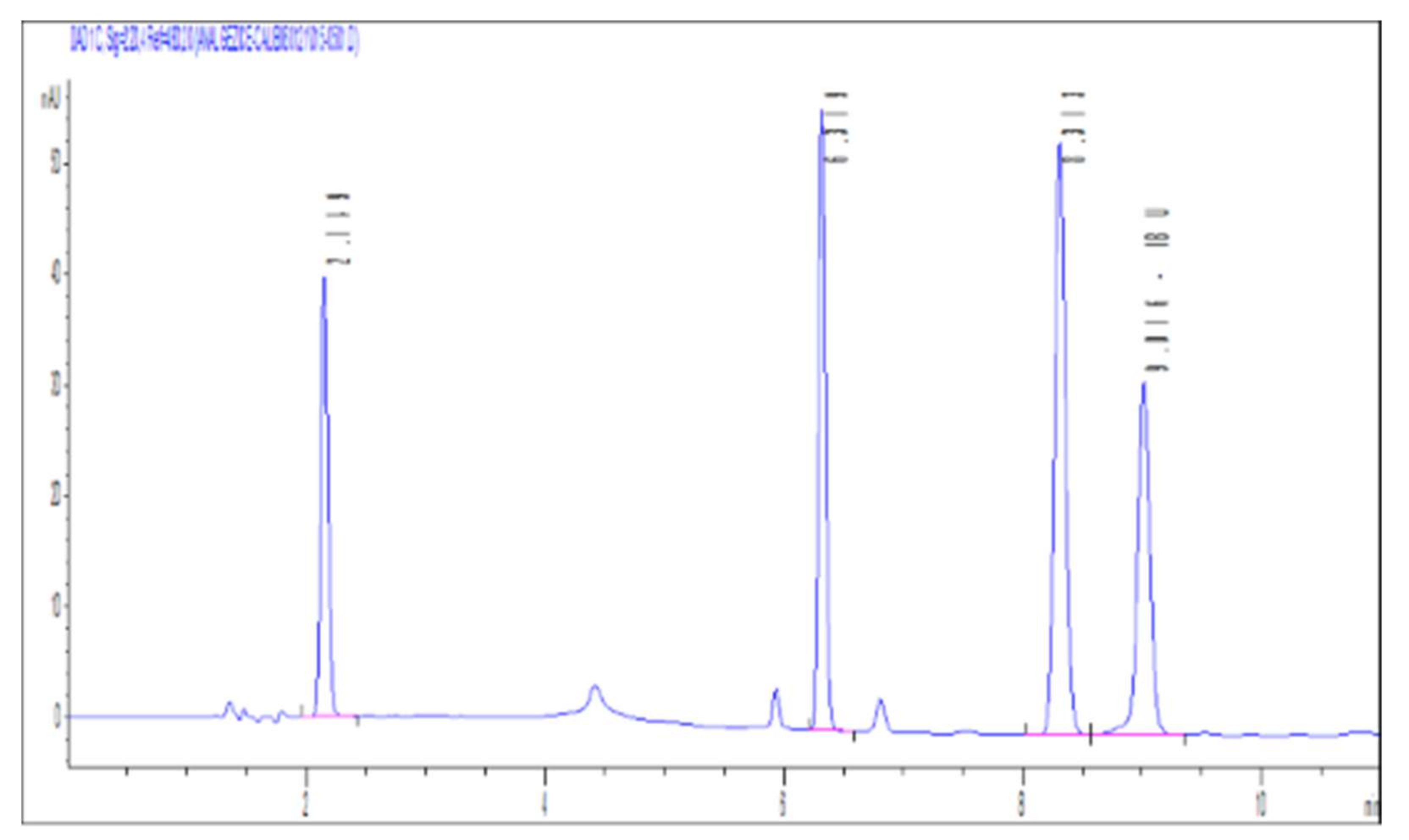
3.1. Analytical Method (HPLC (High Performance Liquid Chromatography))
3.1.1. Linearity
3.1.2. The Accuracy of the Analytical Method
3.1.3. The Precision of the Analytical Method
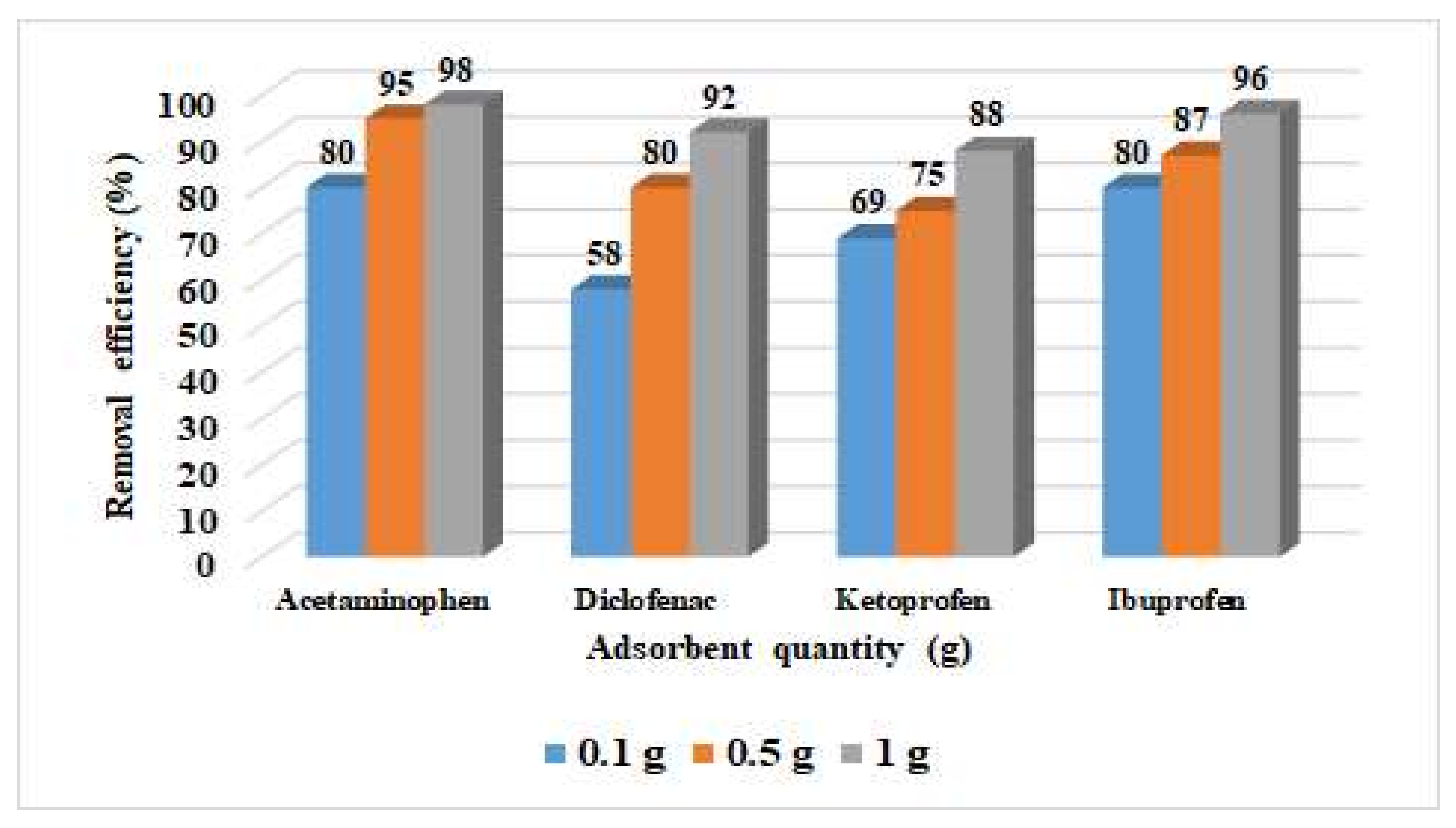
3.2. Adsorption Studies
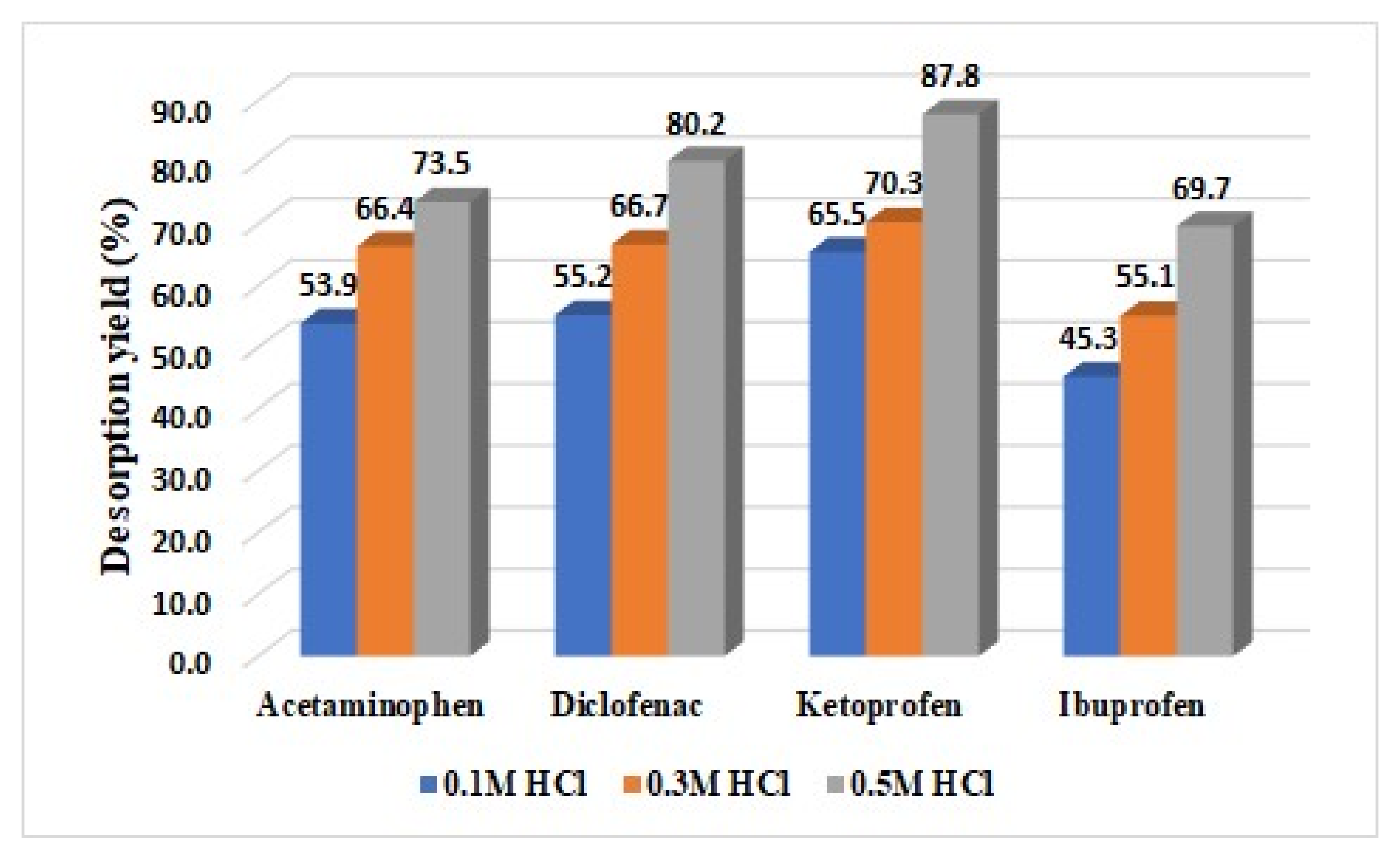
3.3. Desorption Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aitken, R.J.; Koopman, P.; Lewis, S.E.M. Seeds of concern. Nature 2004, 432, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Gaylarde, C.C.; Belinaso, M.L.; Manfio, G.P. Aspectos biológicos e tecnicos da biorremediação de xenobióticos. Biotecnol. Ciência Desensolvimento 2005, 34, 36–43. [Google Scholar]
- Jyoti Sen, D.; Patel, J.G. Logarithmic Partition Coefficient Comparison Study and Molecular Weight of Synthesized Prodrugs of Ibuprofen+Paracetamol, Diclofenac Sodium+ Paracetamol and Ibuprofen+ Diclofenac Sodium. Am. J. Adv. Drug Deliv. 2016, 5, 064–068. [Google Scholar] [CrossRef]
- Li, W.C. Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ. Pollut. 2014, 187, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Der Beek, T.A.; Weber, F.-A.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the environment-Global occurrences and perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Alsbaiee, A.; Smith, B.J.; Xiao, L.L.; Ling, Y.H.; Helbling, D.E.; Dichtel, W.R. Rapid removal of organic micropollutants from water by a porous beta-cyclodextrin polymer. Nature 2016, 529, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Alothman, Z.A.; Wabaidur, S.M. Application of carbon nanotubes in extraction and chromatographic analysis: A review. Arab. J. Chem. 2019, 12, 633–651. [Google Scholar] [CrossRef]
- Covaliu, C.I.; Matei, E.; Georgescu, G.; Malaeru, T.; Biris, S.S. Evaluation of powdered activated carbon performance for wastewater treatment containing organic (C6H6 and C6H5-CH3) and inorganic (Pb+2 and Zn+2) pollutants. Environ. Eng. Manag. J. 2016, 15, 1003–1008. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011; pp. 1–35. [Google Scholar]
- Binoy, S.; Sanchita, M.; Yiu, F.T.; Pawan, K.; Ki-Hyun, K.; Yong, S.O. Designer carbon nanotubes for contaminant removal in water and wastewater: A critical review. Sci. Total Environ. 2018, 612, 561–581. [Google Scholar]
- Jung, C.; Son, A.; Her, N.; Zoh, K.D.; Cho, J.; Yoon, Y. Removal of endocrine disrupting compounds, pharmaceuticals, and personal care products in water using carbon nanotubes: A review. J. Ind. Eng. Chem. 2015, 27, 1–11. [Google Scholar] [CrossRef]
- Pirvu, F.; Covaliu, C.I.; Paraschiv, G.; Paun, I.; Catrina, G.A.C. Preliminary Study of the Removal of Acetaminophen from Wastewater. In Proceedings of the International Symposium “The Environment and the Industry”, E-SIMI 2020, BOOK OF ABSTRACTS, Bucharest, Romania, 24–25 September 2020. [Google Scholar] [CrossRef]
- Aguilar, C.; Montalvo, C.; Ceron, J.; Moctezuma, E. Photocatalytic Degradation of Acetaminophen. Int. J. Environ. Resour. 2011, 5, 1071–1078. [Google Scholar]
- Gotostos, M.J.N.; Chia-Chi, S.; Mark, D.G.; Ming-chun, L. Kinetic study of acetaminophen degradation by visible light photocatalysis. J. Environ. Sci. Health Part A 2014, 49, 892–899. [Google Scholar] [CrossRef] [PubMed]
- WHO. Pharmaceuticals in Drinking-water. In Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Ashton, D.; Hilton, M.; Thomas, K.V. Investigating the environmental transport of human pharmaceuticals to streams in the United Kingdom. Sci. Total Environ. 2004, 333, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.H.; Thomas, K.V. The occurrence of selected pharmaceuticals in wastewater effluent and surface waters of the lower Tyne catchment. Sci. Total Environ. 2006, 356, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Bound, J.P.; Voulvoulis, N. Predicted and measured concentrations for selected pharmaceuticals in UK rivers: Implications for risk assessment. Water Res. 2006, 40, 2885–2892. [Google Scholar] [CrossRef] [PubMed]
- Ternes, T.A.; Janex-Habibi, M.L.; Knacker, T.; Kreuzinger, N.; Siegrist, H. Assessment of technologies for the removal of pharmaceuticals and personal care products in sewage and drinking water to improve the indirect potable water reuse. In POSEIDON Project Detailed Report; EU Contract No. EVK1-CT-2000-00047. 2005. Available online: https://www.semanticscholar.org/paper/Assessment-of-Technologies-for-the-Removal-of-and-Ternes-Kreuzinger/4bee85c2bc6fdc1ee63c8d45fcb7f3e54d25cd4d (accessed on 2 December 2021).
- Monal, D.; Uttiya, D.; Saurav, M.; Suparna, B.; Remanisha, K.; Ratan, B. Adsorption of acetaminophen by using tea waste derived activated carbon. Int. J. Environ. Sci. 2015, 6, 270–281. [Google Scholar]
- Saucier, C.; Karthickeyan, P.; Ranjithkumar, V.; Lima, E.C.; dos Reis, G.S.; de Brum, I.A.S. Efficient removal of amoxicillin and paracetamol from aqueous solutions using magnetic activated carbon. Environ. Sci. Pollut. Res. 2017, 24, 5918–5932. [Google Scholar] [CrossRef] [PubMed]
- Ocampo-Perez, R.; Aguilar-Madera, C.; Díaz-Blancas, V. 3D modeling of overall adsorption rate of acetaminophen on activated carbon pellets. Chem. Eng. J. 2017, 321, 510–520. [Google Scholar] [CrossRef]
- Rodriguez, O.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment technologies for emerging contaminants in water: A review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef] [Green Version]
- ICH Topic Q 2 (R1), Validation of Analytical Procedures: Text and Methodology, European Medicine Agency, June 1995, CPMP/ICH/381/95. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-2-r1-validation-analytical-procedures-text-methodology-step-5_en.pdf (accessed on 2 December 2021).
- Tanase, I.G.; Pana, A.; Radu, G.L.; Buleandra, M. Validation of analytical methods. In Theoretical Principles and Case Studies; Printech: Bucharest, Romania, 2007; pp. 188–203. [Google Scholar]
- Kim, L.; Cernica, G.; Staicu, V.; Popescu, M.; Covaliu, C.I. Removal of Metals from Aqueous Solutions Using Sea Buckthorn Waste from Dietary Supplement Technology. Sustainability 2021, 13, 1441. [Google Scholar] [CrossRef]
| Compound | Effluent (Maximum Concentration, ng/L) | Rivers Waters (Maximum Concentration, ng/L) | References |
|---|---|---|---|
| Diclofenac | 2349 | 568 | [16] |
| 598 | <LOQ | [17] | |
| Ibuprofen | 27,256 | 5044 | [16] |
| 4239 | 2370 | [17] | |
| Acetaminophen | <20 | - | [17] |
| - | 555 | [18] |
| Compound | Austria Maximum Conc. (ng/L) | Finland Maximum Conc. (ng/L) | France Maximum Conc. (ng/L) | Germany Maximum Conc. (ng/L) | Ref. |
|---|---|---|---|---|---|
| Diclofenac | 64 | 40 | 41 | 1200 | [19] |
| Ibuprofen | nd | 65 | 120 | 530 | [19] |
| Drugs | Structural Formula | Molecular Formula | Molecular Mass (g/mol) | Log Kow |
|---|---|---|---|---|
| Ibuprofen (IBF) | 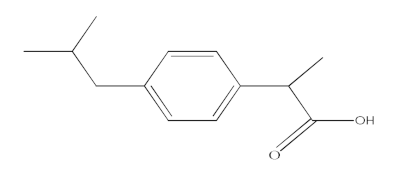 | C13H18O2 | 206.13 | 3.97 |
| Ketoprofen (KTF) | 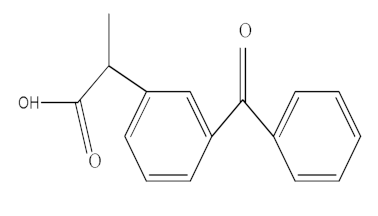 | C16H14O3 | 254.09 | 3.12 |
| Diclofenac (DCF) | 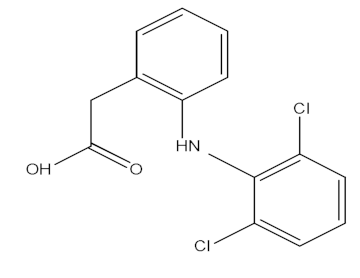 | C14H11Cl2NO2 | 295.02 | 4.51 |
| Acetaminophen (ACF) |  | C8H9NO2 | 151.06 | 0.46 |
| Analyte | Regression Equation | R2 | LOD (µg/L) | LOQ (µg/L) |
|---|---|---|---|---|
| Acetaminophen | y = 38.94x + 11.68 | 0.9991 | 0.10 | 0.30 |
| Ketoprofen | y = 33.13x + 0.26 | 1.0000 | 0.20 | 0.60 |
| Diclofenac | y = 17.03x + 3.89 | 0.9997 | 0.10 | 0.65 |
| Ibuprofen | y = 21.75x + 8.98 | 0.9991 | 0.03 | 0.15 |
| Analyte | Added Concentration (µg/L) | Obtained Concentration (µg/L) | Recovery (%) |
|---|---|---|---|
| Acetaminophen | 10 | 9.599 | 95.99 |
| Diclofenac | 10 | 8.145 | 81.45 |
| Ketoprofen | 10 | 8.392 | 83.92 |
| Ibuprofen | 10 | 9.022 | 90.22 |
| Analyte | Concentration (µg/L) | Repeatability (RSD %) (n = 6) | Reproducibility (RSD %) (n = 12) |
|---|---|---|---|
| Acetaminophen | 5 | 0.15 | 0.29 |
| Ketoprofen | 5 | 0.11 | 0.28 |
| Ibuprofen | 5 | 0.29 | 0.40 |
| Diclofenac | 5 | 0.17 | 0.23 |
| Mathematical Model | Equation | References |
|---|---|---|
| Langmuir | equilibrium parameter (RL) | [21] |
| Freundlich | [21] |
| Compound | Initial Concentration (mg/L) | ||
|---|---|---|---|
| 1 | 5 | 10 | |
| Acetaminophen | 0.6623 | 0.2817 | 0.1639 |
| Diclofenac | 0.0132 | 0.0027 | 0.0013 |
| Ketoprofen | 0.7737 | 0.4061 | 0.2548 |
| Ibuprofen | 0.5000 | 0.1667 | 0.0909 |
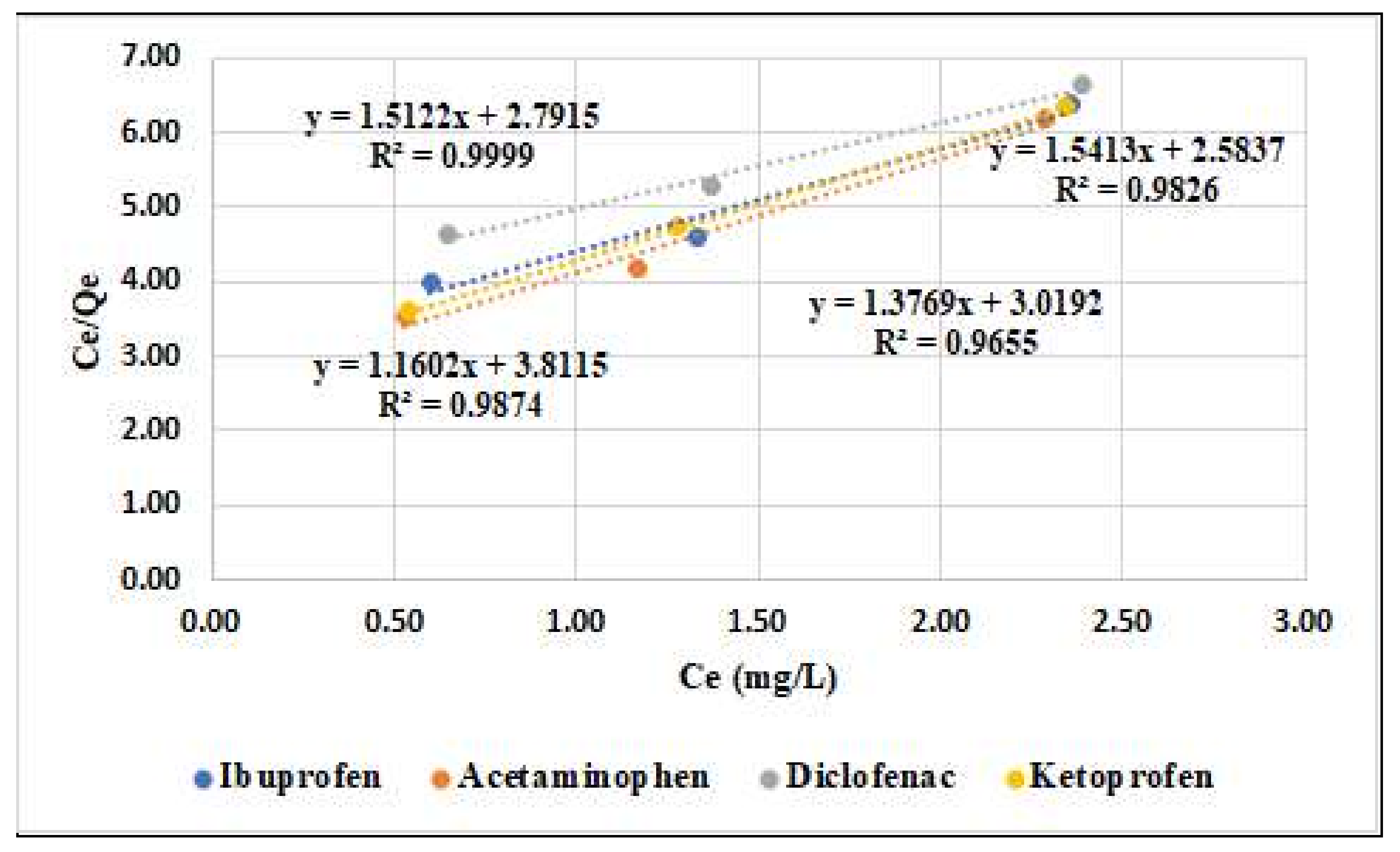
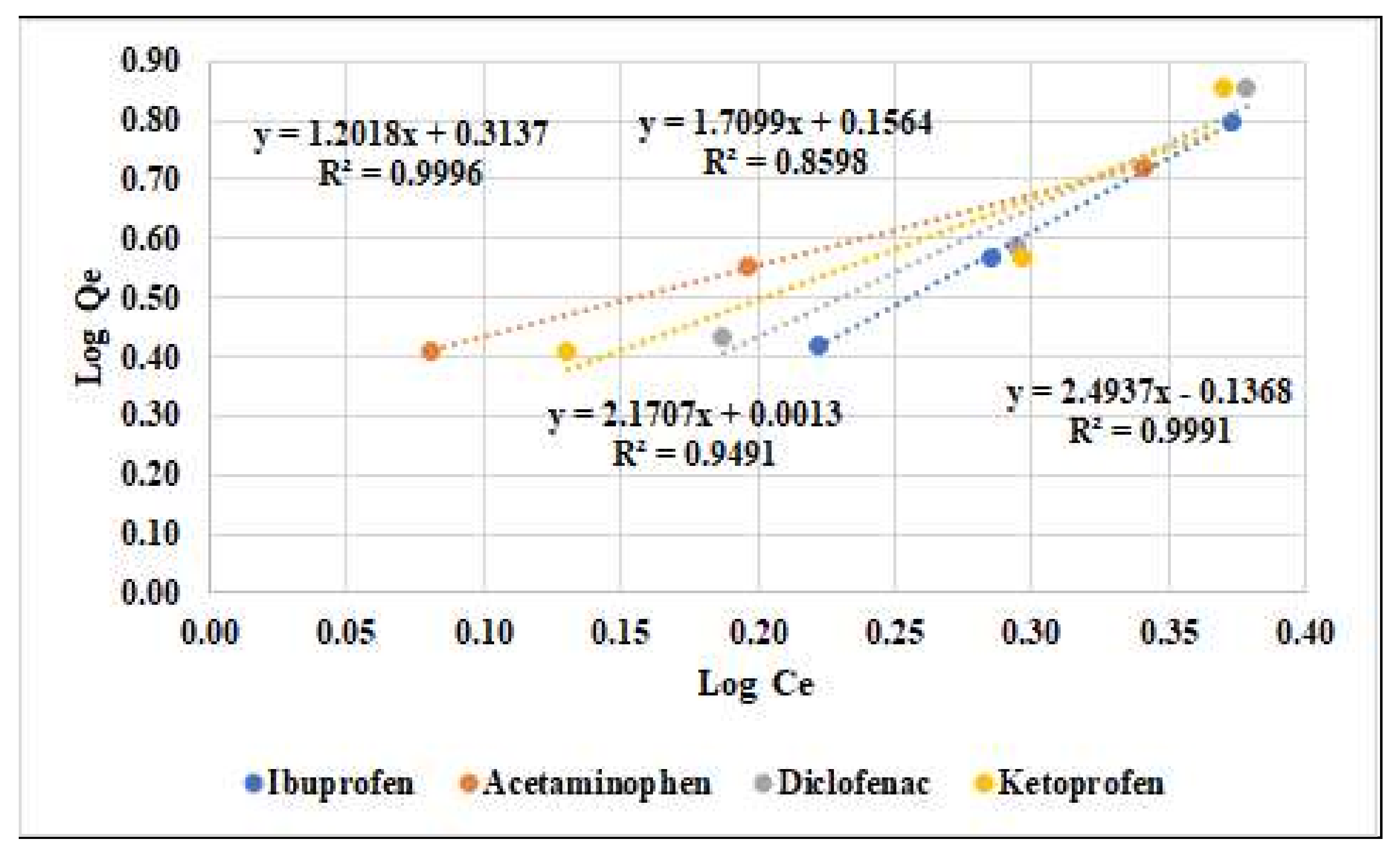
| Adsorbent Material | Langmuir Parameters | Freundlich Parameters | ||||
|---|---|---|---|---|---|---|
| Qmax (mg/g) | KL (L/g) | R2 | KF (m/g) | 1/n | R2 | |
| Ibuprofen | 0.70 | 2.07 | 0.9655 | 2.49 | 0.14 | 0.9991 |
| Acetaminophen | 0.64 | 1.62 | 0.9826 | 1.20 | 0.31 | 0.9996 |
| Diclofenac | 0.85 | 3.23 | 0.9874 | 1.55 | 0.21 | 0.9491 |
| Ketoprofen | 0.66 | 1.85 | 0.9999 | 1.71 | 0.16 | 0.8598 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirvu, F.; Covaliu-Mierlă, C.I.; Paun, I.; Paraschiv, G.; Iancu, V. Treatment of Wastewater Containing Nonsteroidal Anti-Inflammatory Drugs Using Activated Carbon Material. Materials 2022, 15, 559. https://doi.org/10.3390/ma15020559
Pirvu F, Covaliu-Mierlă CI, Paun I, Paraschiv G, Iancu V. Treatment of Wastewater Containing Nonsteroidal Anti-Inflammatory Drugs Using Activated Carbon Material. Materials. 2022; 15(2):559. https://doi.org/10.3390/ma15020559
Chicago/Turabian StylePirvu, Florinela, Cristina Ileana Covaliu-Mierlă, Iuliana Paun, Gigel Paraschiv, and Vasile Iancu. 2022. "Treatment of Wastewater Containing Nonsteroidal Anti-Inflammatory Drugs Using Activated Carbon Material" Materials 15, no. 2: 559. https://doi.org/10.3390/ma15020559
APA StylePirvu, F., Covaliu-Mierlă, C. I., Paun, I., Paraschiv, G., & Iancu, V. (2022). Treatment of Wastewater Containing Nonsteroidal Anti-Inflammatory Drugs Using Activated Carbon Material. Materials, 15(2), 559. https://doi.org/10.3390/ma15020559







