Polypyrrole Polyethylene Composite for Controllable Linear Actuators in Different Organic Electrolytes
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals
2.2. Electropolymerization
2.3. Characterizations
3. Results and Discussion
3.1. Characterization of PPy/DBS and PPy-PEO Films
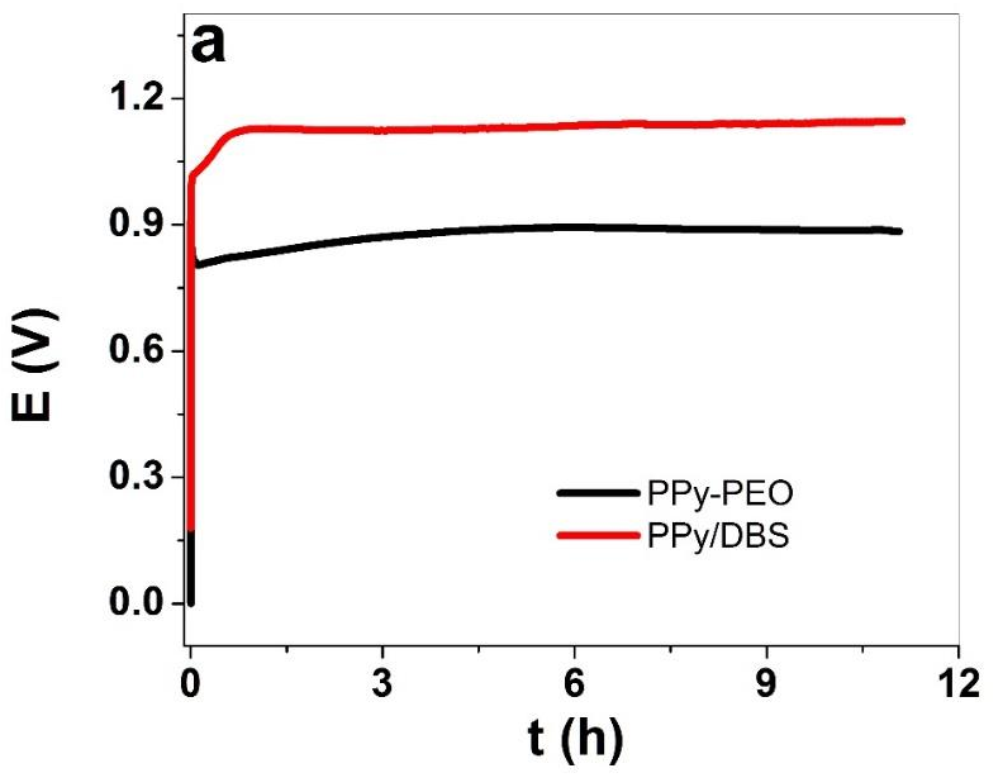
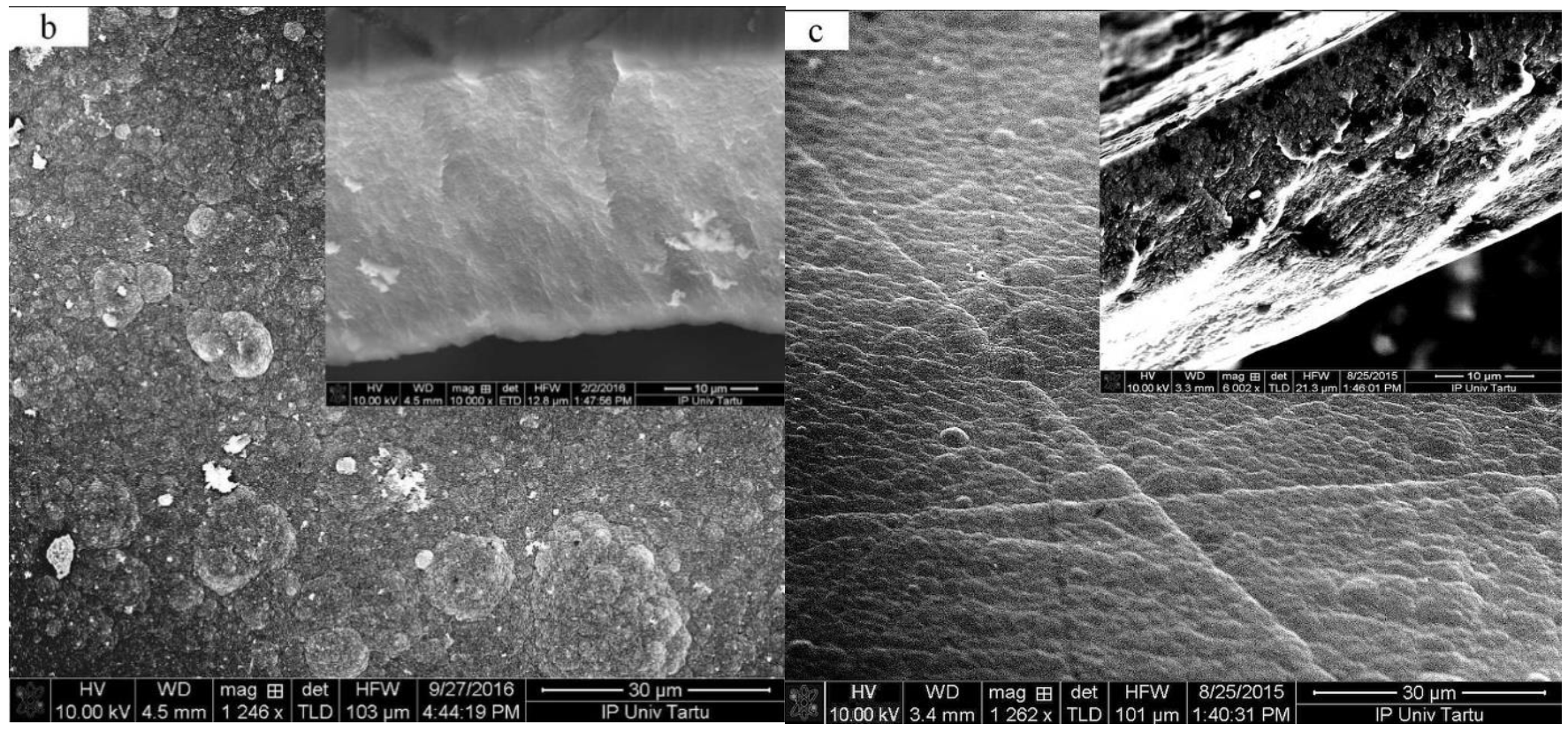
3.1.1. Electropolymerization, SEM Images and Electronic Conductivity
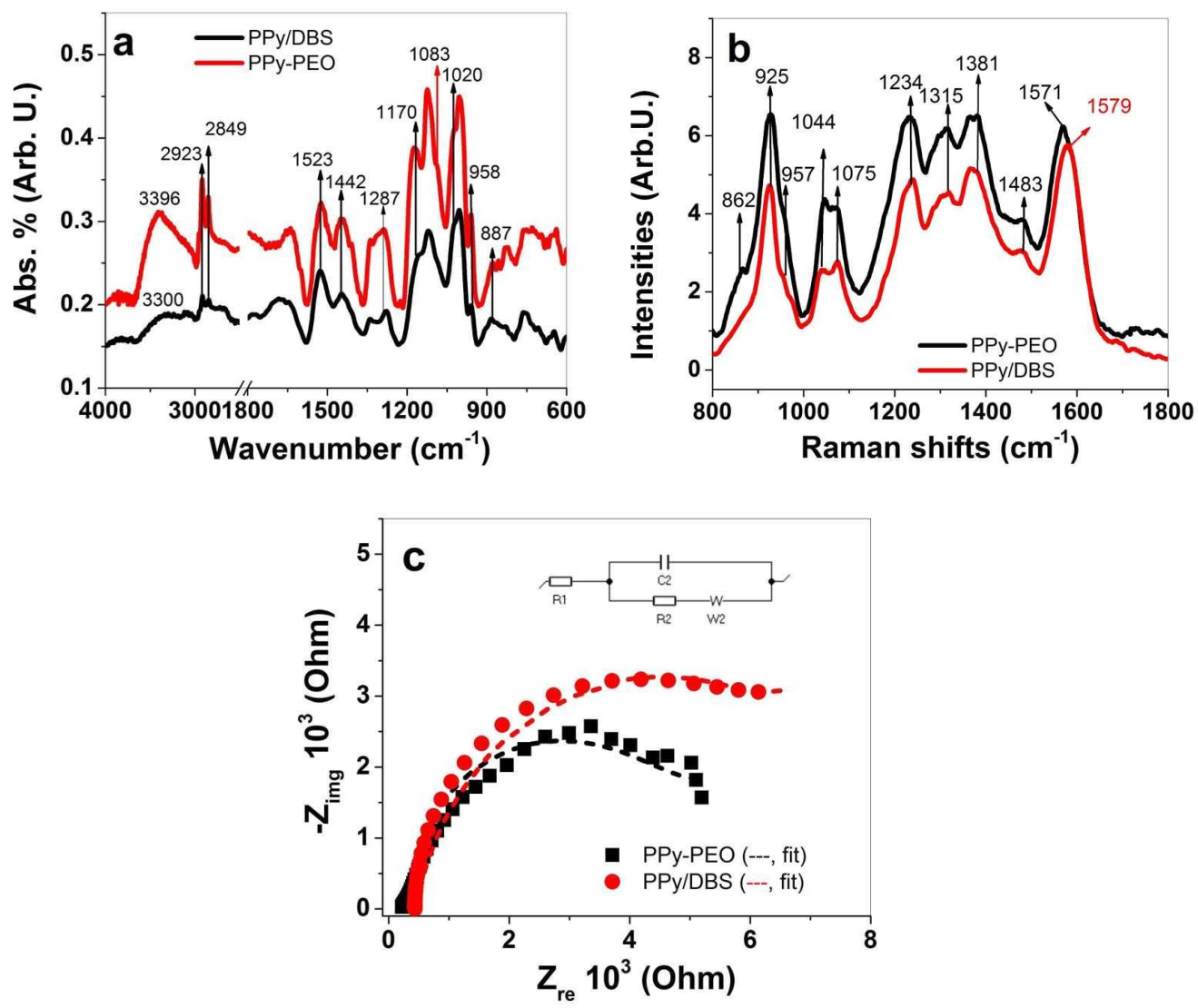
3.1.2. FTIR, Raman and EIS Spectroscopy
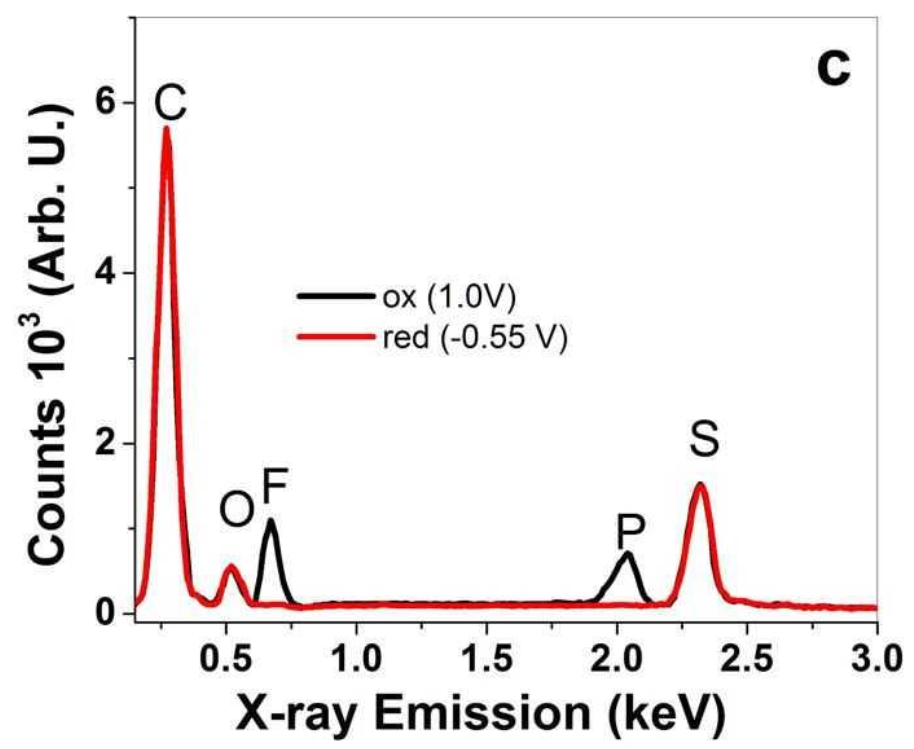
3.1.3. EDX Spectroscopy
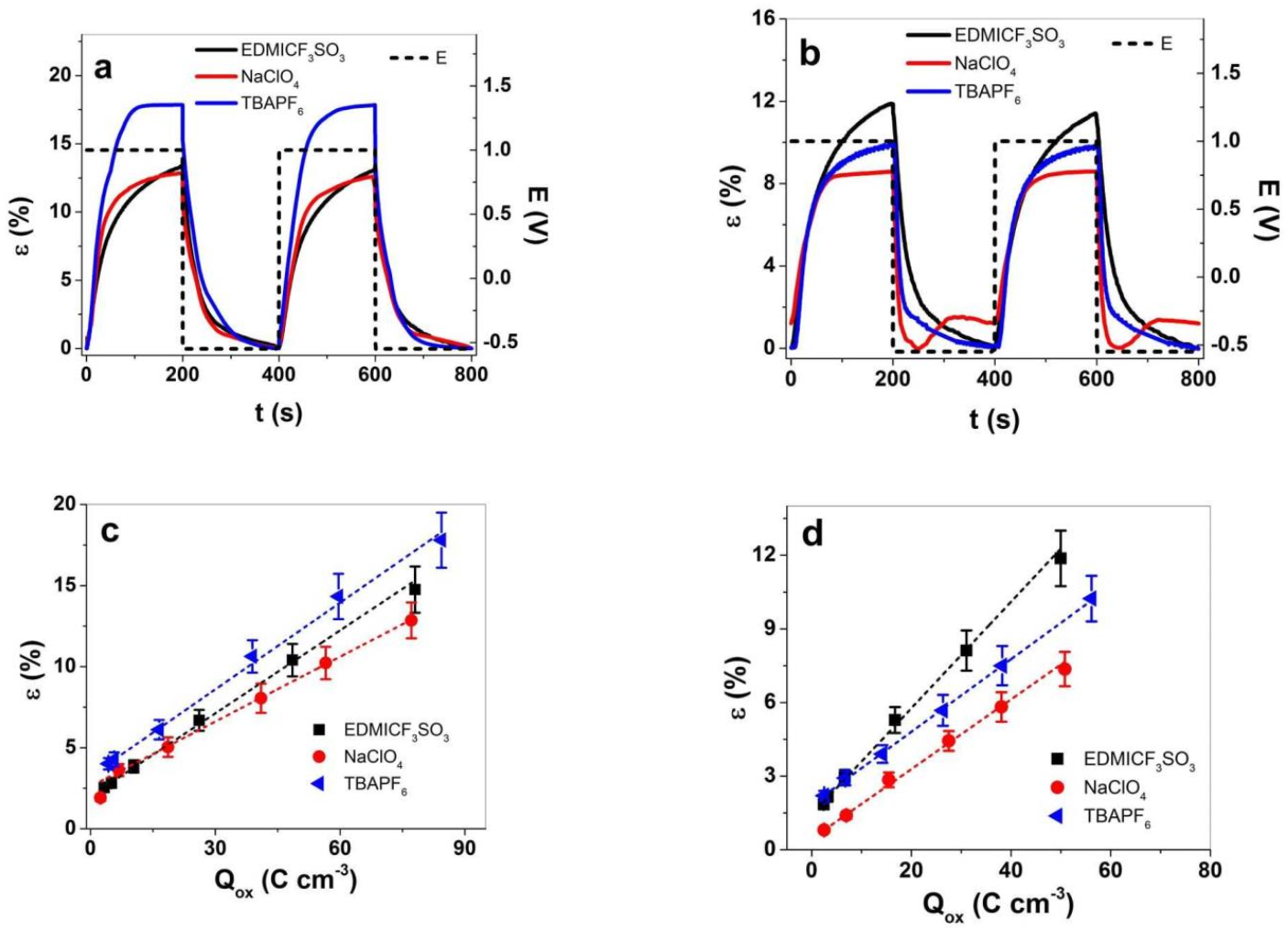
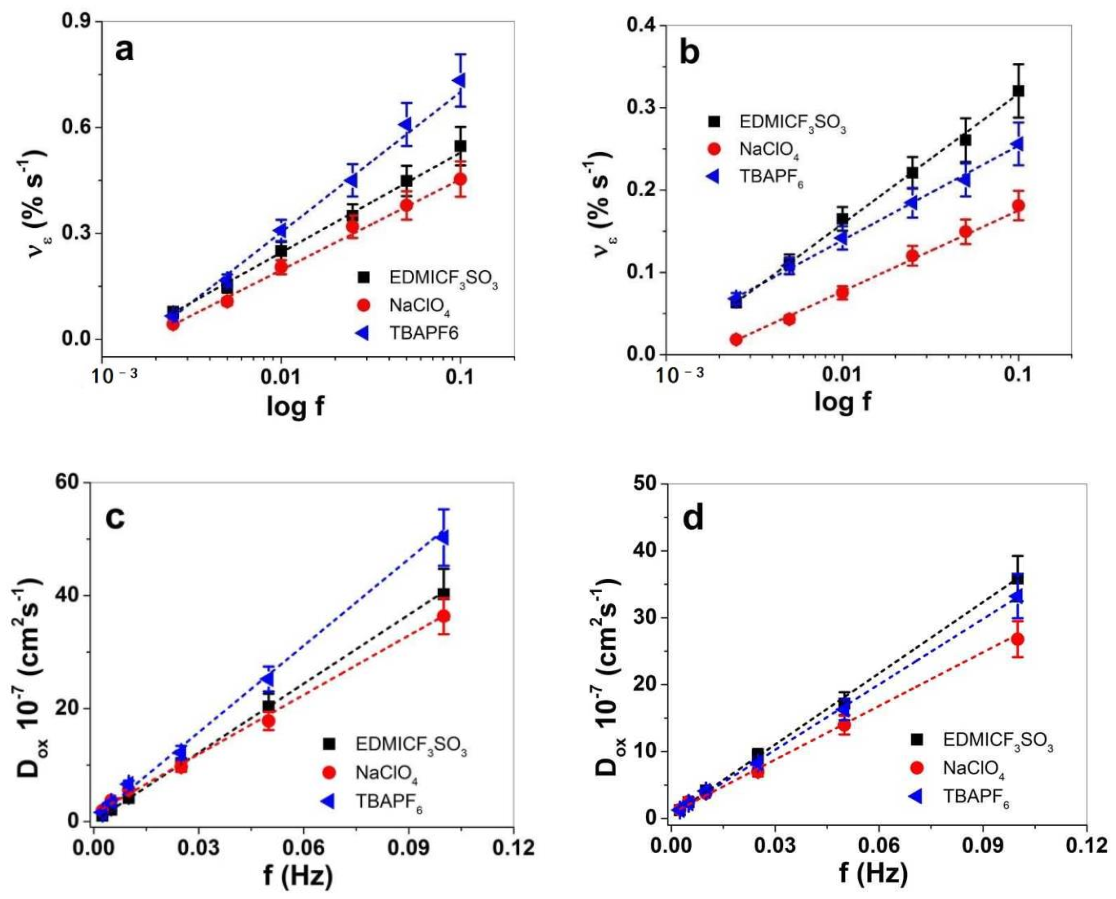
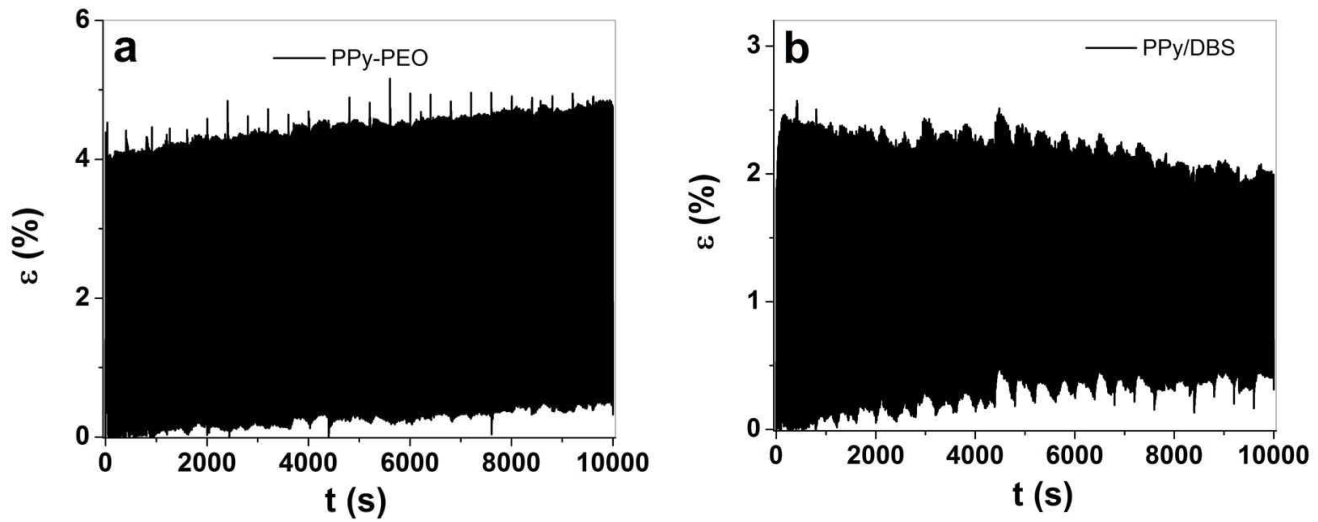
3.2. Linear Actuation Properties of PPy-PEO and PPy/DBS
3.2.1. Cyclic Voltammetry
3.2.2. Square Potential Steps
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jager, E.W.H.; Smela, E.; Ingana, O. Microfabricating Conjugated Polymer Actuators. Science 2000, 290, 1540–1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jager, E.W.H. Microrobots for Micrometer-Size Objects in Aqueous Media: Potential Tools for Single-Cell Manipulation. Science 2000, 288, 2335–2338. [Google Scholar] [CrossRef]
- Smela, E. Conjugated polymer actuators for biomedical applications. Adv. Mater. 2003, 15, 481–494. [Google Scholar] [CrossRef]
- Maziz, A.; Concas, A.; Khaldi, A.; Stålhand, J.; Persson, N.-K.; Jager, E.W.H. Knitting and weaving artificial muscles. Sci. Adv. 2017, 3, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Melling, D.; Martinez, J.G.; Jager, E.W.H. Conjugated Polymer Actuators and Devices: Progress and Opportunities. Adv. Mater. 2019, 31, 1808210. [Google Scholar] [CrossRef]
- Bay, L.; Jacobsen, T.; Skaarup, S.; West, K. Mechanism of actuation in conducting polymers: Osmotic expansion. J. Phys. Chem. B 2001, 105, 8492–8497. [Google Scholar] [CrossRef]
- Khuyen, N.Q.; Zondaka, Z.; Harjo, M.; Torop, J.; Tamm, T.; Kiefer, R. Comparative Analysis of Fluorinated Anions for Polypyrrole Linear Actuator Electrolytes. Polymers 2019, 11, 849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidanapathirana, K.P.; Careem, M.A.; Skaarup, S.; West, K. Ion movement in polypyrrole/dodecylbenzenesulphonate films in aqueous and non-aqueous electrolytes. Solid State Ionics 2002, 154–155, 331–335. [Google Scholar] [CrossRef]
- Kivilo, A.; Zondaka, Z.; Kesküla, A.; Rasti, P.; Tamm, T.; Kiefer, R. Electro-chemo-mechanical deformation properties of polypyrrole/dodecylbenzenesulfate linear actuators in aqueous and organic electrolyte. RSC Adv. 2016, 6, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Sadki, S.; Schottland, P.; Brodiec, N.; Sabouraud, G. The mechanisms of pyrrole electropolymerization. Chem. Soc. Rev. 2000, 29, 283–293. [Google Scholar] [CrossRef]
- Khanh, T.T.; Kesküla, A.; Zondaka, Z.; Harjo, M.; Kivilo, A.; Khorram, M.S.; Tamm, T.; Kiefer, R. Role of polymerization temperature on the performance of polypyrrole/dodecylbenzenesulphonate linear actuators. Synth. Met. 2019, 247, 53–58. [Google Scholar] [CrossRef]
- Ferreira, C.A.; Aeiyach, S.; Delamar, M.; Lacaze, P.C. Electropolymerization of pyrrole on iron electrodes. Influence of solvent and electrolyte on the nature of the deposits. J. Electroanal. Chem. 1990, 284, 351–369. [Google Scholar] [CrossRef]
- Martinez, J.G.; Otero, T.F.; Jager, E.W.H. Effect of the electrolyte concentration and substrate on conducting polymer actuators. Langmuir 2014, 30, 3894–3904. [Google Scholar] [CrossRef] [PubMed]
- Kesküla, A.; Peikolainen, A.L.; Kiefer, R.; Tamm, T. Consistent response from conducting polymer actuators: Potential window and embedded charges to avoid mixed ion transport. Synth. Met. 2020, 268, 116502. [Google Scholar] [CrossRef]
- Kiefer, R.; Chu, S.Y.; Kilmartin, P.A.; Bowmaker, G.A.; Cooney, R.P.; Travas-Sejdic, J. Mixed-ion linear actuation behaviour of polypyrrole. Electrochim. Acta 2007, 52, 2386–2391. [Google Scholar] [CrossRef]
- Kiefer, R.; Weis, D.G.; Aabloo, A.; Urban, G.; Heinze, J. Dependence of polypyrrole bilayer deflection upon polymerization potential. Synth. Met. 2013, 172, 37–43. [Google Scholar] [CrossRef]
- Kiefer, R.; Bowmaker, G.A.; Cooney, R.P.; Kilmartin, P.A.; Travas-Sejdic, J. Cation driven actuation for free standing PEDOT films prepared from propylene carbonate electrolytes containing TBACF3SO3. Electrochim. Acta 2008, 53, 2593–2599. [Google Scholar] [CrossRef]
- Alici, G.; Punning, A.; Shea, H.R. Enhancement of actuation ability of ionic-type conducting polymer actuators using metal ion implantation. Sens. Actuators B Chem. 2011, 157, 72–84. [Google Scholar] [CrossRef]
- Quek, G.; Roehrich, B.; Su, Y.; Sepunaru, L.; Bazan, G.C. Conjugated Polyelectrolytes: Underexplored Materials for Pseudocapacitive Energy Storage. Adv. Mater. 2021, 2104206. [Google Scholar] [CrossRef] [PubMed]
- Luangaramvej, P.; Poungsripong, P.; Dubas, S.T. Synthesis of Janus polyaniline–polyelectrolyte complex membrane via in situ confined polymerization. Polym. Int. 2022, 71, 139–145. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Z.; Zhao, S.; Westover, A.S.; Belharouak, I.; Cao, P.F. Single-Ion Conducting Polymer Electrolytes for Solid-State Lithium–Metal Batteries: Design, Performance, and Challenges. Adv. Energy Mater. 2021, 11, 2003836. [Google Scholar] [CrossRef]
- Fannir, A.; Plesse, C.; Nguyen, G.T.M.; Vidal, F. Electro-interpenetration as tool for high strain trilayer conducting polymer actuator. Smart Mater. Struct. 2021, 30. [Google Scholar] [CrossRef]
- Khadka, R.; Zhang, P.; Nguyen, N.T.; Tamm, T.; Travas-Sejdic, J.; Otero, T.F.; Kiefer, R. Role of polyethylene oxide content in polypyrrole linear actuators. Mater. Today Commun. 2020, 23, 100908. [Google Scholar] [CrossRef]
- Tran, C.B.; Zondaka, Z.; Le, Q.B.; Velmurugan, B.K.; Kiefer, R. Polypyrrole with phosphor tungsten acid and carbide-derived carbon: Change of solvent in electropolymerization and linear actuation. Materials 2021, 14, 6302. [Google Scholar] [CrossRef]
- Weis, D.G.; Kiefer, R.; Zondaka, Z.; Tamm, T.; Urban, G. Polypyrrole and poly (3, 4-ethylenedioxythiophene) on silicon cantilever: Role of formation potential in bending displacement. Synth. Met. 2021, 271, 116653. [Google Scholar] [CrossRef]
- Harjo, M.; Tamm, T.; Anbarjafari, G.; Kiefer, R. Hardware and Software Development for Isotonic Strain and Isometric Stress Measurements of Linear Ionic Actuators. Polymers 2019, 11, 1054. [Google Scholar] [CrossRef] [Green Version]
- Suárez, I.J.; Otero, T.F.; Márquez, M. Diffusion coefficients in swelling polypyrrole: ESCR and cottrell models. J. Phys. Chem. B 2005, 109, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Otero, T.F.; Boyano, I. Comparative study of conducting polymers by the ESCR model. J. Phys. Chem. B 2003, 107, 6730–6738. [Google Scholar] [CrossRef]
- Otero, T.F.; Martinez, J.G. Activation energy for polypyrrole oxidation: Film thickness influence. J. Solid State Electrochem. 2011, 15, 1169–1178. [Google Scholar] [CrossRef]
- Kiefer, R.; Nguyen, N.T.; Le, Q.B.; Anbarjafari, G.; Tamm, T. Antagonist concepts of polypyrrole actuators: Bending hybrid actuator and mirrored trilayer linear actuator. Polymers 2021, 13, 861. [Google Scholar] [CrossRef]
- Kiefer, R.; Martinez, J.G.; Kesküla, A.; Anbarjafari, G.; Aabloo, A.; Otero, T.F. Polymeric actuators: Solvents tune reaction-driven cation to reaction-driven anion actuation. Sens. Actuators B Chem. 2016, 233, 461–469. [Google Scholar] [CrossRef]
- Liu, Y.C. Method of evaluating the ionic conductance of polypyrrole films and improvement of ionic conductance of polyethylene oxide-incorporated polypyrrole composite. Mater. Chem. Phys. 2003, 77, 791–795. [Google Scholar] [CrossRef]
- Zhou, M.; Pagels, M.; Geschke, B.; Heinze, J. Electropolymerization of pyrrole and electrochemical study of polypyrrole. 5. Controlled electrochemical synthesis and solid-state transition of well-defined polypyrrole variants. J. Phys. Chem. B 2002, 106, 10065–10073. [Google Scholar] [CrossRef]
- Theron, S.A.; Zussman, E.; Yarin, A.L. Experimental investigation of the governing parameters in the electrospinning of polyethylene oxide solution. Polymer (Guildf). 2004, 45, 2017–2030. [Google Scholar] [CrossRef]
- Gade, V.K.; Shirale, D.J.; Gaikwad, P.D.; Kakde, P.; Savale, P.A.; Kharat, H.J. Synthesis and Characterization of Ppy-PVS, Ppy-pTS, and Ppy-. Int. J. Polym. Mater. Polym. Biomater. 2007, 56, 37–41. [Google Scholar] [CrossRef]
- Omastová, M.; Trchová, M.; Kovářová, J.; Stejskal, J. Synthesis and structural study of polypyrroles prepared in the presence of surfactants. Synth. Met. 2003, 138, 447–455. [Google Scholar] [CrossRef]
- Li, J.; Cui, L.; Zhang, X. Preparation and electrochemistry of one-dimensional nanostructured MnO2/PPy composite for electrochemical capacitor. Appl. Surf. Sci. 2010, 256, 4339–4343. [Google Scholar] [CrossRef]
- Zhang, M.; Nautiyal, A.; Du, H.; Li, J.; Liu, Z.; Zhang, X.; Wang, R. Polypyrrole film based flexible supercapacitor: Mechanistic insight into influence of acid dopants on electrochemical performance. Electrochim. Acta 2020, 357, 136877. [Google Scholar] [CrossRef]
- Lee, G.J.; Lee, S.H.; Ahn, K.S.; Kim, K.H. Synthesis and characterization of soluble polypyrrole with improved electrical conductivity. J. Appl. Polym. Sci. 2002, 84, 2583–2590. [Google Scholar] [CrossRef]
- Calabrò, E.; Magazù, S. Demicellization of polyethylene oxide in water solution under static magnetic field exposure studied by FTIR spectroscopy. Adv. Phys. Chem. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.C.; Hwang, B.J. Enhancement of conductivity stability of polypyrrole films modified by valence copper and polyethylene oxide in an oxygen atmosphere. Thin Solid Films 2000, 360, 1–9. [Google Scholar] [CrossRef]
- Han, G.; Shi, G. Electrochemical actuator based on single-layer polypyrrole film. Sens. Actuators B Chem. 2006, 113, 259–264. [Google Scholar] [CrossRef]
- Gupta, S. Hydrogen bubble-assisted syntheses of polypyrrole micro/nanostructures using electrochemistry: Structural and physical property characterization. J. Raman Spectrosc. 2008, 39, 1343–1355. [Google Scholar] [CrossRef]
- Maxfield, J.; Shepherd, I.W. Conformation of poly(ethylene oxide) in the solid state, melt and solution measured by Raman scattering. Polymer (Guildf). 1975, 16, 505–509. [Google Scholar] [CrossRef]
- Khadka, R.; Aydemir, N.; Kesküla, A.; Tamm, T.; Travas-Sejdic, J.; Kiefer, R. Enhancement of polypyrrole linear actuation with poly(ethylene oxide). Synth. Met. 2017, 232, 1–7. [Google Scholar] [CrossRef]
- Valero, L.; Otero, T.F.; Martinez, J.G.; Martínez, J.G. Exchanged Cations and Water during Reactions in Polypyrrole Macroions from Artificial Muscles. ChemPhysChem 2014, 15, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Baughman, R.H. Conducting polymer artificial muscles. Synth. Met. 1996, 78, 339–353. [Google Scholar] [CrossRef]
- Otero, T.F.; Cortes, M.T. Artificial muscle: Movement and position control. Chem. Commun. 2004, 4, 284–285. [Google Scholar] [CrossRef]
- Otero, T.F.; Martinez, J.G. Ionic exchanges, structural movements and driven reactions in conducting polymers from bending artificial muscles. Sens. Actuators B Chem. 2014, 199, 27–30. [Google Scholar] [CrossRef]
- Chaban, V. Solvation of the fluorine containing anions and their lithium salts in propylene carbonate and dimethoxyethane. J. Mol. Model. 2015, 21, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Barthel, J.; Buchner, R.; Wismeth, E. FTIR spectroscopy of ion solvation of LiClO4 and LiSCN in acetonitrile, benzonitrile, and propylene carbonate. J. Solut. Chem. 2000, 29, 937–954. [Google Scholar] [CrossRef]
- West, B.J.; Otero, T.F.; Shapiro, B.; Smela, E. Chronoamperometric study of conformational relaxation in PPy(DBS). J. Phys. Chem. B 2009, 113, 1277–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otero, T.F.; Grande, H.; Rodrîguez, J. Conformational relaxation during polypyrrole oxidation: From experiment to theory. Electrochim. Acta 1996, 41, 1863–1869. [Google Scholar] [CrossRef]
- Valero, L.; Martinez, J.G.; Otero, T.F. Creeping and structural effects in Faradaic artificial muscles. J. Solid State Electrochem. 2015, 19, 2683–2689. [Google Scholar] [CrossRef] [Green Version]
- Madden, J.D.; Rinderknecht, D.; Anquetil, P.A.; Hunter, I.W. Creep and cycle life in polypyrrole actuators. Sens. Actuators A Phys. 2007, 133, 210–217. [Google Scholar] [CrossRef]



| PPy Films | Pristine (S cm−1) | EDMICF3SO3-PC (S cm−1) | NaClO4-PC (S cm−1) | TBAPF6-PC (S cm−1) |
|---|---|---|---|---|
| PPy/DBS. | 2.1 ± 0.2 | 6.4 ± 0.7 | 4.6 ± 0.4 | 6.2 ± 0.6 |
| PPy-PEO | 3.9 ± 0.8 | 11.4 ± 0.9 | 6.3 ± 0.5 | 13.1 ± 1.2 |
| Electrolytes in PC | ε (%) | νε (% s−1) | Dox 10−7 (cm2 s−1) | |||
|---|---|---|---|---|---|---|
| PPy-PEO | PPy/DBS | PPy-PEO | PPy/DBS | PPy-PEO | PPy/DBS | |
| EDMI-CF3SO3 | 2.54 ± 0.22 | 1.85 ± 0.15 | 0.55 ± 0.05 | 0.32 ± 0.03 | 40 ± 4.5 | 35 ± 3.4 |
| NaClO4 | 1.91 ± 0.20 | 0.8 ± 0.07 | 0.45 ± 0.04 | 0.18 ± 0.02 | 36 ± 3.5 | 27 ± 2.4 |
| TBAPF6 | 4.0 ± 0.35 | 2.2 ± 0.2 | 0.73 ± 0.07 | 0.26 ± 0.03 | 50 ± 4.8 | 33 ± 3.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khuyen, N.Q.; Nguyen, N.T.; Kiefer, R. Polypyrrole Polyethylene Composite for Controllable Linear Actuators in Different Organic Electrolytes. Materials 2022, 15, 540. https://doi.org/10.3390/ma15020540
Khuyen NQ, Nguyen NT, Kiefer R. Polypyrrole Polyethylene Composite for Controllable Linear Actuators in Different Organic Electrolytes. Materials. 2022; 15(2):540. https://doi.org/10.3390/ma15020540
Chicago/Turabian StyleKhuyen, Nguyen Quang, Ngoc Tuan Nguyen, and Rudolf Kiefer. 2022. "Polypyrrole Polyethylene Composite for Controllable Linear Actuators in Different Organic Electrolytes" Materials 15, no. 2: 540. https://doi.org/10.3390/ma15020540
APA StyleKhuyen, N. Q., Nguyen, N. T., & Kiefer, R. (2022). Polypyrrole Polyethylene Composite for Controllable Linear Actuators in Different Organic Electrolytes. Materials, 15(2), 540. https://doi.org/10.3390/ma15020540






