Highly Stable Liposomes Based on Tetraether Lipids as a Promising and Versatile Drug Delivery System
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
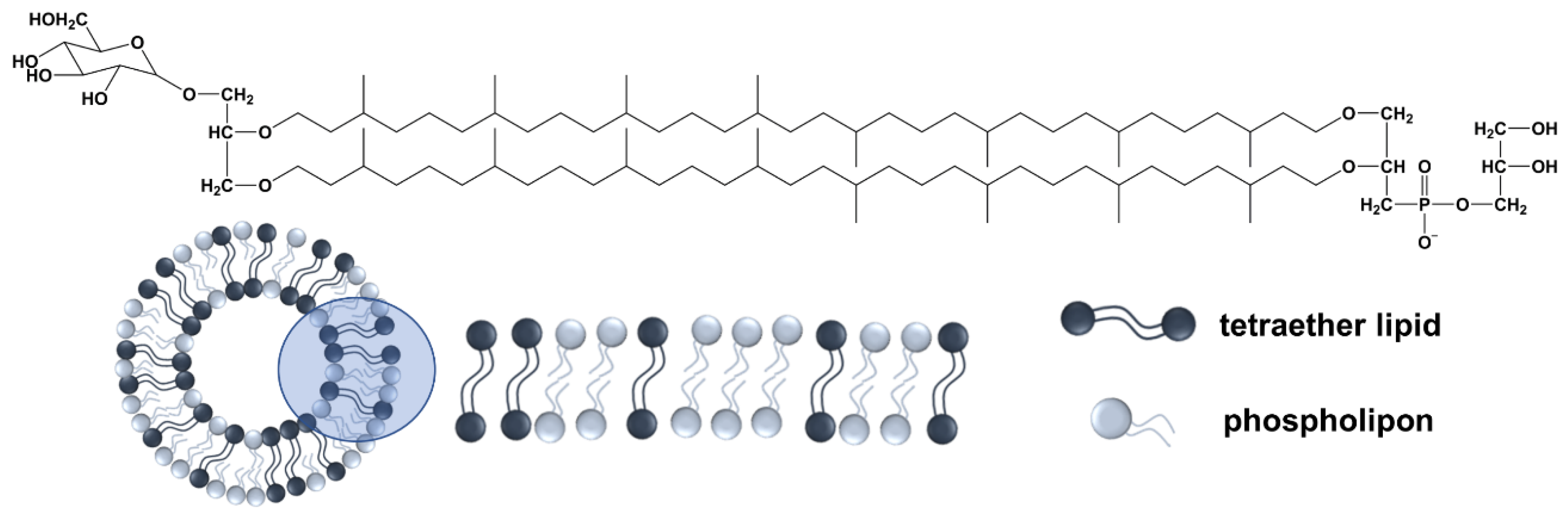
2.2. Extraction of the Tetraether Lipids
2.3. Preparation of Liposomes
2.4. Preparation of 5(6)-Carboxyfluorescein-Loaded Liposomes
2.5. Vesicle Size and Zeta Potential Determination
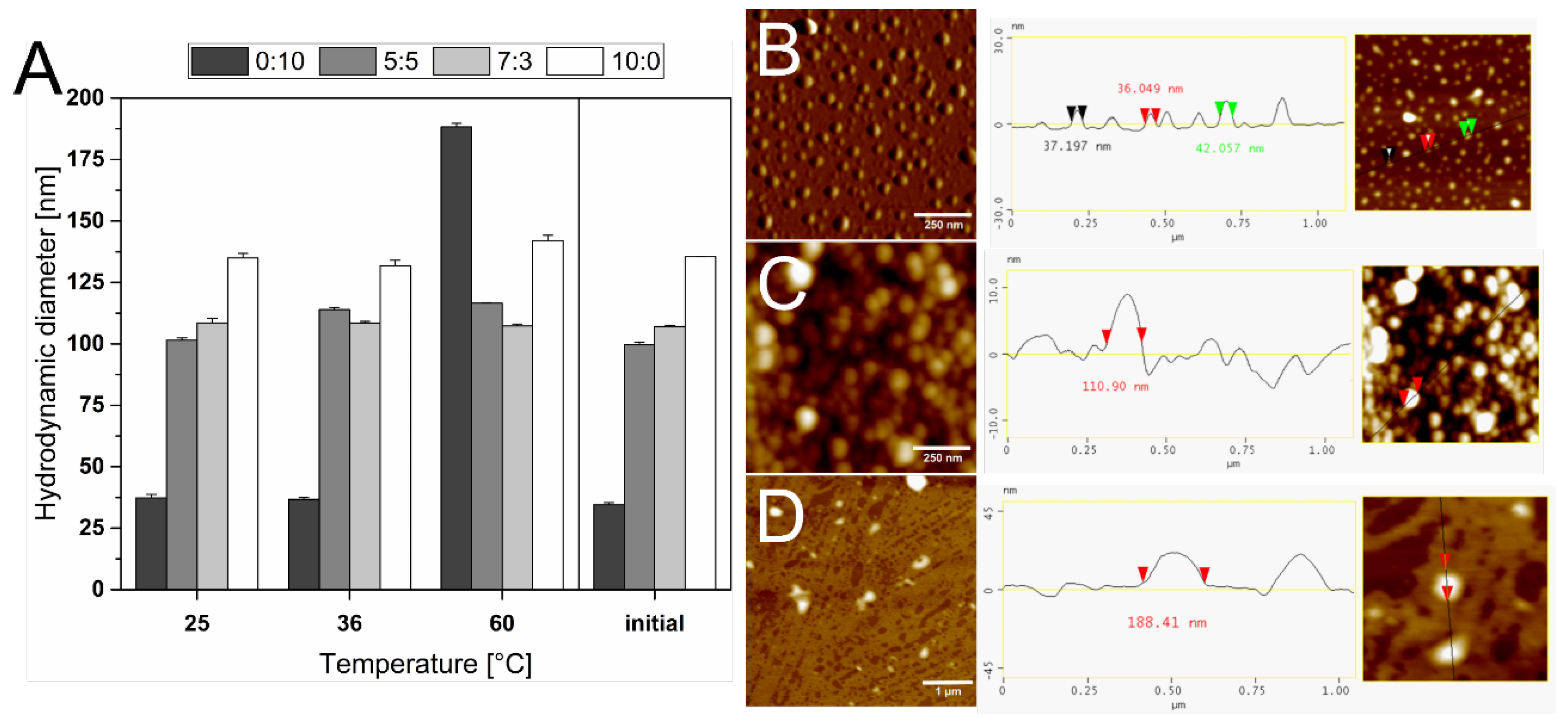
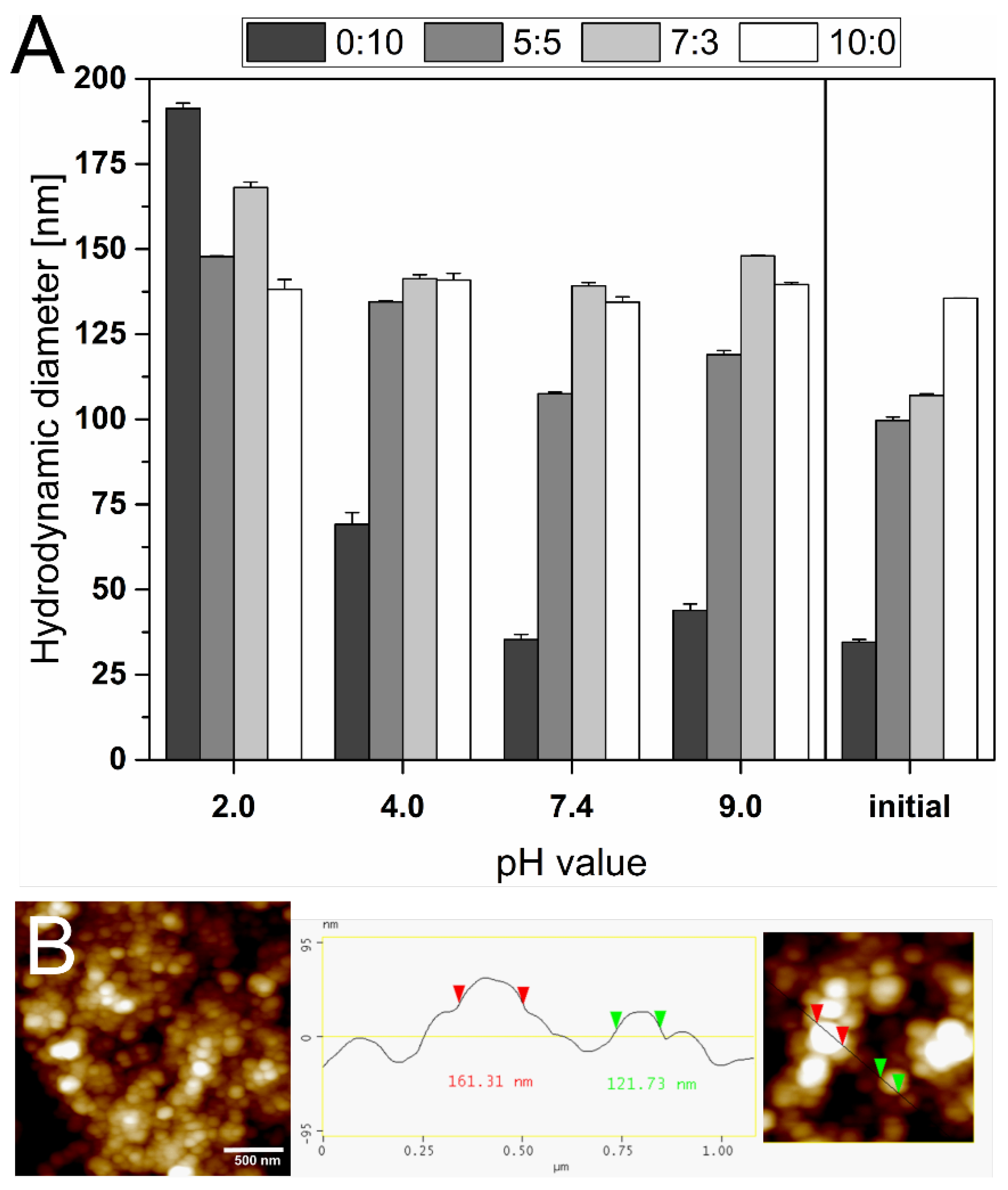
2.6. Atomic Force Microscopy
2.7. Thermostability
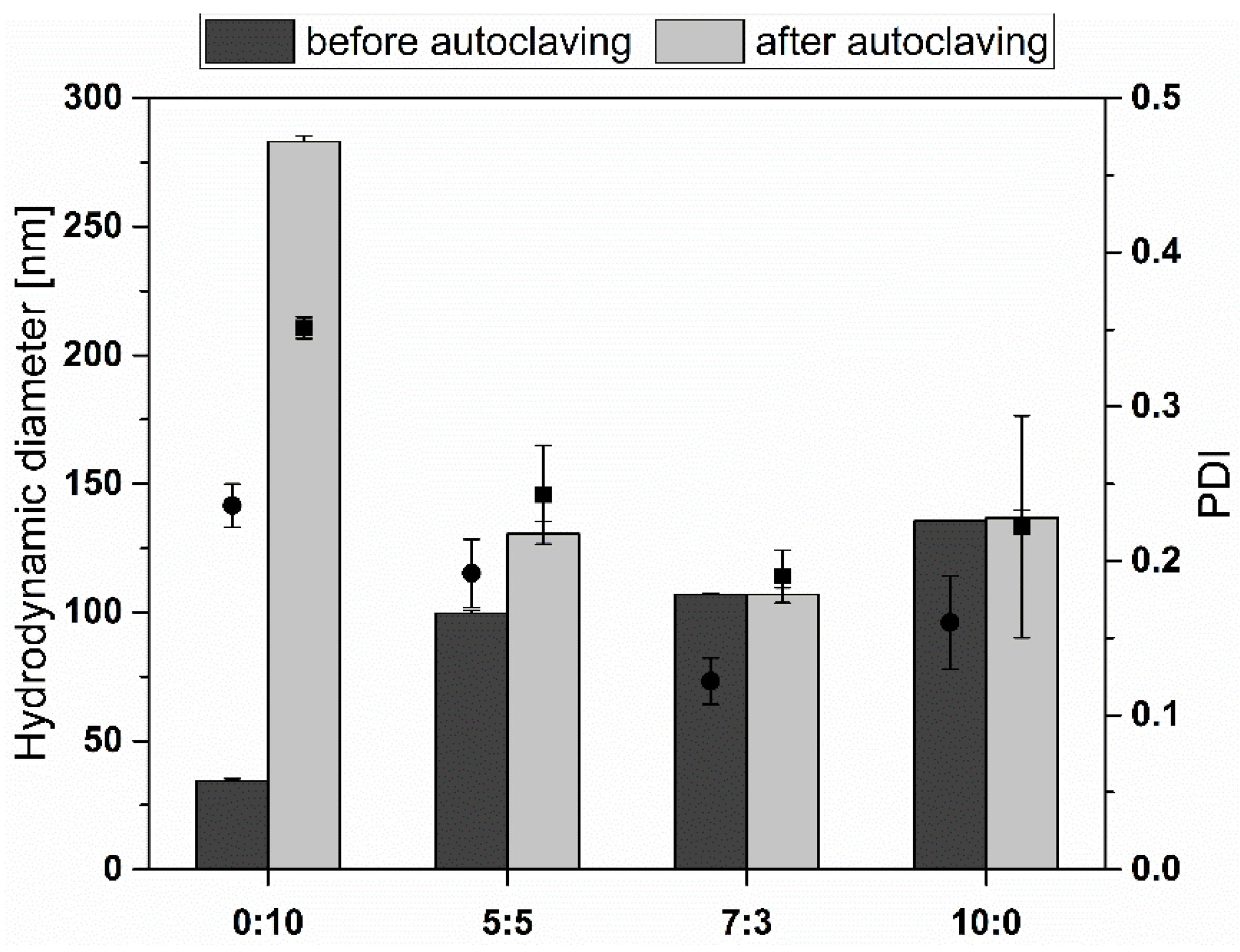
2.8. Stability during Autoclavation
2.9. pH Stability
2.10. Stability in Lung Surfactant
2.11. Stability against FCS
2.12. Statistical Analysis
3. Results
3.1. Physicochemical Characterization
3.2. Thermostability of the Liposomes
3.3. Stability during Autoclavation
3.4. pH Stability
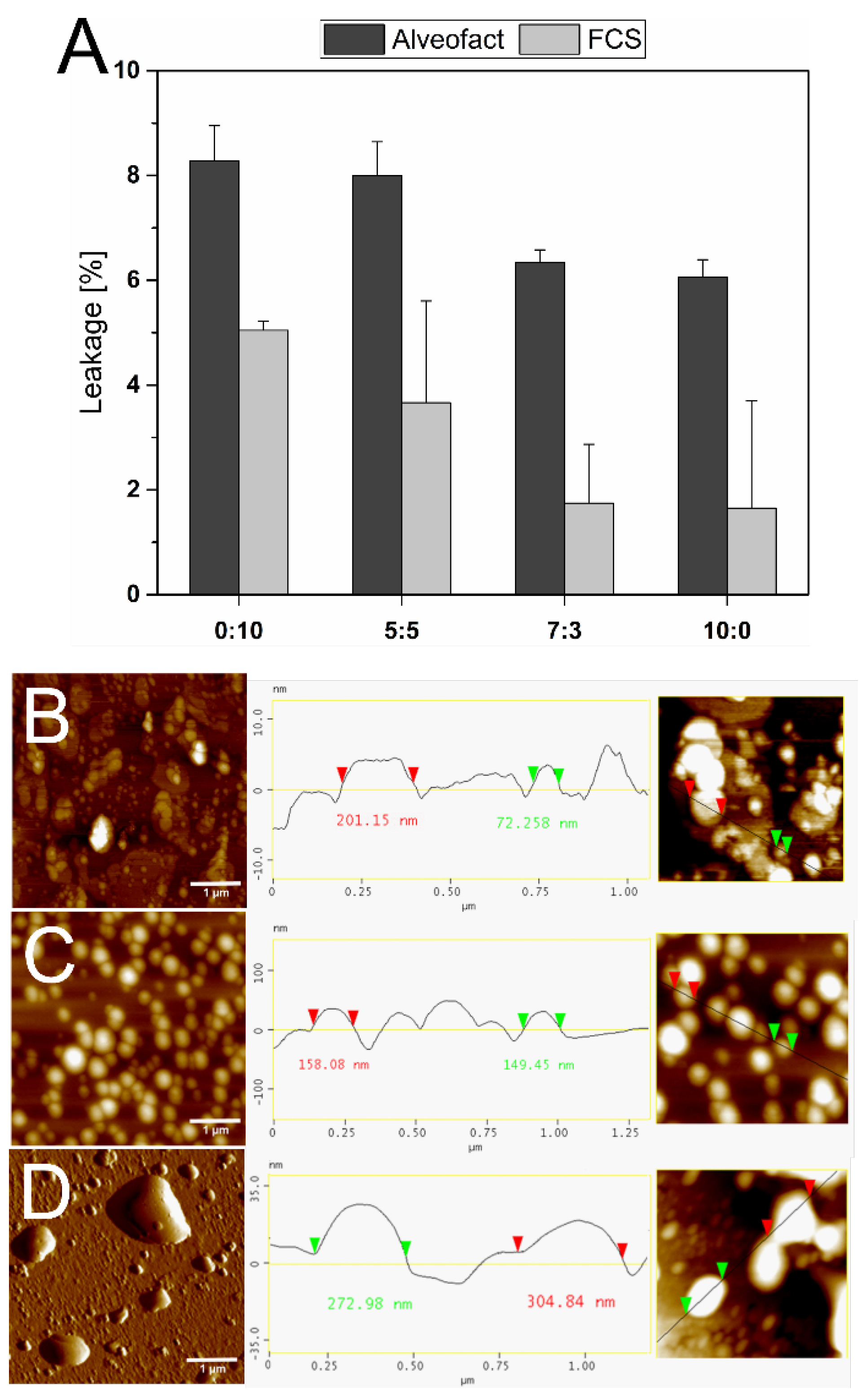
3.5. Stability against Alveofact and FCS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252. [Google Scholar] [CrossRef]
- Bunker, A.; Magarkar, A.; Viitala, T. Rational design of liposomal drug delivery systems, a review: Combined experimental and computational studies of lipid membranes, liposomes and their PEGylation. Biochim. Biophys. Acta 2016, 1858, 2334–2352. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Du, C.; Guo, N.; Teng, Y.; Meng, X.; Sun, H.; Li, S.; Yu, P.; Galons, H. Composition design and medical application of liposomes. Eur. J. Med. Chem. 2019, 164, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, R.; Han, G.; Liu, X.; Huang, T.; Diao, J.; Sun, Y. Super-resolution analyzing spatial organization of lysosomes with an organic fluorescent probe. Exploration 2022, 2, 20210215. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Li, K.; Hu, B.; Li, C.; Zhang, M.; Hussain, A.; Wang, X.; Cheng, Q.; Yang, F.; Ge, K.; et al. Membrane-destabilizing ionizable lipid empowered imaging-guided siRNA delivery and cancer treatment. Exploration 2021, 1, 35–49. [Google Scholar] [CrossRef]
- Marxer, E.E.J.; Brüssler, J.; Becker, A.; Schümmelfeder, J.; Schubert, R.; Nimsky, C.; Bakowsky, U. Development and characterization of new nanoscaled ultrasound active lipid dispersions as contrast agents. Eur. J. Pharm. Biopharm. 2011, 77, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, M.M.; Radomska, A.; Gobbo, O.L.; Bakowsky, U.; Radomski, M.W.; Ehrhardt, C. Targeted delivery of transferrin-conjugated liposomes to an orthotopic model of lung cancer in nude rats. J. Aerosol. Med. Pulm. Drug Deliv. 2012, 25, 310–318. [Google Scholar] [CrossRef]
- Zylberberg, C.; Matosevic, S. Pharmaceutical liposomal drug delivery: A review of new delivery systems and a look at the regulatory landscape. Drug Deliv. 2016, 23, 3319–3329. [Google Scholar] [CrossRef]
- Panahi, Y.; Farshbaf, M.; Mohammadhosseini, M.; Mirahadi, M.; Khalilov, R.; Saghfi, S.; Akbarzadeh, A. Recent advances on liposomal nanoparticles: Synthesis, characterization and biomedical applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Ewe, A.; Panchal, O.; Pinnapireddy, S.R.; Bakowsky, U.; Przybylski, S.; Temme, A.; Aigner, A. Liposome-polyethylenimine complexes (DPPC-PEI lipopolyplexes) for therapeutic siRNA delivery in vivo. Nanomedicine 2017, 13, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Preis, E.; Schulze, J.; Gutberlet, B.; Pinnapireddy, S.R.; Jedelská, J.; Bakowsky, U. The chorioallantoic membrane as a bio-barrier model for the evaluation of nanoscale drug delivery systems for tumour therapy. Adv. Drug Deliv. Rev. 2021, 174, 317–336. [Google Scholar] [CrossRef]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New Developments in Liposomal Drug Delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef] [PubMed]
- Wang, G. Liposomes as Drug Delivery Vehicles. In Drug Delivery: Principles and Applications, 2nd ed.; Wang, B., Hu, L., Siahaan, T., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 272–298. ISBN 9781118833322. [Google Scholar]
- Fricker, G.; Kromp, T.; Wendel, A.; Blume, A.; Zirkel, J.; Rebmann, H.; Setzer, C.; Quinkert, R.-O.; Martin, F.; Müller-Goymann, C. Phospholipids and lipid-based formulations in oral drug delivery. Pharm. Res. 2010, 27, 1469–1486. [Google Scholar] [CrossRef]
- Kraft, J.C.; Freeling, J.P.; Wang, Z.; Ho, R.J. Emerging Research and Clinical Development Trends of Liposome and Lipid Nanoparticle Drug Delivery Systems. J. Pharm. Sci. 2014, 103, 29–52. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, A.-C.; Jensen, S.M.; Fricker, G.; Brandl, M.; Treusch, A.H. Archaeal lipids in oral delivery of therapeutic peptides. Eur. J. Pharm. Sci. 2017, 108, 101–110. [Google Scholar] [CrossRef]
- Benvegnu, T.; Réthoré, G.; Brard, M.; Richter, W.; Plusquellec, D. Archaeosomes based on novel synthetic tetraether-type lipids for the development of oral delivery systems. Chem. Commun. 2005, 5536–5538. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.L.-G. Archaebacterial bipolar tetraether lipids: Physico-chemical and membrane properties. Chem. Phys. Lipids 2010, 163, 253–265. [Google Scholar] [CrossRef]
- Müller, S.; Gruhle, K.; Meister, A.; Hause, G.; Drescher, S. Bolalipid-Doped Liposomes: Can Bolalipids Increase the Integrity of Liposomes Exposed to Gastrointestinal Fluids? Pharmaceutics 2019, 11, 646. [Google Scholar] [CrossRef]
- Ozcetin, A.; Mutlu, S.; Bakowsky, U. Archaebacterial tetraetherlipid liposomes. Methods Mol. Biol. 2010, 605, 87–96. [Google Scholar] [CrossRef]
- Darland, G.; Brock, T.D.; Samsonoff, W.; Conti, S.F. A thermophilic, acidophilic mycoplasma isolated from a coal refuse pile. Science 1970, 170, 1416–1418. [Google Scholar] [CrossRef] [PubMed]
- Bakowsky, U.; Rothe, U.; Antonopoulos, E.; Martini, T.; Henkel, L.; Freisleben, H.-J. Monomolecular organization of the main tetraether lipid from Thermoplasma acidophilum at the water–air interface. Chem. Phys. Lipids 2000, 105, 31–42. [Google Scholar] [CrossRef]
- Mahmoud, G.; Jedelská, J.; Strehlow, B.; Omar, S.; Schneider, M.; Bakowsky, U. Photo-responsive tetraether lipids based vesicles for prophyrin mediated vascular targeting and direct phototherapy. Colloids Surf. B Biointerfaces 2017, 159, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.; Pfannmöller, M.; Teuscher, N.; Heilmann, A.; Rothe, U. New Method for Surface Modification of Nanoporous Aluminum Oxide Membranes Using Tetraether Lipid. J. Biomed. Nanotechnol. 2006, 2, 16–22. [Google Scholar] [CrossRef]
- Vitkova, V.; Mitkova, D.; Yordanova, V.; Pohl, P.; Bakowsky, U.; Staneva, G.; Batishchev, O. Elasticity and phase behaviour of biomimetic membrane systems containing tetraether archaeal lipids. Colloids Surf. A Physicochem. Eng. Asp. 2020, 601, 124974. [Google Scholar] [CrossRef]
- Plenagl, N.; Seitz, B.S.; Duse, L.; Pinnapireddy, S.R.; Jedelska, J.; Brüßler, J.; Bakowsky, U. Hypericin inclusion complexes encapsulated in liposomes for antimicrobial photodynamic therapy. Int. J. Pharm. 2019, 570, 118666. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, G.; Jedelská, J.; Strehlow, B.; Bakowsky, U. Bipolar tetraether lipids derived from thermoacidophilic archaeon Sulfolobus acidocaldarius for membrane stabilization of chlorin e6 based liposomes for photodynamic therapy. Eur. J. Pharm. Biopharm. 2015, 95, 88–98. [Google Scholar] [CrossRef]
- Parmentier, J.; Hofhaus, G.; Thomas, S.; Cuesta, L.C.; Gropp, F.; Schröder, R.; Hartmann, K.; Fricker, G. Improved oral bioavailability of human growth hormone by a combination of liposomes containing bio-enhancers and tetraether lipids and omeprazole. J. Pharm. Sci. 2014, 103, 3985–3993. [Google Scholar] [CrossRef]
- Li, Z.; Chen, J.; Sun, W.; Xu, Y. Investigation of archaeosomes as carriers for oral delivery of peptides. Biochem. Biophys. Res. Commun. 2010, 394, 412–417. [Google Scholar] [CrossRef]
- Parmentier, J.; Thewes, B.; Gropp, F.; Fricker, G. Oral peptide delivery by tetraether lipid liposomes. Int. J. Pharm. 2011, 415, 150–157. [Google Scholar] [CrossRef]
- Uhl, P.; Pantze, S.; Storck, P.; Parmentier, J.; Witzigmann, D.; Hofhaus, G.; Huwyler, J.; Mier, W.; Fricker, G. Oral delivery of vancomycin by tetraether lipid liposomes. Eur. J. Pharm. Sci. 2017, 108, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Uhl, P.; Helm, F.; Hofhaus, G.; Brings, S.; Kaufman, C.; Leotta, K.; Urban, S.; Haberkorn, U.; Mier, W.; Fricker, G. A liposomal formulation for the oral application of the investigational hepatitis B drug Myrcludex B. Eur. J. Pharm. Biopharm. 2016, 103, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Higaki, K.; Sezaki, H. Transmucosal passage of liposomally-entrapped drugs in rat small intestine. Pharm. Res. 1984, 1, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Gilligan, C.A.; Li Wan Po, A. Oral vaccines: Design and delivery. Int. J. Pharm. 1991, 75, 1–24. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Huang, L.; Gauthier, M.; Yang, G.; Wang, Q. Recent advances in liposome surface modification for oral drug delivery. Nanomedicine 2016, 11, 1169–1185. [Google Scholar] [CrossRef]
- Richards, M.H.; Gardner, C.R. Effects of bile salts on the structural integrity of liposomes. Biochim. Et Biophys. Acta (BBA) Gen. Subj. 1978, 543, 508–522. [Google Scholar] [CrossRef]
- Freisleben, H.-J.; Zwicker, K.; Jezek, P.; John, G.; Bettin-Bogutzki, A.; Ring, K.; Nawroth, T. Reconstitution of bacteriorhodopsin and ATP synthase from Micrococcus luteus into liposomes of the purified main tetraether lipid from Thermoplasma acidophilum: Proton conductance and light-driven ATP synthesis. Chem. Phys. Lipids 1995, 78, 137–147. [Google Scholar] [CrossRef]
- Niven, R.W. Delivery of Biotherapeutics by Inhalation Aerosol. Crit. Rev. Ther. Drug Carr. Syst. 1995, 12, 151–231. [Google Scholar] [CrossRef]
- Anabousi, S.; Kleemann, E.; Bakowsky, U.; Kissel, T.; Schmehl, T.; Gessler, T.; Seeger, W.; Lehr, C.-M.; Ehrhardt, C. Effect of PEGylation on the Stability of Liposomes During Nebulisation and in Lung Surfactant. J. Nanosci. Nanotechnol. 2006, 6, 3010–3016. [Google Scholar] [CrossRef]
- Lehmann, J.; Agel, M.R.; Engelhardt, K.H.; Pinnapireddy, S.R.; Agel, S.; Duse, L.; Preis, E.; Wojcik, M.; Bakowsky, U. Improvement of Pulmonary Photodynamic Therapy: Nebulisation of Curcumin-Loaded Tetraether Liposomes. Pharmaceutics 2021, 13, 1243. [Google Scholar] [CrossRef]
- Schulze, J.; Lehmann, J.; Agel, S.; Amin, M.U.; Schaefer, J.; Bakowsky, U. In Ovo Testing Method for Inhalants on a Chorio-Allantoic Membrane. ACS Appl. Bio Mater. 2021, 4, 7764–7768. [Google Scholar] [CrossRef] [PubMed]
- Bode, M.L.; Buddoo, S.R.; Minnaar, S.H.; Du Plessis, C.A. Extraction, isolation and NMR data of the tetraether lipid calditoglycerocaldarchaeol (GDNT) from Sulfolobus metallicus harvested from a bioleaching reactor. Chem. Phys. Lipids 2008, 154, 94–104. [Google Scholar] [CrossRef]
- Ishii, F.; Nagasaka, Y. Simple and Convenient Method for Estimation of Marker Entrapped in Liposomes. J. Dispers. Sci. Technol. 2001, 22, 97–101. [Google Scholar] [CrossRef]
- Sitterberg, J.; Özcetin, A.; Ehrhardt, C.; Bakowsky, U. Utilising atomic force microscopy for the characterisation of nanoscale drug delivery systems. Eur. J. Pharm. Sci. 2010, 74, 2–13. [Google Scholar] [CrossRef]
- Abu-Dahab, R.; Schäfer, U.F.; Lehr, C.-M. Lectin-functionalized liposomes for pulmonary drug delivery: Effect of nebulization on stability and bioadhesion. Eur. J. Pharm. Sci. 2001, 14, 37–46. [Google Scholar] [CrossRef]
- Kronberg, B.; Dahlman, A.; Carlfors, J.; Karlsson, J.; Artursson, P. Preparation and evaluation of sterically stabilized liposomes: Colloidal stability, serum stability, macrophage uptake, and toxicity. J. Pharm. Sci. 1990, 79, 667–671. [Google Scholar] [CrossRef]
- Rufini, S.; Cesaroni, P.; Desideri, A.; Farias, R.; Gubensek, F.; Gutiérrez, J.M.; Luly, P.; Massoud, R.; Morero, R.; Pedersen, J.Z. Calcium ion independent membrane leakage induced by phospholipase-like myotoxins. Biochemistry 1992, 31, 12424–12430. [Google Scholar] [CrossRef]
- Elferink, M.G.; de Wit, J.G.; Driessen, A.J.; Konings, W.N. Stability and proton-permeability of liposomes composed of archaeal tetraether lipids. Biochim. Et Biophys. Acta (BBA) Biomembr. 1994, 1193, 247–254. [Google Scholar] [CrossRef]
- Jarrell, H.C.; Zukotynski, K.A.; Sprott, G. Lateral diffusion of the total polar lipids from Thermoplasma acidophilum in multilamellar liposomes. Biochim. Et Biophys. Acta (BBA) Biomembr. 1998, 1369, 259–266. [Google Scholar] [CrossRef][Green Version]
- Komatsu, H.; Chong, P.L. Low permeability of liposomal membranes composed of bipolar tetraether lipids from thermoacidophilic archaebacterium Sulfolobus acidocaldarius. Biochemistry 1998, 37, 107–115. [Google Scholar] [CrossRef]
- Choquet, C.G.; Patel, G.B.; Beveridge, T.J.; Sprott, G.D. Stability of pressure-extruded liposomes made from archaeobacterial ether lipids. Appl. Microbiol. Biotechnol. 1994, 42, 375–384. [Google Scholar] [CrossRef]
- Kikuchi, H.; Carlsson, A.; Yachi, K.; Hirota, S. Possibility of heat sterilization of liposomes. Chem. Pharm. Bull. 1991, 39, 1018–1022. [Google Scholar] [CrossRef]
- Lukyanov, A.N.; Torchilin, V.P. Autoclaving of liposomes. J. Microencapsul. 1994, 11, 669–672. [Google Scholar] [CrossRef]
- Goldbach, P.; Brochart, H.; Wehrlé, P.; Stamm, A. Sterile filtration of liposomes: Retention of encapsulated carboxyfluorescein. Int. J. Pharm. 1995, 117, 225–230. [Google Scholar] [CrossRef]
- Cobley, J.G.; Cox, J.C. Energy conservation in acidophilic bacteria. Microbiol. Rev. 1983, 47, 579–595. [Google Scholar] [CrossRef]
- Krulwich, T.A.; Guffanti, A.A. Physiology of Acidophilic and Alkalophilic Bacteria. In Advances in Microbial Physiology; Rose, A.H., Morris, J.G., Tempest, D.W., Eds.; Academic Press: New York, NY, USA, 1983; pp. 173–214. ISBN 9780120277247. [Google Scholar]
- de Rosa, M.; Gambacorta, A.; Nicolaus, B. A New type of cell membrane, in thermophilic archaebacteria, based on bipolar ether lipids. J. Membr. Sci. 1983, 16, 287–294. [Google Scholar] [CrossRef]
- Anabousi, S.; Laue, M.; Lehr, C.-M.; Bakowsky, U.; Ehrhardt, C. Assessing transferrin modification of liposomes by atomic force microscopy and transmission electron microscopy. Eur. J. Pharm. Biopharm. 2005, 60, 295–303. [Google Scholar] [CrossRef]
- Patel, G.B.; Agnew, B.J.; Deschatelets, L.; Fleming, L.; Sprott, G. In vitro assessment of archaeosome stability for developing oral delivery systems. Int. J. Pharm. 2000, 194, 39–49. [Google Scholar] [CrossRef]
| Formulation | Hydrodynamic Diameter [nm] | PDI | Zeta Potential [mV] |
|---|---|---|---|
| 0:10 TEL:Phospholipon 100H | 34.56 ± 0.77 | 0.24 ± 0.01 | −19.40 ± 0.03 |
| 5:5 TEL:Phospholipon 100H | 99.64 ± 1.04 | 0.19 ± 0.02 | −42.50 ± 1.41 |
| 7:3 TEL:Phospholipon 100H | 107.00 ± 0.53 | 0.12 ± 0.02 | −45.50 ± 0.63 |
| 10:0 TEL:Phospholipon 100H | 135.60 ± 0.03 | 0.16 ± 0.03 | −49.90 ± 0.91 |
| Formulation | PDI | ||
|---|---|---|---|
| 25 °C | 36 °C | 60 °C | |
| 0:10 TEL:Phospholipon 100H | 0.24 ± 0.04 | 0.25 ± 0.08 | 0.44 ± 0.11 |
| 5:5 TEL:Phospholipon 100H | 0.16 ± 0.01 | 0.15 ± 0.06 | 0.18 ± 0.01 |
| 7:3 TEL:Phospholipon 100H | 0.12 ± 0.02 | 0.13 ± 0.01 | 0.14 ± 0.02 |
| 10:0 TEL:Phospholipon 100H | 0.15 ± 0.03 | 0.17 ± 0.04 | 0.17 ± 0.05 |
| Formulation | PDI | |||
|---|---|---|---|---|
| pH 2.0 | pH 4.0 | pH 7.4 | pH 9.0 | |
| 0:10 TEL:Phospholipon 100H | 0.43 ± 0.02 | 0.31 ± 0.05 | 0.20 ± 0.11 | 0.28 ± 0.04 |
| 5:5 TEL:Phospholipon 100H | 0.22 ± 0.09 | 0.23 ± 0.02 | 0.12 ± 0.02 | 0.14 ± 0.02 |
| 7:3 TEL:Phospholipon 100H | 0.06 ± 0.05 | 0.12 ± 0.02 | 0.13 ± 0.02 | 0.15 ± 0.07 |
| 10:0 TEL:Phospholipon 100H | 0.14 ± 0.02 | 0.15 ± 0.03 | 0.14 ± 0.04 | 0.24 ± 0.03 |
| Formulation | Encapsulation Efficiency (EE) |
|---|---|
| 0:10 TEL:Phospholipon 100H | 18.80 ± 3.81% |
| 5:5 TEL:Phospholipon 100H | 22.73 ± 0.21% |
| 7:3 TEL:Phospholipon 100H | 20.43 ± 5.71% |
| 10:0 TEL:Phospholipon 100H | 20.48 ± 4.94% |
| Formulation | FCS | Alveofact | ||
|---|---|---|---|---|
| Diameter [nm] | PDI | Diameter [nm] | PDI | |
| 0:10 TEL:Phospholipon 100H | 187.25 ± 7.95 | 0.38 ± 0.06 | 252.56 ± 6.88 | 0.44 ± 0.07 |
| 5:5 TEL:Phospholipon 100H | 132.21 ± 2.38 | 0.17 ± 0.04 | 151.71 ± 3.22 | 0.22 ± 0.03 |
| 7:3 TEL:Phospholipon 100H | 148.65 ± 3.31 | 0.19 ± 0.02 | 155.67 ± 3.45 | 0.20 ± 0.04 |
| 10:0 TEL:Phospholipon 100H | 145.70 ± 0.03 | 0.19 ± 0.03 | 142.35 ± 2.64 | 0.18 ± 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hemetsberger, A.; Preis, E.; Engelhardt, K.; Gutberlet, B.; Runkel, F.; Bakowsky, U. Highly Stable Liposomes Based on Tetraether Lipids as a Promising and Versatile Drug Delivery System. Materials 2022, 15, 6995. https://doi.org/10.3390/ma15196995
Hemetsberger A, Preis E, Engelhardt K, Gutberlet B, Runkel F, Bakowsky U. Highly Stable Liposomes Based on Tetraether Lipids as a Promising and Versatile Drug Delivery System. Materials. 2022; 15(19):6995. https://doi.org/10.3390/ma15196995
Chicago/Turabian StyleHemetsberger, Aybike, Eduard Preis, Konrad Engelhardt, Bernd Gutberlet, Frank Runkel, and Udo Bakowsky. 2022. "Highly Stable Liposomes Based on Tetraether Lipids as a Promising and Versatile Drug Delivery System" Materials 15, no. 19: 6995. https://doi.org/10.3390/ma15196995
APA StyleHemetsberger, A., Preis, E., Engelhardt, K., Gutberlet, B., Runkel, F., & Bakowsky, U. (2022). Highly Stable Liposomes Based on Tetraether Lipids as a Promising and Versatile Drug Delivery System. Materials, 15(19), 6995. https://doi.org/10.3390/ma15196995








