Electrocaloric Effect in Different Oriented BaZr0.15Ti0.85O3 Single Crystals
Abstract
1. Introduction
2. Theoretical Approach
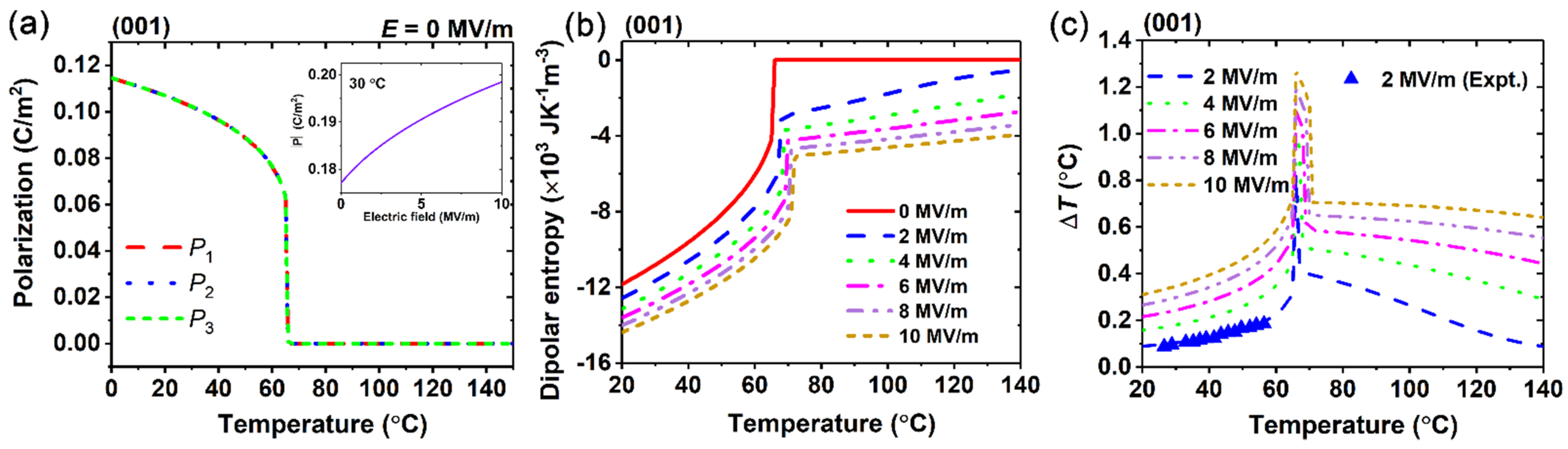
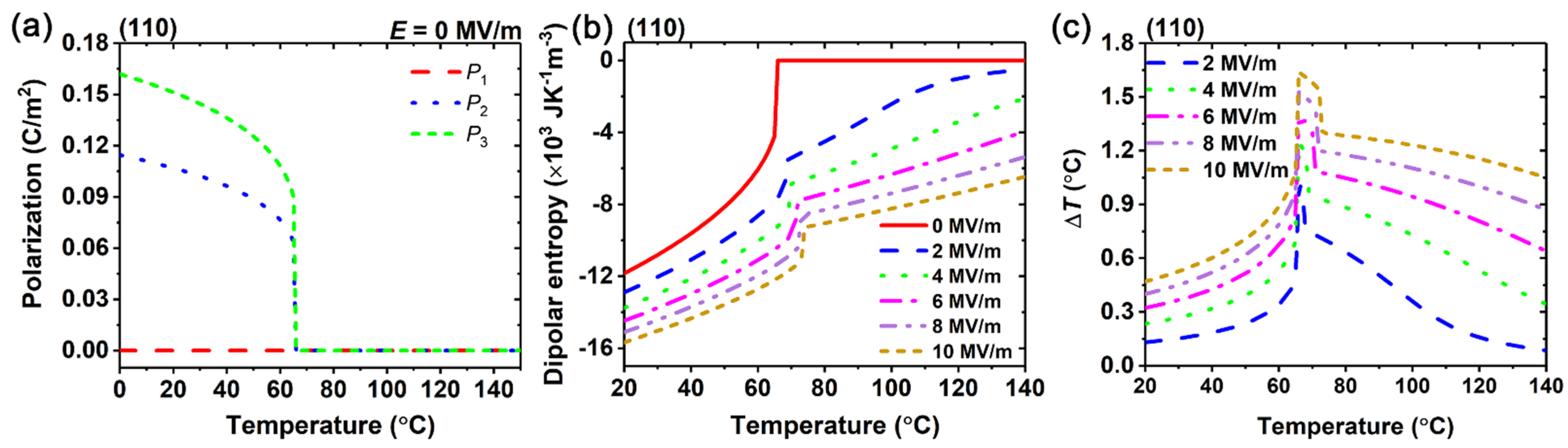
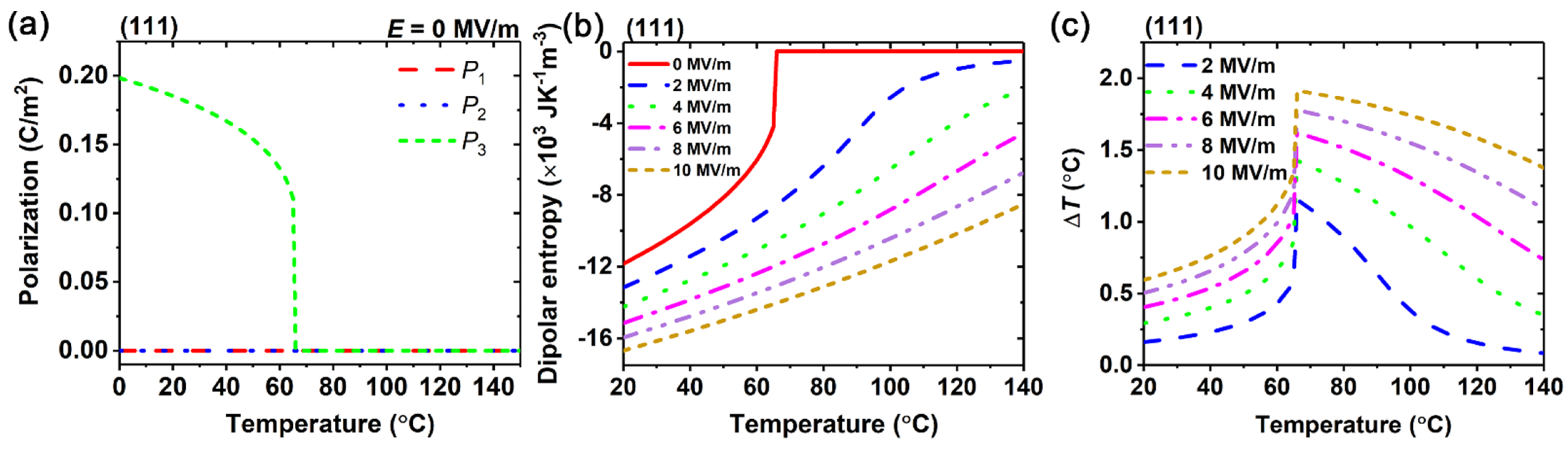
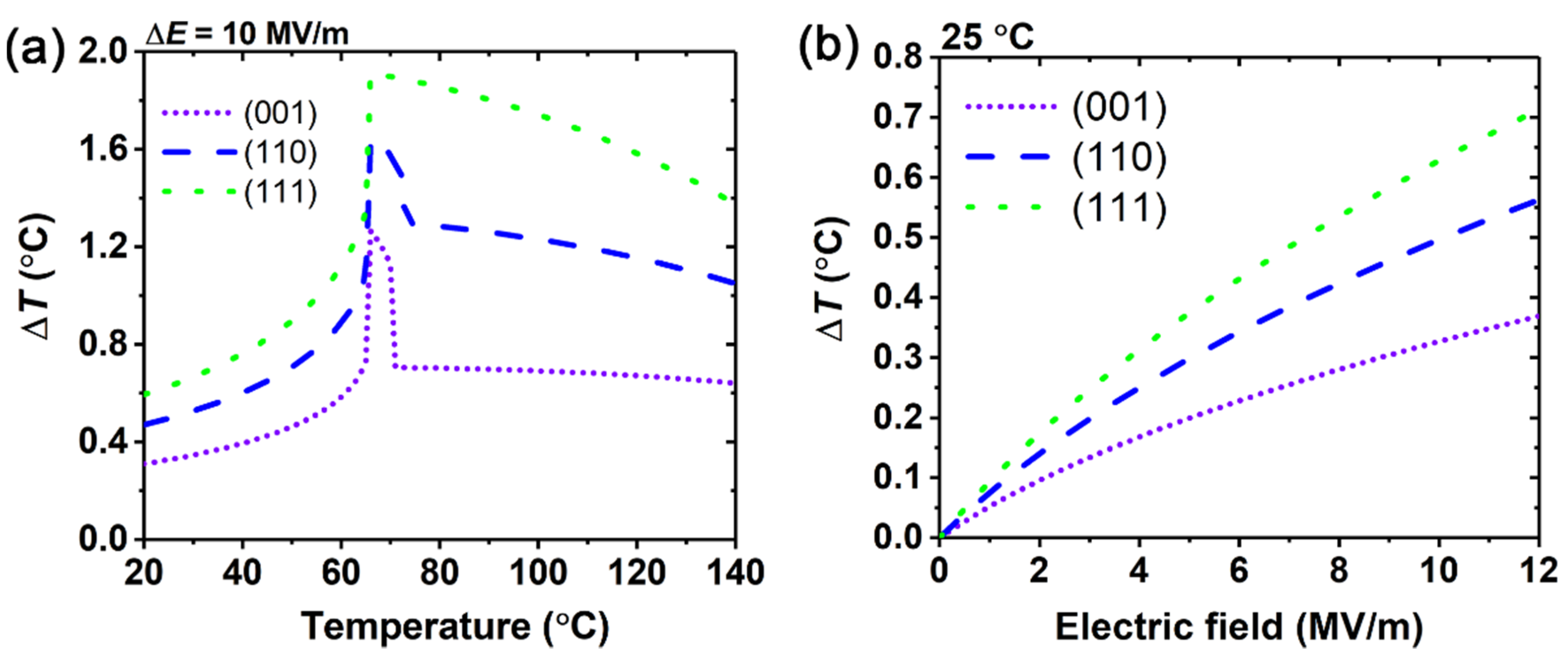
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Zhang, Z.; Usui, T.; Benedict, M.; Hirose, S.; Lee, J.; Kalb, J.; Schwartz, D. A high-performance solid-state electrocaloric cooling system. Science 2020, 370, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.S.; Han, D.L.; Zheng, L.R.; Chen, J.; Tyagi, M.; Li, Q.; Du, F.H.; Zheng, S.; Huang, X.Y.; Zhang, S.H.; et al. High-entropy polymer produces a giant electrocaloric effect at low fields. Nature 2021, 600, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.Y.; Han, D.L.; Li, Z.C.; Yang, L.; Lu, S.G.; Zhong, Z.F.; Chen, J.P.; Zhang, Q.M.; Qian, X.S. Electrocaloric cooling materials and devices for zero-global-warming-potential, high-efficiency refrigeration. Joule 2019, 3, 1200–1225. [Google Scholar] [CrossRef]
- Li, X.Y.; Lu, S.G.; Chen, X.Z.; Gu, H.M.; Qian, X.S.; Zhang, Q.M. Pyroelectric and electrocaloric materials. J. Mater. Chem. C 2013, 1, 23–37. [Google Scholar] [CrossRef]
- Scott, J.F. Electrocaloric materials. Annu. Rev. Mater. Res. 2011, 41, 229–240. [Google Scholar] [CrossRef]
- Shi, J.; Zhu, R.F.; Liu, X.; Fang, B.J.; Yuan, N.Y.; Ding, J.N.; Luo, H.S. Large electrocaloric effect in lead-free (Ba0.85Ca0.15)(Zr0.1Ti0.9)O3 ceramics prepared via citrate route. Materials 2017, 10, 1093. [Google Scholar] [CrossRef] [PubMed]
- Asbani, B.; Gagou, Y.; Ben Moumen, S.; Dellis, J.-L.; Lahmar, A.; Amjoud, M.B.; Mezzane, D.; El Marssi, M.; Rozic, B.; Kutnjak, Z. Large electrocaloric responsivity and energy storage response in the lead-free Ba(GexTi1−x)O3 ceramics. Materials 2022, 15, 5227. [Google Scholar] [CrossRef]
- Hou, X.; Li, H.Y.; Shimada, T.; Kitamura, T.; Wang, J. Effect of geometric configuration on the electrocaloric properties of nanoscale ferroelectric materials. J. Appl. Phys. 2018, 123, 124103. [Google Scholar] [CrossRef]
- Correia, T.; Zhang, Q. Electrocaloric Materials: New Generation of Coolers; Springer: Berlin, Germany, 2014. [Google Scholar]
- Moya, X.; Mathur, N.D. Caloric materials for cooling and heating. Science 2020, 370, 797–803. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, Z.Y.; Wu, H.X.; Wu, R.Y.; Wu, J.H.; Wang, H.L.; Pei, Q.B. A cascade electrocaloric cooling device for large temperature lift. Nat. Energy 2020, 5, 996–1002. [Google Scholar] [CrossRef]
- Mischenko, A.S.; Zhang, Q.; Scott, J.F.; Whatmore, R.W.; Mathur, N.D. Giant electrocaloric effect in thin-film PbZr0.95Ti0.05O3. Science 2006, 311, 1270–1271. [Google Scholar] [CrossRef] [PubMed]
- Moya, X.; Stern-Taulats, E.; Crossley, S.; Gonzalez-Alonso, D.; Kar-Narayan, S.; Planes, A.; Manosa, L.; Mathur, N.D. Giant electrocaloric strength in single-crystal BaTiO3. Adv. Mater. 2013, 25, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
- Shan, D.L.; Pan, K.; Lei, C.H.; Peng, J.L.; He, N.B.; Pan, J.Y.; Jin, H.Y.; Liu, Y.Y. Large electrocaloric response over a broad temperature range near room temperature in BaxSr1−xTiO3 single crystals. J. Appl. Phys. 2019, 126, 204103. [Google Scholar] [CrossRef]
- Moya, X.; Kar-Narayan, S.; Mathur, N.D. Caloric materials near ferroic phase transitions. Nat. Mater. 2014, 13, 439–450. [Google Scholar] [CrossRef]
- Bai, G.; Qin, X.S.; Xie, Q.Y.; Gao, C.F. Electric-field-induced phase transition and electrocaloric effect in PZT near morphotropic phase boundary. Phys. B 2019, 560, 208–214. [Google Scholar] [CrossRef]
- Neese, B.; Chu, B.J.; Lu, S.G.; Wang, Y.; Furman, E.; Zhang, Q.M. Large electrocaloric effect in ferroelectric polymers near room temperature. Science 2008, 321, 821–823. [Google Scholar] [CrossRef]
- Wu, H.H.; Cohen, R.E. Electric-field-induced phase transition and electrocaloric effect in PMN-PT. Phys. Rev. B 2017, 96, 054116. [Google Scholar] [CrossRef]
- Qian, X.S.; Ye, H.J.; Zhang, Y.T.; Gu, H.M.; Li, X.Y.; Randall, C.A.; Zhang, Q.M. Giant electrocaloric response over a broad temperature range in modified BaTiO3 ceramics. Adv. Funct. Mater. 2014, 24, 1300–1305. [Google Scholar] [CrossRef]
- Qian, J.F.; Hu, P.H.; Liu, C.; Jiang, J.Y.; Dan, Z.K.; Ma, J.; Lin, Y.H.; Nan, C.W.; Shen, Y. High electrocaloric cooling power of relaxor ferroelectric BaZrxTi1–xO3 ceramics within broad temperature range. Sci. Bull. 2018, 63, 356–361. [Google Scholar] [CrossRef]
- Jian, X.D.; Lu, B.; Li, D.D.; Yao, Y.B.; Tao, T.; Liang, B.; Guo, J.H.; Zeng, Y.J.; Chen, J.L.; Lu, S.G. Direct measurement of large electrocaloric effect in Ba(ZrxTi1−x)O3 ceramics. ACS Appl. Mater. Inter. 2018, 10, 4801–4807. [Google Scholar] [CrossRef]
- Shan, D.L.; Cai, Y.C.; Lei, C.H.; Peng, J.L.; He, N.B.; Pan, K.; Liu, Y.Y.; Li, J.Y. Electric-field-driven coexistence of positive and negative electrocaloric effects near room temperature for high-efficiency two-stage cooling. Appl. Phys. Lett. 2021, 118, 122905. [Google Scholar] [CrossRef]
- Peng, J.L.; Shan, D.L.; Liu, Y.Y.; Pan, K.; Lei, C.H.; He, N.B.; Zhang, Z.Y.; Yang, Q. A thermodynamic potential for barium zirconate titanate solid solutions. npj Comput. Mater. 2018, 4, 66. [Google Scholar] [CrossRef]
- Co, K.; Khassaf, H.; Alpay, S.P. Electrocaloric and pyroelectric properties of barium zirconate titanate. J. Appl. Phys. 2020, 127, 174102. [Google Scholar] [CrossRef]
- Xu, R.J.; Zhang, J.L.; Chen, Z.H.; Martin, L.W. Orientation-dependent structural phase diagrams and dielectric properties of PbZr1−xTixO3 polydomain thin films. Phys. Rev. B 2015, 91, 144106. [Google Scholar] [CrossRef]
- Xu, R.J.; Liu, S.; Grinberg, I.; Karthik, J.; Damodaran, A.R.; Rappe, A.M.; Martin, L.W. Ferroelectric polarization reversal via successive ferroelastic transitions. Nat. Mater. 2015, 14, 79–86. [Google Scholar] [CrossRef]
- Li, J.T.; Yin, R.W.; Su, X.P.; Wu, H.H.; Li, J.J.; Qin, S.Q.; Sun, S.D.; Chen, J.; Su, Y.J.; Qiao, L.J.; et al. Complex phase transitions and associated electrocaloric effects in different oriented PMN-30PT single crystals under multi-fields of electric field and temperature. Acta Mater. 2020, 182, 250–256. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Zhu, Z.X.; Li, J.F.; Li, J.Y. Misfit strain modulated phase structures of epitaxial Pb(Zr1−xTix)O3 thin films: The effect of substrate and film thickness. Mech. Mater. 2010, 42, 816–826. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Li, J.Y. Shear-driven morphotropic phase boundary in epitaxial ferroelectric thin films. Phys. Rev. B 2011, 84, 132104. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Yang, L.; Li, J.Y. Strain-engineered orthorhombic-rhombohedral phase boundary in epitaxial bismuth ferrite films. J. Appl. Phys. 2013, 113, 183524. [Google Scholar] [CrossRef]
- Li, Y.L.; Cross, L.E.; Chen, L.Q. A phenomenological thermodynamic potential for BaTiO3 single crystals. J. Appl. Phys. 2005, 98, 064101. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Vasudevan, R.K.; Pan, K.; Xie, S.H.; Liang, W.I.; Kumar, A.; Jesse, S.; Chen, Y.C.; Chu, Y.H.; Nagarajan, V.; et al. Controlling magnetoelectric coupling by nanoscale phase transformation in strain engineered bismuth ferrite. Nanoscale 2012, 4, 3175–3183. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Su, Y.; Weng, G.J. A phase-field study on the hysteresis behaviors and domain patterns of nanocrystalline ferroelectric polycrystals. J. Appl. Phys. 2013, 113, 204106. [Google Scholar] [CrossRef]
- Pertsev, N.A.; Zembilgotov, A.G.; Tagantsev, A.K. Effect of mechanical boundary conditions on phase diagrams of epitaxial ferroelectric thin films. Phys. Rev. Lett. 1998, 80, 1988–1991. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Li, J.Y. Seeing is believing: Negative capacitance captured at both nano- and macro-scales. Sci. Bull. 2019, 64, 361–363. [Google Scholar] [CrossRef]
- Pirc, R.; Kutnjak, Z.; Blinc, R.; Zhang, Q.M. Electrocaloric effect in relaxor ferroelectrics. J. Appl. Phys. 2011, 110, 074113. [Google Scholar] [CrossRef]
- Liu, Y.; Scott, J.F.; Dkhil, B. Direct and indirect measurements on electrocaloric effect: Recent developments and perspectives. Appl. Phys. Rev. 2016, 3, 031102. [Google Scholar] [CrossRef]
- Shan, D.L.; Lei, C.H.; Cai, Y.C.; Pan, K.; Liu, Y.Y. Mechanical control of electrocaloric response in epitaxial ferroelectric thin films. Int. J. Solids Struct. 2021, 216, 59–67. [Google Scholar] [CrossRef]
- Peng, J.L.; Li, Q.; Shan, D.L.; Pan, K.; Yu, G.S.; Liu, Y.Y. Phenomenological thermodynamic potentials for bulk and thin-film Ba(Zr0.08Ti0.92)O3 single crystals. J. Appl. Phys. 2016, 119, 204103. [Google Scholar] [CrossRef]
- Jian, X.D.; Lu, B.; Li, D.D.; Yao, Y.B.; Tao, T.; Liang, B.; Lu, S.G. Large electrocaloric effect in lead-free Ba(ZrxTi1−x)O3 thick film ceramics. J. Alloys Compd. 2018, 742, 165–171. [Google Scholar] [CrossRef]
- Mahesh, M.L.V.; Prasad, V.V.B.; James, A.R. Enhanced dielectric and ferroelectric properties of lead-free Ba(Zr0.15Ti0.85)O3 ceramics compacted by cold isostatic pressing. J. Alloys Compd. 2014, 611, 43–49. [Google Scholar] [CrossRef]
- Yu, Z.; Guo, R.Y.; Bhalla, A.S. Dielectric behavior of Ba(Ti1−xZrx)O3 single crystals. J. Appl. Phys. 2000, 88, 410–415. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, Y.; Lei, C.; Shan, D. Electrocaloric Effect in Different Oriented BaZr0.15Ti0.85O3 Single Crystals. Materials 2022, 15, 7018. https://doi.org/10.3390/ma15197018
Ou Y, Lei C, Shan D. Electrocaloric Effect in Different Oriented BaZr0.15Ti0.85O3 Single Crystals. Materials. 2022; 15(19):7018. https://doi.org/10.3390/ma15197018
Chicago/Turabian StyleOu, Yun, Chihou Lei, and Dongliang Shan. 2022. "Electrocaloric Effect in Different Oriented BaZr0.15Ti0.85O3 Single Crystals" Materials 15, no. 19: 7018. https://doi.org/10.3390/ma15197018
APA StyleOu, Y., Lei, C., & Shan, D. (2022). Electrocaloric Effect in Different Oriented BaZr0.15Ti0.85O3 Single Crystals. Materials, 15(19), 7018. https://doi.org/10.3390/ma15197018





