A Long-Term Study on the Content of Polycyclic Aromatic Hydrocarbons in Rubber from End-of-Life Tires of Passenger Cars and Trucks
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization of the Sample Material
2.2. Sampling Procedure
2.2.1. Truck Tires, Whole (TW)
2.2.2. Truck Tires, Tread (TT)
2.2.3. Passenger Car Tires, Whole (PW)
2.2.4. Measurement Frequency
2.3. Extraction and Quantification of PAH
2.3.1. Analytical Method
2.3.2. Chemicals and Materials
2.3.3. Extraction of PAH from ELT Materials
2.3.4. Sample Preparation of the Extract
2.3.5. Instrumental PAH analysis
3. Results
3.1. Notes on the Presentation of Results
- Each measured value of a discrete PAH species and the sum values in the three lower columns are to be considered separately. The sum values were calculated separately for each individual measurement and then the statistical characteristic values were derived from these discrete individual sums. In this respect, the sums of the discrete PAH will not necessarily correspond to the respective sum values in the lower three lines.
- The margin of error e corresponds to half the width of the two-sided confidence interval of the mean value at the confidence level of 95% (α = 0.05).
- The maximum value represents, for each individual PAH or for the respective sums, the highest value measured in an individual measurement over the entire measurement series.
- On the basis of the p-value it was examined whether a normal distribution of the measured values [wx,y~ N(µ, σ2)] could be excluded. The Anderson–Darling test used for this purpose compares the measured values with the theoretical distribution of the values in relation to the normal distribution. If the p-value is less than 0.05, the hypothesis that the values correspond to a normal distribution should be rejected (in this case the fields were marked red). In contrast, a p-value greater than 0.05 does not necessarily mean that the data is normal distributed. The Anderson–Darling test can be used for a sample size of n ≥ 8, which is why an evaluation for buffings of truck tread (n = 3) was omitted.
- In addition to testing the results for normal distribution [wx,y~ N(µ, σ2)], the test for log-normal distribution [wx,y~ LN(µ*, σ2*)] was also carried out. For this purpose, the data was transformed by logarithmizing each individual measurement result: w*x,y = ln(wx,y). The corresponding fields were marked red if the p-value is less than 0.05, thus indicating that a log-normal distribution can be rejected.
- The respective higher result for the p-value of wx,y or w*x,y was marked green if they were higher than 0.05.
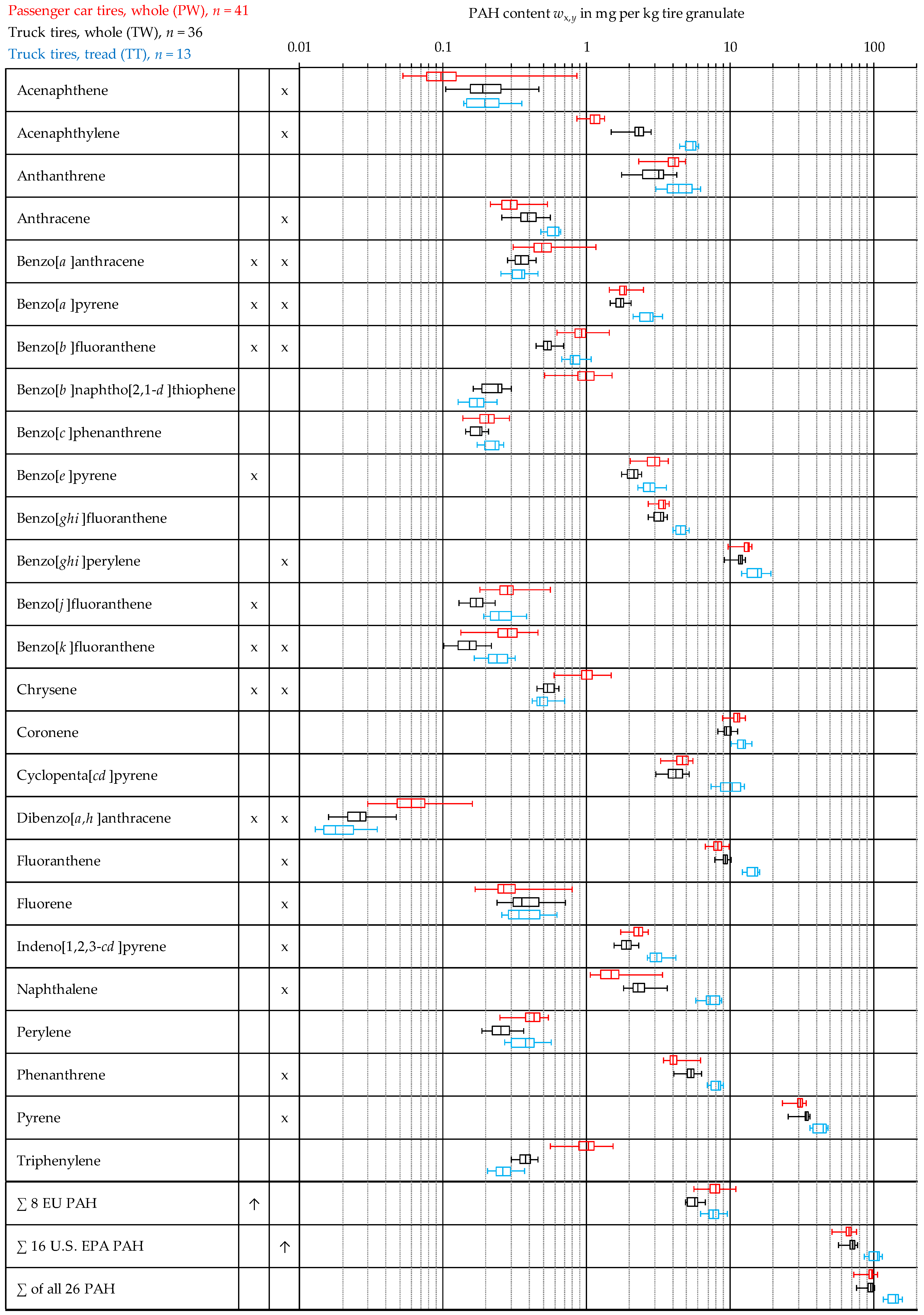
3.2. Overview of the Measurement Results for all Three Analyzed Tire Materials
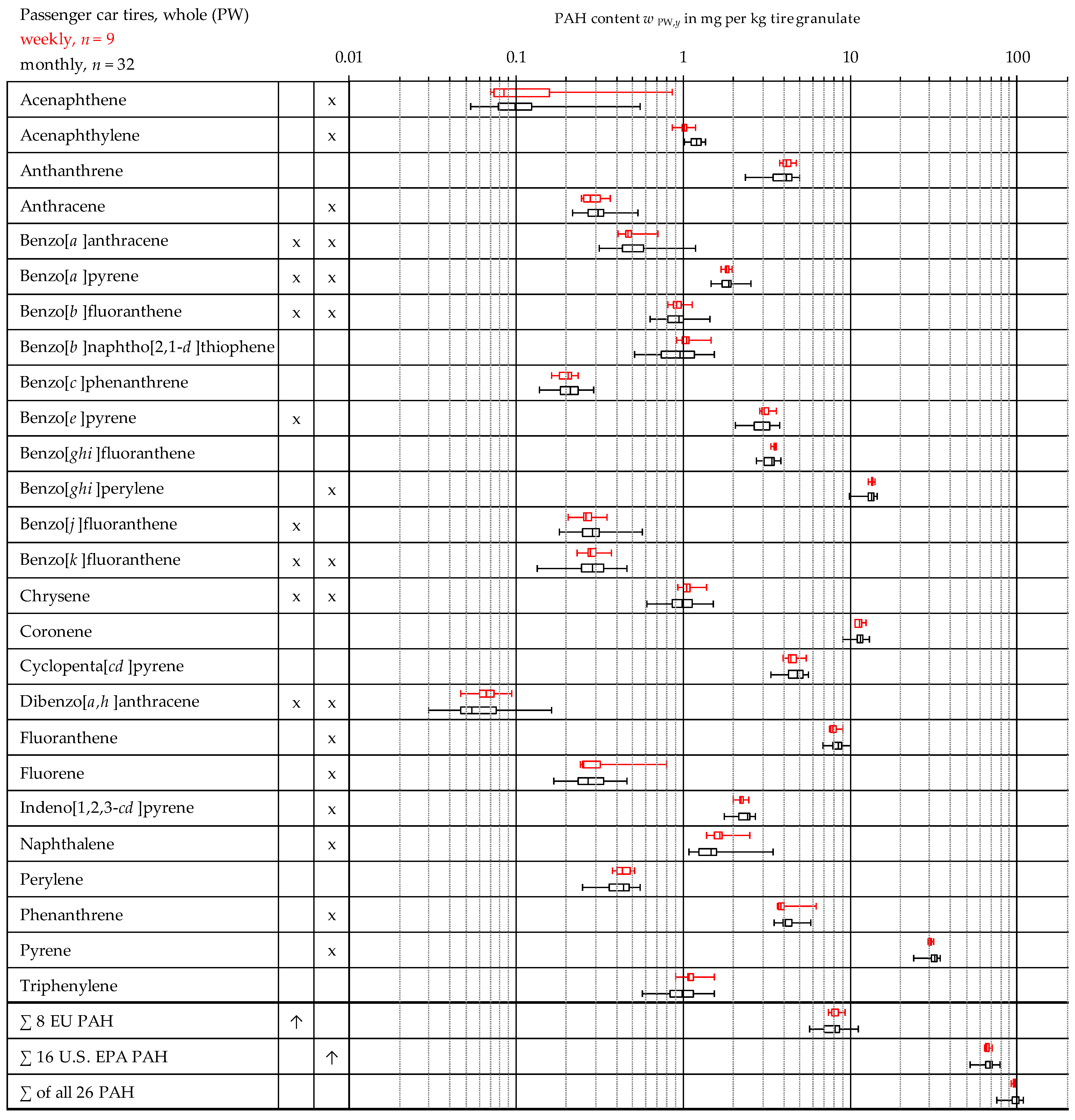
3.3. Comparison of the Weekly and Monthly Mixed Samples from Passenger Car Tires
4. Discussion
5. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kreider, M.L.; Panko, J.M.; McAtee, B.L.; Sweet, L.I.; Finley, B.L. Physical and chemical characterization of tire-related particles: Comparison of particles generated using different methodologies. Sci. Total Environ. 2010, 408, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Skoczynska, E.; Leonards, P.E.G.; Llompart, M.; de Boer, J. Analysis of recycled rubber: Development of an analytical method and determination of polycyclic aromatic hydrocarbons and heterocyclic aromatic compounds in rubber matrices. Chemosphere 2021, 276, 130076. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, A.; Archibong, A.E.; Hood, D.B.; Guo, Z.; Loganathan, B.G. Global Environmental Distribution and Human Health Effects of Polycyclic Aromatic Hydrocarbons. In Global Contamination Trends of Persistent Organic Chemicals; CRC Press: Boca Raton, FL, USA, 2011; pp. 95–125. [Google Scholar] [CrossRef]
- Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006, ANNEX XVII, Point 50, Column 2. Available online: https://eur-lex.europa.eu/eli/reg/2006/1907 (accessed on 11 July 2022).
- Bergmann, C.; Trimbach, J.; Haase-Held, M.; Seidel, A. Consequences of European directive 2005/69/EC for tire industry. KGK Kautsch. Gummi Kunstst. 2011, 64, 24–35. [Google Scholar]
- German National and Regional Committee for the Safety of Chemicals (Bund/Länder-Arbeitsgemeinschaft Chemikaliensicherheit BLAC). Bericht Zum Nationalen Überwachungsschwerpunkt “PAK in Reifen“. 2012. Available online: www.blac.de/documents/bericht_pak_in_reifen_1503997053.pdf (accessed on 11 July 2022).
- European Tyre and Rubber Manufacturers’ Association (ETRMA). ETRMA High-PAH Oil Testing Programme Q&A. 2011. Available online: www.etrma.org/wp-content/uploads/2011/03/20110301_-_ETRMA_Q-A__PAH_Oil_testing_programme.pdf (accessed on 11 July 2022).
- European Tyre and Rubber Manufacturers’ Association (ETRMA). Second Testing Program Confirms: REACH Compliance Tests Continue to Give Failing Grades to Tyre Imports. 2011. Available online: www.etrma.org/wp-content/uploads/2019/09/pah-2nd-round_press-release_2011-10-17.pdf (accessed on 11 July 2022).
- Ministry of the Environment, Climate Protection and the Energy Sector of the German Regional State of Baden-Württemberg (UM BW). Ergebnisse der Marktüberwachung 2010 im Bereich Chemikaliensicherheit. 2010. Available online: http://www.blac.de/documents/blac_jahresbericht_2010_1503996441.pdf (accessed on 11 July 2022).
- Ministry of the Environment and Forests of the German Regional State of Rhineland-Palatinate (MUEEF). Marktüberwachung PAK in Reifen. 2011. Available online: https://mkuem.rlp.de/fileadmin/mulewf/Themen/Umweltschutz/Chemikaliensicherheit/PAK_in_reifen_Stand_Nov__2011.pdf (accessed on 11 July 2022).
- COMMISSION REGULATION (EU) No 1272/2013 of 6 December 2013 Amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as Regards Polycyclic Aromatic Hydrocarbons. Available online: http://data.europa.eu/eli/reg/2013/1272/oj (accessed on 11 July 2022).
- REGULATION (EC) No 1907/2006 of The European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), Establishing a European Chemicals Agency, Amending Directive 1999/45/EC and Repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. ANNEX XVII, Number 50. Available online: http://data.europa.eu/eli/reg/2006/1907/oj (accessed on 11 July 2022).
- Commission Regulation (EU) 2021/1199 of 20 July 2021 Amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council as regards Polycyclic-Aromatic Hydrocarbons (PAHs) in Granules or Mulches Used as Infill Material in Synthetic Turf Pitches or in Loose form on Playgrounds or in Sport Applications. Available online: http://data.europa.eu/eli/reg/2021/1199/oj (accessed on 11 July 2022).
- German Federal Institute for Risk Assessment (BfR). PAHs in Consumer Products Must Be Reduced as much as Possible. Updated BfR Expert Opinion 2009, No. 025/2009. Available online: https://mobil.bfr.bund.de/cm/349/pahs_in_consumer_products_must_be_reduced_as_much_as_possible.pdf (accessed on 11 July 2022).
- Diekmann, A.; Giese, U.; Schaumann, I. Polycyclic aromatic hydrocarbons in consumer goods made from recycled rubber material: A review. Chemosphere 2019, 220, 1163–1178. [Google Scholar] [CrossRef] [PubMed]
- European Chemicals Agency (ECHA). ANNEX XV INVESTIGATION REPORT, Investigation of the Available Analytical Methods to Measure Content and Migration of Polycyclic Aromatic Hydrocarbons, Limit Values in Rubber and Plastic Articles in Paragraphs 5 and 6 of Entry 50 of Annex XVII to REACH, and Alternative Low-PAH Raw Materials. 2020, Draft 2.1. Available online: https://echa.europa.eu/documents/10162/13641/rest_pah_investigation_en.pdf/53877b6e-239b-fcb8-6560-e86f5b27349b (accessed on 11 July 2022).
- Geiss, O.; Senaldi, C.; Bianchi, I.; Lucena, A.; Tirendi, S.; Barrero-Moreno, J. A fast and selective method for the determination of 8 carcinogenic polycyclic aromatic hydrocarbons in rubber and plastic materials. J. Chromatogr. A 2018, 1566, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Keith, L. The Source of U.S. EPA’s sixteen PAH priority pollutants. Polycycl. Aromat. Compd. 2015, 35, 147–160. [Google Scholar] [CrossRef]
- Grimmer, G.; Jacob, J.; Naujack, K.-W. Atmospheric emission of polycylic aromatic hydrocarbons in sampling areas of the German enviromental specimen bank. Method for the precise measurement of gaseous and particle-associated polycyclic aromatic hydrocarbons in the sub-nanogram range using deuterated internal standards. Chemosphere 1997, 34, 2213–2226. [Google Scholar] [CrossRef]
- Marsili, L.; Coppola, D.; Bianchi, N.; Maltese, S.; Bianchi, M.; Fossi, M.C. Release of polycyclic aromatic hydrocarbons and heavy metals from rubber crumb in synthetic turf fields: Preliminary hazard assessment for athletes. J. Environ. Anal. Toxicol. 2014, 5, 265. [Google Scholar] [CrossRef]
- Sadiktsis, I.; Bergvall, C.; Johansson, C.; Westerholm, R. Automobile tires—A potential source of highly carcinogenic dibenzopyrenes to the environment. Environ. Sci. Toxicol. 2012, 46, 3326–3334. [Google Scholar] [CrossRef] [PubMed]
- Menichini, E.; Abate, V.; Attias, L.; De Luca, S.; di Domenico, A.; Fochi, I.; Forte, G.; Iacovella, N.; Iamicelli, A.L.; Izzo, P.; et al. Artificial-turf playing fields: Contents of metals, PAHs, PCBs, PCDDs and PCDFs, inhalation exposure to PAHs and related preliminary risk assessment. Sci. Total Environ. 2011, 409, 4950–4957. [Google Scholar] [CrossRef] [PubMed]
- Sommerfeld, T.; Jung, C.; Riedel, J.; Mauch, T.; Sauer, A.; Koch, M. Development of a certified reference material for the determination of polycyclic aromatic hydrocarbons (PAHs) in rubber toy. Anal. Bioanal. Chem. 2022, 414, 4369–4378. [Google Scholar] [CrossRef] [PubMed]
- Hamm, S.; Frey, T.; Weinand, R.; Moninot, G.; Petiniot, N. Investigations on the extraction and migration behavior of polycyclic aromatic hydrocarbons (PAHs) from cured rubber formulations containing carbon black as reinforcing agent. Rubber Chem. Technol. 2009, 82, 214–228. [Google Scholar] [CrossRef]
- Hamm, S.; Hölscher, K.; Gruenberger, T.M.; Moninot, G.; Örtel, W.; Petiniot, N. Unsuitability of the ZEK/AFPS test method for the determination of polycyclic aromatic hydrocarbons in carbon black. Rubber Chem. Technol. 2018, 91, 225–250. [Google Scholar] [CrossRef]
- Taylor, G.T.; Redington, T.E.; Bailey, M.J.; Buddingh, F.; Nau, C.A. Solvent extracts of carbon black—Determination of total extractables and analysis for benzo(a)pyrene. Am. Ind. Hyg. Assoc. J. 1980, 41, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Celeiro, M.; Lamas, J.P.; Garcia-Jares, C.; Dagnac, T.; Ramos, L.; Llompart, M. Investigation of PAH and other hazardous contaminant occurrence in recycled tyre rubber surfaces. Case-study: Restaurant playground in an indoor shopping centre. Int. J. Environ. Anal. Chem. 2014, 94, 1264–1271. [Google Scholar] [CrossRef]
- Depaoloni, A.R.; Bianchi, G.; Fornai, D.; Cardelli, A.; Badalassi, M.; Cardelli, C.; Davoli, E. Physical and chemical characterization of representative samples of recycled rubber from end-of-life tires. Chemosphere 2017, 184, 1320–1326. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency (U.S. EPA). Synthetic Turf Field Recycled Tire Crumb Rubber Research Under the Federal Research Action Plan. 2019. Available online: https://www.epa.gov/chemical-research/july-2019-report-tire-crumb-rubber-characterization-0 (accessed on 11 July 2022).
- Bartsch, N.; Heidler, J.; Vieth, B.; Hutzler, C.; Luch, A. Skin permeation of polycyclic aromatic hydrocarbons: A solvent-based in vitro approach to assess dermal exposures against benzo[a]pyrene and dibenzopyrenes. J. Occup. Environ. Hyg. 2016, 13, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Geiss, O.; Bianchi, I.; Senaldi, C.; Lucena, A.; Tirendi, S.; Barrero-Moreno, J. Skin surface film liquid as new migration medium for the determination of PAHs released from rubber containing consumer goods. Polycycl. Aromat. Compd. 2020, 40, 553–562. [Google Scholar] [CrossRef]
- French Agency for Food, Environmental and Occupational Health & Safety (ANSES). Scientific and Technical Support on the Possible Risks Related to the Use of Materials Derived from the Recycling of Used Tyres in Synthetic Sports Grounds and Similar Uses. AST Report 2018. Available online: https://www.anses.fr/en/system/files/CONSO2018SA0033RaEN.pdf (accessed on 11 July 2022).
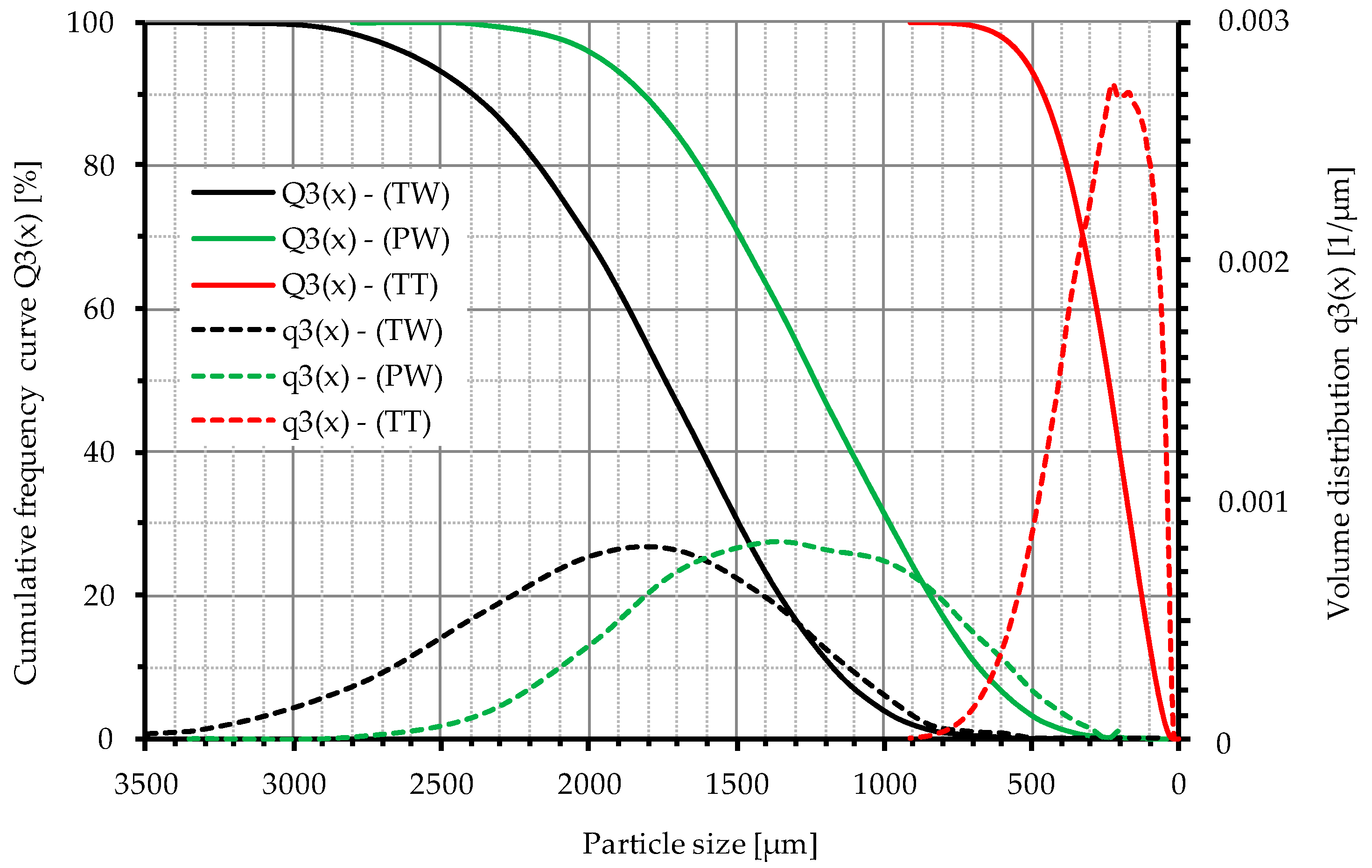

| Truck Tires, Whole (TW) | Truck Tires, Tread (TT) | Passenger Car Tires, Whole (PW) | |
|---|---|---|---|
| Grain size | 3000–800 µm | 700–0 µm | 2500–400 µm |
| Mesh size | 2000–500 µm | <400 µm | 2000–500 µm |
| Supplier | MRH | MRH | PVP |
| Year | 2017 | 2018 | 2019 | ∑ | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Month | 7 | 8 | 9 | 10 | 11 | 12 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Truck tires, whole (TW) | / | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 36 |
| Truck tires, tread (TT) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | / | / | / | / | / | / | / | / | / | / | 3* | / | 13/3 |
| Car tires, whole (PW) | 2 | 4 | 3 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 41 |
 | ||||||||||||||||||||||||||
| Week number | 30–31 | 32–35 | 36–38 | * Buffings | ||||||||||||||||||||||
| PW: Passenger Car Tires, Whole (n = 41) | TW: Truck Tires, Whole (n = 36) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arithmetic Mean | Margin of Error e | Sample Standard Deviation s | Maximum Value | p-Value wPW, y | p-Value for w*PW, y = ln(w PW, y) | Arithmetic Mean | Margin of Error e | Sample Standard Deviation s | Maximum Value | p-Value wTW, y | p-Value for w*TW, y = ln(w TW, y) | |
| Acenaphthene | 0.13 | 0.04 | 0.14 | 0.87 | 0.00 | 0.00 | 0.21 | 0.03 | 0.08 | 0.46 | 0.02 | 0.51 |
| Acenaphthylene | 1.15 | 0.04 | 0.12 | 1.35 | 0.68 | 0.56 | 2.34 | 0.09 | 0.27 | 2.82 | 0.17 | 0.05 |
| Anthanthrene | 3.92 | 0.22 | 0.69 | 4.88 | 0.00 | 0.00 | 2.99 | 0.22 | 0.64 | 4.27 | 0.01 | 0.00 |
| Anthracene | 0.31 | 0.02 | 0.06 | 0.54 | 0.08 | 0.67 | 0.39 | 0.02 | 0.07 | 0.56 | 0.44 | 0.34 |
| Benzo[a]anthracene | 0.51 | 0.05 | 0.15 | 1.18 | 0.00 | 0.27 | 0.36 | 0.02 | 0.05 | 0.45 | 0.10 | 0.24 |
| Benzo[a]pyrene | 1.82 | 0.06 | 0.19 | 2.50 | 0.02 | 0.01 | 1.72 | 0.05 | 0.14 | 2.04 | 0.81 | 0.66 |
| Benzo[b]fluoranthene | 0.92 | 0.05 | 0.16 | 1.44 | 0.34 | 0.28 | 0.54 | 0.02 | 0.06 | 0.69 | 0.05 | 0.17 |
| Benzo[b]naphtho[2,1-d]thiophene | 0.99 | 0.08 | 0.26 | 1.53 | 0.36 | 0.02 | 0.23 | 0.01 | 0.04 | 0.30 | 0.00 | 0.00 |
| Benzo[c]phenanthrene | 0.21 | 0.01 | 0.03 | 0.29 | 0.93 | 0.66 | 0.17 | 0.01 | 0.02 | 0.21 | 0.01 | 0.00 |
| Benzo[e]pyrene | 2.96 | 0.14 | 0.43 | 3.74 | 0.32 | 0.06 | 2.10 | 0.07 | 0.20 | 2.43 | 0.07 | 0.04 |
| Benzo[ghi]fluoranthene | 3.34 | 0.09 | 0.29 | 3.77 | 0.00 | 0.00 | 3.23 | 0.09 | 0.26 | 3.64 | 0.00 | 0.00 |
| Benzo[ghi]perylene | 12.95 | 0.37 | 1.16 | 14.31 | 0.00 | 0.00 | 11.58 | 0.34 | 1.01 | 12.73 | 0.00 | 0.00 |
| Benzo[j]fluoranthene | 0.29 | 0.02 | 0.07 | 0.56 | 0.01 | 0.19 | 0.17 | 0.01 | 0.02 | 0.23 | 0.35 | 0.63 |
| Benzo[k]fluoranthene | 0.29 | 0.02 | 0.06 | 0.46 | 0.89 | 0.46 | 0.15 | 0.01 | 0.03 | 0.22 | 0.80 | 0.37 |
| Chrysene | 1.01 | 0.06 | 0.20 | 1.49 | 0.63 | 0.43 | 0.55 | 0.02 | 0.05 | 0.64 | 0.07 | 0.12 |
| Coronene | 11.22 | 0.28 | 0.87 | 12.82 | 0.17 | 0.05 | 9.60 | 0.25 | 0.74 | 11.25 | 0.41 | 0.61 |
| Cyclopenta[cd]pyrene | 4.59 | 0.20 | 0.62 | 5.52 | 0.02 | 0.00 | 4.16 | 0.22 | 0.64 | 5.22 | 0.56 | 0.40 |
| Dibenzo[a,h]anthracene | 0.07 | 0.01 | 0.03 | 0.16 | 0.00 | 0.31 | 0.03 | 0.00 | 0.01 | 0.05 | 0.09 | 0.59 |
| Fluoranthene | 8.26 | 0.22 | 0.70 | 9.94 | 0.54 | 0.70 | 9.18 | 0.21 | 0.63 | 10.17 | 0.00 | 0.00 |
| Fluorene | 0.29 | 0.03 | 0.11 | 0.79 | 0.00 | 0.03 | 0.39 | 0.04 | 0.12 | 0.72 | 0.01 | 0.18 |
| Indeno[1,2,3-cd]pyrene | 2.30 | 0.07 | 0.22 | 2.69 | 0.27 | 0.17 | 1.90 | 0.07 | 0.20 | 2.32 | 0.63 | 0.67 |
| Naphthalene | 1.62 | 0.16 | 0.52 | 3.39 | 0.00 | 0.00 | 2.36 | 0.12 | 0.35 | 3.64 | 0.05 | 0.29 |
| Perylene | 0.42 | 0.02 | 0.07 | 0.55 | 0.36 | 0.02 | 0.26 | 0.01 | 0.04 | 0.36 | 0.25 | 0.30 |
| Phenanthrene | 4.19 | 0.18 | 0.58 | 6.29 | 0.00 | 0.00 | 5.34 | 0.15 | 0.44 | 6.29 | 0.57 | 0.30 |
| Pyrene | 30.39 | 0.88 | 2.77 | 34.10 | 0.00 | 0.00 | 32.88 | 1.11 | 3.28 | 36.10 | 0.00 | 0.00 |
| Triphenylene | 1.02 | 0.07 | 0.23 | 1.54 | 0.56 | 0.24 | 0.37 | 0.01 | 0.04 | 0.46 | 0.77 | 0.43 |
| ∑ 8 EU PAH | 7.87 | 0.37 | 1.16 | 11.10 | 0.35 | 0.09 | 5.62 | 0.17 | 0.51 | 6.71 | 0.08 | 0.06 |
| ∑ 16 U.S. EPA PAH | 66.21 | 1.76 | 5.57 | 77.10 | 0.00 | 0.00 | 69.92 | 1.95 | 5.78 | 77.01 | 0.00 | 0.00 |
| ∑ of all 26 PAH | 95.18 | 2.53 | 8.02 | 107.4 | 0.00 | 0.00 | 93.22 | 2.61 | 7.70 | 102.1 | 0.00 | 0.00 |
| TT: Truck Tire Tread, Cryo Powder (n = 13) | TT: Truck Tread, Buffings (n = 3) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Arithmetic Mean | Margin of Error e | Sample Standard Deviation s | Maximum Value | p-Value for w TT, y | p-Value for w* TT, y = ln(w TT, y) | Arithmetic Mean | Margin of error e | Sample standard deviation s | Maximum value | |
| Acenaphthene | 0.21 | 0.04 | 0.06 | 0.36 | 0.26 | 0.49 | 0.10 | 0.01 | 0.01 | 0.11 |
| Acenaphthylene | 5.35 | 0.33 | 0.54 | 6.10 | 0.53 | 0.44 | 4.69 | 1.09 | 0.44 | 5.06 |
| Anthanthrene | 4.55 | 0.62 | 1.02 | 6.26 | 0.53 | 0.55 | 1.72 | 0.58 | 0.23 | 1.99 |
| Anthracene | 0.58 | 0.04 | 0.06 | 0.67 | 0.35 | 0.24 | 0.41 | 0.08 | 0.03 | 0.44 |
| Benzo[a]anthracene | 0.34 | 0.03 | 0.06 | 0.46 | 0.87 | 0.90 | 0.24 | 0.10 | 0.04 | 0.29 |
| Benzo[a]pyrene | 2.68 | 0.22 | 0.37 | 3.38 | 0.59 | 0.61 | 1.85 | 0.56 | 0.23 | 2.10 |
| Benzo[b]fluoranthene | 0.84 | 0.07 | 0.11 | 1.08 | 0.88 | 0.95 | 0.63 | 0.19 | 0.07 | 0.72 |
| Benzo[b]naphtho[2,1-d]thiophene | 0.18 | 0.02 | 0.03 | 0.24 | 0.75 | 0.90 | 0.24 | 0.09 | 0.04 | 0.28 |
| Benzo[c]phenanthrene | 0.22 | 0.02 | 0.03 | 0.27 | 0.28 | 0.18 | 0.19 | 0.01 | 0.00 | 0.20 |
| Benzo[e]pyrene | 2.79 | 0.22 | 0.36 | 3.59 | 0.66 | 0.77 | 2.15 | 0.45 | 0.18 | 2.35 |
| Benzo[ghi]fluoranthene | 4.56 | 0.24 | 0.40 | 5.21 | 0.30 | 0.32 | 3.45 | 0.11 | 0.04 | 3.49 |
| Benzo[ghi]perylene | 15.12 | 1.29 | 2.14 | 19.27 | 0.55 | 0.57 | 10.67 | 2.12 | 0.86 | 11.61 |
| Benzo[j]fluoranthene | 0.26 | 0.03 | 0.06 | 0.39 | 0.33 | 0.46 | 0.18 | 0.05 | 0.02 | 0.20 |
| Benzo[k]fluoranthene | 0.24 | 0.03 | 0.05 | 0.32 | 0.69 | 0.79 | 0.16 | 0.06 | 0.03 | 0.19 |
| Chrysene | 0.51 | 0.05 | 0.08 | 0.71 | 0.04 | 0.09 | 0.54 | 0.26 | 0.10 | 0.66 |
| Coronene | 12.16 | 0.76 | 1.26 | 14.27 | 0.50 | 0.39 | 8.15 | 1.28 | 0.51 | 8.74 |
| Cyclopenta[cd]pyrene | 10.24 | 1.07 | 1.77 | 12.60 | 0.15 | 0.13 | 7.05 | 2.40 | 0.96 | 7.94 |
| Dibenzo[a,h]anthracene | 0.02 | 0.00 | 0.01 | 0.04 | 0.19 | 0.53 | 0.00 | 0.00 | 0.00 | 0.00 |
| Fluoranthene | 14.43 | 0.83 | 1.38 | 15.98 | 0.12 | 0.10 | 11.38 | 1.29 | 0.52 | 11.72 |
| Fluorene | 0.40 | 0.08 | 0.13 | 0.63 | 0.09 | 0.23 | 0.25 | 0.02 | 0.01 | 0.26 |
| Indeno[1,2,3-cd]pyrene | 3.15 | 0.26 | 0.43 | 4.22 | 0.24 | 0.43 | 1.65 | 0.43 | 0.18 | 1.85 |
| Naphthalene | 7.48 | 0.57 | 0.95 | 8.78 | 0.47 | 0.50 | 6.74 | 1.11 | 0.45 | 7.16 |
| Perylene | 0.38 | 0.05 | 0.09 | 0.57 | 0.39 | 0.53 | 0.25 | 0.06 | 0.03 | 0.27 |
| Phenanthrene | 8.09 | 0.40 | 0.66 | 9.00 | 0.15 | 0.12 | 6.96 | 0.97 | 0.39 | 7.40 |
| Pyrene | 42.52 | 2.65 | 4.38 | 47.82 | 0.04 | 0.04 | 32.23 | 2.17 | 0.87 | 32.74 |
| Triphenylene | 0.27 | 0.03 | 0.05 | 0.37 | 0.24 | 0.54 | 0.30 | 0.08 | 0.03 | 0.33 |
| ∑ 8 EU PAH | 7.69 | 0.57 | 0.94 | 9.57 | 0.80 | 0.78 | 5.76 | 1.61 | 0.65 | 6.51 |
| ∑ 16 U.S. EPA PAH | 102.0 | 6.31 | 10.44 | 115.0 | 0.08 | 0.08 | 78.50 | 9.66 | 3.89 | 82.3 |
| ∑ of all 26 PAH | 137.6 | 8.89 | 14.71 | 157.0 | 0.08 | 0.08 | 102.2 | 14.24 | 5.73 | 108.1 |
| Benzo[a]pyrene | ∑ 16 U.S. EPA PAH | |||||
|---|---|---|---|---|---|---|
| 1 | s 2 | Min/Max 3 | 1 | s 2 | Min/Max 3 | |
| Grimmer method 4 | 1.61 | 0.19 | 1.45/1.9 | 55.06 | 3.39 | 51.1/60.2 |
| DIN ISO 18287 5 | 0.72 | 0.08 | 0.6/0.8 | 39.16 | 5.17 | 36.2/47.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoyer, S.; Kroll, L.; Lippert, K.; Seidel, A. A Long-Term Study on the Content of Polycyclic Aromatic Hydrocarbons in Rubber from End-of-Life Tires of Passenger Cars and Trucks. Materials 2022, 15, 7017. https://doi.org/10.3390/ma15197017
Hoyer S, Kroll L, Lippert K, Seidel A. A Long-Term Study on the Content of Polycyclic Aromatic Hydrocarbons in Rubber from End-of-Life Tires of Passenger Cars and Trucks. Materials. 2022; 15(19):7017. https://doi.org/10.3390/ma15197017
Chicago/Turabian StyleHoyer, Stefan, Lothar Kroll, Kirsten Lippert, and Albrecht Seidel. 2022. "A Long-Term Study on the Content of Polycyclic Aromatic Hydrocarbons in Rubber from End-of-Life Tires of Passenger Cars and Trucks" Materials 15, no. 19: 7017. https://doi.org/10.3390/ma15197017
APA StyleHoyer, S., Kroll, L., Lippert, K., & Seidel, A. (2022). A Long-Term Study on the Content of Polycyclic Aromatic Hydrocarbons in Rubber from End-of-Life Tires of Passenger Cars and Trucks. Materials, 15(19), 7017. https://doi.org/10.3390/ma15197017







