Biocompatible Nano-Hydroxyapatites Regulate Macrophage Polarization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
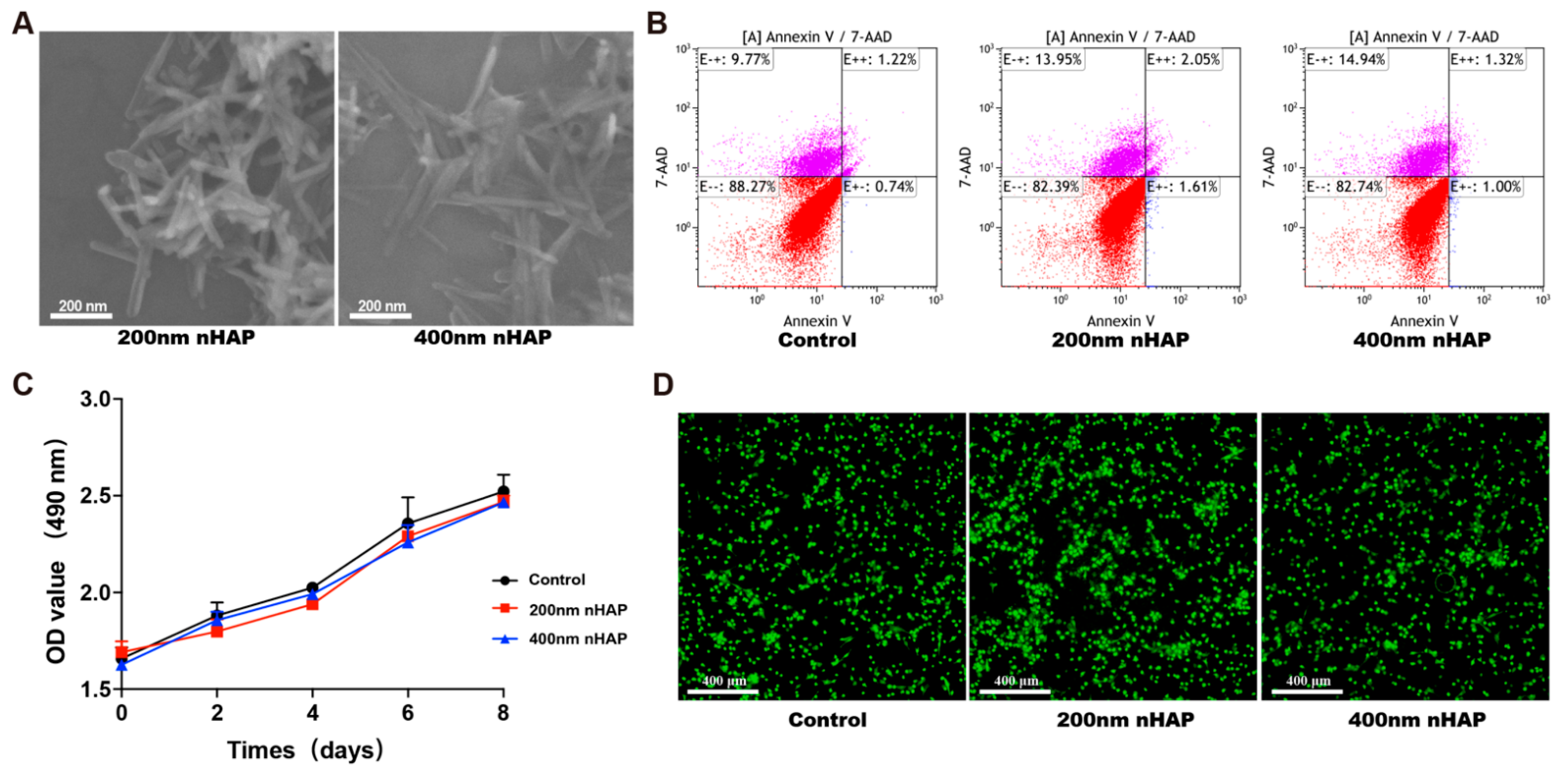
2.2.1. Fabrication and Morphology of nHAP
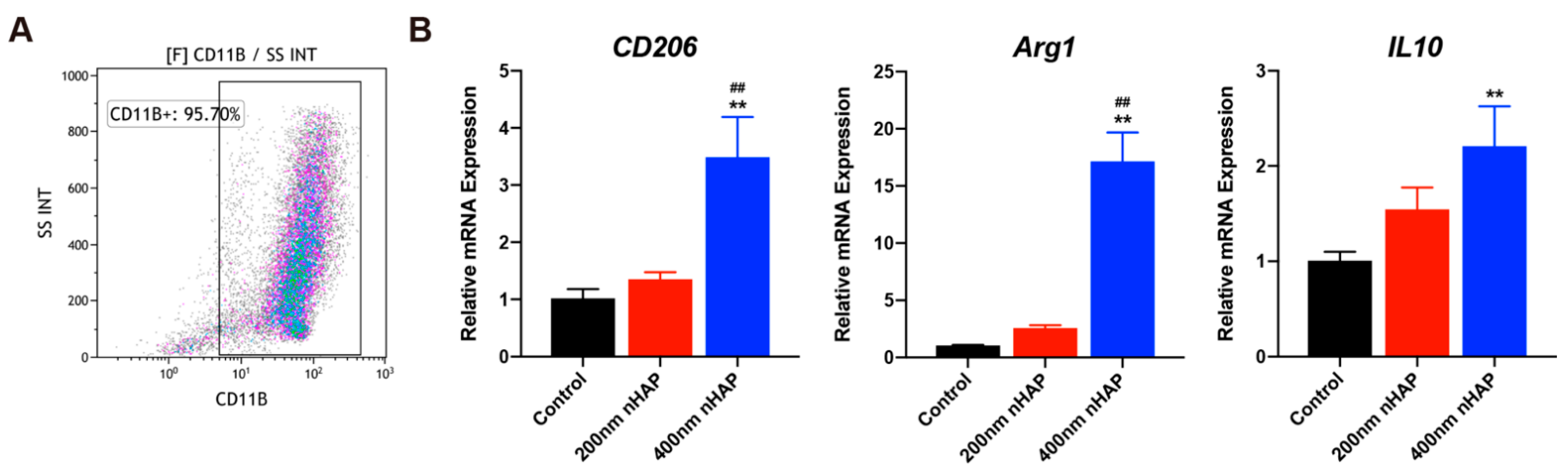
2.2.2. Rat Macrophage Isolation, Culture, and Identification
2.2.3. Cell Apoptosis
2.2.4. Methyl Thiazolyl Tetrazolium (MTT) Assay
2.2.5. Live/Dead Staining
2.2.6. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
- CD206: Forward 5′-GAGGACTGCGTGGTGATGAA-3′ and reverse 5′-CATGCCGTTTCCAGCCTTTC-3′;
- Arg1: Forward 5′-AAGACAGGGCTACTTTCAGGAC-3′ and reverse 5′-ACCTTCCCGTTTCGTTCCAA-3′;
- IL10: Forward 5′-TAACTGCACCCACTTCCCAG-3′ and reverse 5′-TGGCAACCCAAGTAACCCTTAAA-3′;
- β-actin: Forward 5′-CCTCTATGACAACACAGT-3′ and reverse 5′-AGCCACCAATCCACACAG-3′.
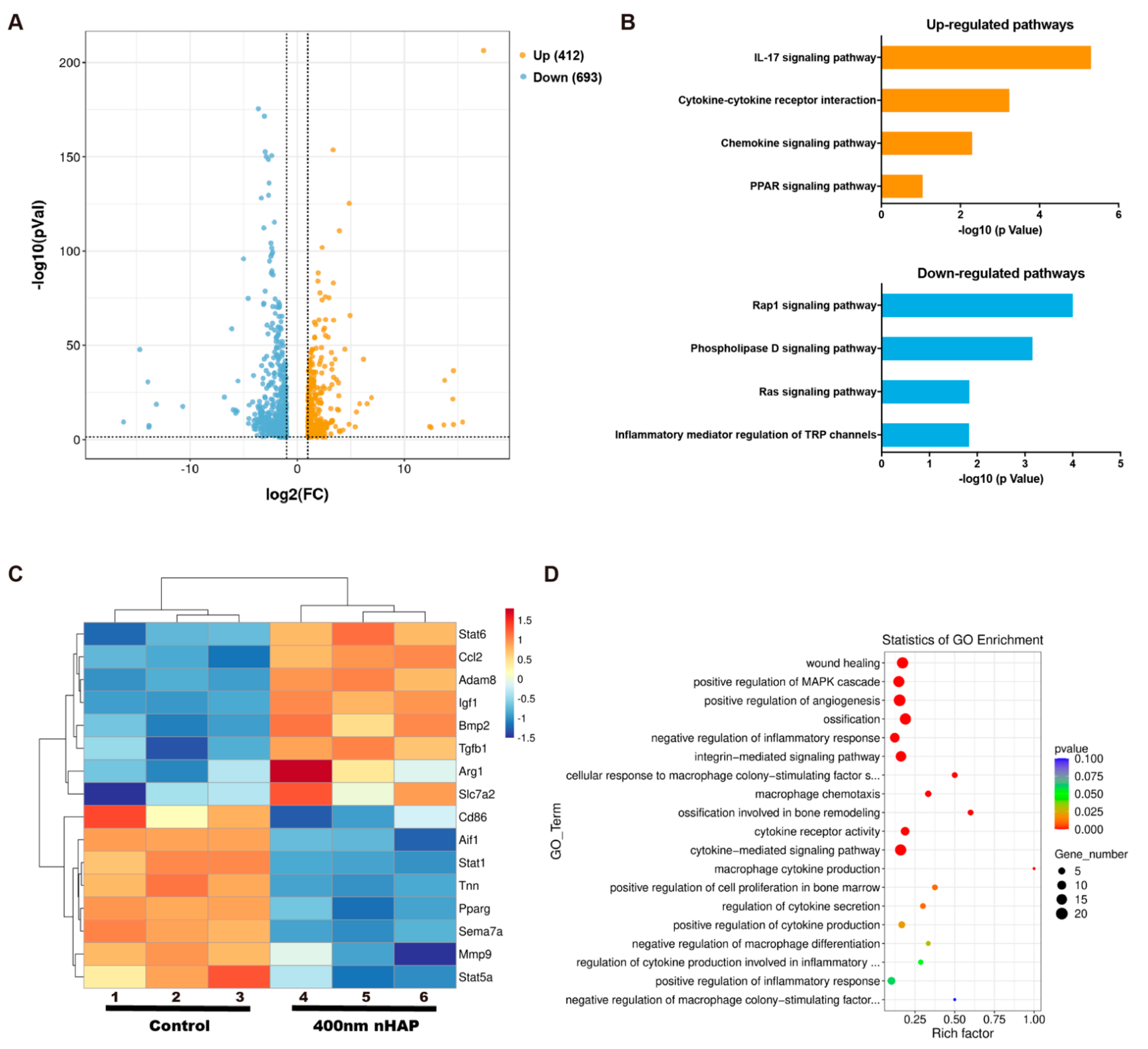
2.2.7. RNA-Sequence
2.2.8. Statistical Analysis
3. Results
3.1. Characterization and Biocompatibility of nHAPs
3.2. Evaluation of Macrophage Characterization and Polarization
3.3. RNA-Sequence
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bai, L.; Liu, Y.; Du, Z.; Weng, Z.; Yao, W.; Zhang, X.; Xiao, Y. Differential effect of hydroxyapatite nano-particle versus nano-rod decorated titanium micro-surface on osseointegration. Acta Biomater. 2018, 76, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Du, Z.; Du, J.; Yao, W.; Zhang, J.; Weng, Z.; Xiao, Y. A multifaceted coating on titanium dictates osteoimmunomodulation and osteo/angio-genesis towards ameliorative osseointegratioxn. Biomaterials 2018, 162, 154–169. [Google Scholar] [CrossRef]
- Reilly, G.C.; Engler, A.J. Intrinsic extracellular matrix properties regulate stem cell differentiation. J. Biomech. 2010, 43, 55–62. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, H.K.; Kim, K.S. Intrinsic and extrinsic mechanical properties related to the differentiation of mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2016, 473, 752–757. [Google Scholar] [CrossRef]
- Zhao, D.-W.; Liu, C.; Zuo, K.-Q.; Su, P.; Li, L.-B.; Xiao, G.-Y.; Cheng, L. Strontium-zinc phosphate chemical conversion coating improves the osseointegration of titanium implants by regulating macrophage polarization. Chem. Eng. J. 2021, 408, 127362. [Google Scholar] [CrossRef]
- Alipour, M.; Firouzi, N.; Aghazadeh, Z.; Samiei, M.; Montazersaheb, S.; Khoshfetrat, A.B.; Aghazadeh, M. The osteogenic differentiation of human dental pulp stem cells in alginate-gelatin/Nano-hydroxyapatite microcapsules. BMC Biotechnol 2021, 21, 6. [Google Scholar] [CrossRef]
- Grotenhuis, N.; De Witte, S.F.; van Osch, G.J.; Bayon, Y.; Lange, J.F.; Bastiaansen-Jenniskens, Y.M. Biomaterials Influence Macrophage-Mesenchymal Stem Cell Interaction In Vitro. Tissue Eng. Part A 2016, 22, 1098–1107. [Google Scholar] [CrossRef]
- Julier, Z.; Park, A.J.; Briquez, P.S.; Martino, M.M. Promoting tissue regeneration by modulating the immune system. Acta Biomater. 2017, 53, 13–28. [Google Scholar] [CrossRef]
- Hotchkiss, K.M.; Reddy, G.B.; Hyzy, S.L.; Schwartz, Z.; Boyan, B.D.; Olivares-Navarrete, R. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater. 2016, 31, 425–434. [Google Scholar] [CrossRef]
- Glass, G.E.; Chan, J.K.; Freidin, A.; Feldmann, M.; Horwood, N.J.; Nanchahal, J. TNF-alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc. Natl. Acad. Sci. USA 2011, 108, 1585–1590. [Google Scholar] [CrossRef]
- Franz, S.; Rammelt, S.; Scharnweber, D.; Simon, J.C. Immune responses to implants—A review of the implications for the design of immunomodulatory biomaterials. Biomaterials 2011, 32, 6692–6709. [Google Scholar] [CrossRef]
- Mahon, O.R.; Browe, D.C.; Gonzalez-Fernandez, T.; Pitacco, P.; Whelan, I.T.; Von Euw, S.; Hobbs, C.; Nicolosi, V.; Cunningham, K.T.; Mills, K.H.G.; et al. Nano-particle mediated M2 macrophage polarization enhances bone formation and MSC osteogenesis in an IL-10 dependent manner. Biomaterials 2020, 239, 119833. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.S.; He, D.Q.; Luo, D.; Wang, Y.; Yu, M.; Guan, B.; Fu, Y.; Li, Z.X.; Zhang, T.; Zhou, Y.H.; et al. A Biomimetic Hierarchical Nanointerface Orchestrates Macrophage Polarization and Mesenchymal Stem Cell Recruitment To Promote Endogenous Bone Regeneration. ACS Nano 2019, 13, 6581–6595. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, H.; Hao, G.; Zhang, Y.; Ding, H.; Fan, Z.; Sun, L. 3D Printing Hydrogel Scaffolds with Nanohydroxyapatite Gradient to Effectively Repair Osteochondral Defects in Rats. Adv. Funct. Mater. 2020, 31, 2006697. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, X.; Lv, X.; Zhang, M.; Huang, X.; Shi, Y.; Lu, C.; Song, J.; Yang, H. Bioinspired Mineral–Organic Bone Adhesives for Stable Fracture Fixation and Accelerated Bone Regeneratio. Adv. Funct. Mater. 2019, 30, 1908381. [Google Scholar] [CrossRef]
- Ma, B.; Zhang, S.; Liu, F.; Duan, J.; Wang, S.; Han, J.; Sang, Y.; Yu, X.; Li, D.; Tang, W.; et al. One-Dimensional Hydroxyapatite Nanostructures with Tunable Length for Efficient Stem Cell Differentiation Regulation. ACS Appl. Mater. Interfaces 2017, 9, 33717–33727. [Google Scholar] [CrossRef]
- Hubner, W.; Blume, A.; Pushnjakova, R.; Dekhtyar, Y.; Hein, H.J. The influence of X-ray radiation on the mineral/organic matrix interaction of bone tissue: An FT-IR microscopic investigation. Int. J. Artif. Organs 2005, 28, 66–73. [Google Scholar] [CrossRef]
- Glass, K.A.; Link, J.M.; Brunger, J.M.; Moutos, F.T.; Gersbach, C.A.; Guilak, F. Tissue-engineered cartilage with inducible and tunable immunomodulatory properties. Biomaterials 2014, 35, 5921–5931. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.W.; Zuo, K.Q.; Wang, K.; Sun, Z.Y.; Lu, Y.P.; Cheng, L.; Xiao, G.Y.; Liu, C. Interleukin-4 assisted calcium-strontium-zinc-phosphate coating induces controllable macrophage polarization and promotes osseointegration on titanium implant. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111512. [Google Scholar] [CrossRef] [PubMed]
- Pagano, S.; Lombardo, G.; Costanzi, E.; Balloni, S.; Bruscoli, S.; Flamini, S.; Coniglio, M.; Valenti, C.; Cianetti, S.; Marinucci, L. Morpho-functional effects of different universal dental adhesives on human gingival fibroblasts: An in vitro study. Odontology 2021, 109, 524–539. [Google Scholar] [CrossRef]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef]
- Noel, W.; Raes, G.; Ghassabeh, G.H.; De Baetselier, P.; Beschin, A. Alternatively activated macrophages during parasite infections. Trends Parasitol. 2004, 20, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat. Rev. Immunol. 2004, 4, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Gan, S.C.; Zhu, Q.C.; Dai, D.F.; Li, N.; Wang, H.; Chen, X.S.; Hou, D.; Wang, Y.; Pan, Q.; et al. Modulation of M2 macrophage polarization by the crosstalk between Stat6 and Trim24. Nat. Commun. 2019, 10, 4353. [Google Scholar] [CrossRef]
- Sierra-Filardi, E.; Nieto, C.; Dominguez-Soto, A.; Barroso, R.; Sanchez-Mateos, P.; Puig-Kroger, A.; Lopez-Bravo, M.; Joven, J.; Ardavin, C.; Rodriguez-Fernandez, J.L.; et al. CCL2 Shapes Macrophage Polarization by GM-CSF and M-CSF: Identification of CCL2/CCR2-Dependent Gene Expression Profile. J. Immunol. 2014, 192, 3858–3867. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Zhuo, X.; Ma, A. STAT6 Upregulation Promotes M2 Macrophage Polarization to Suppress Atherosclerosis. Med. Sci. Monit. Basic Res. 2017, 23, 240–249. [Google Scholar] [CrossRef]
- Egana-Gorrono, L.; Chinnasamy, P.; Casimiro, I.; Almonte, V.M.; Parikh, D.; Oliveira-Paula, G.H.; Jayakumar, S.; Law, C.; Riascos-Bernal, D.F.; Sibinga, N.E.S. Allograft inflammatory factor-1 supports macrophage survival and efferocytosis and limits necrosis in atherosclerotic plaques. Atherosclerosis 2019, 289, 184–194. [Google Scholar] [CrossRef]
- Lacey, D.C.; Simmons, P.J.; Graves, S.E.; Hamilton, J.A. Proinflammatory cytokines inhibit osteogenic differentiation from stem cells: Implications for bone repair during inflammation. Osteoarthr. Cartil. 2009, 17, 735–742. [Google Scholar] [CrossRef]
- Martino, M.M.; Maruyama, K.; Kuhn, G.A.; Satoh, T.; Takeuchi, O.; Muller, R.; Akira, S. Inhibition of IL-1R1/MyD88 signalling promotes mesenchymal stem cell-driven tissue regeneration. Nat. Commun. 2016, 7, 11051. [Google Scholar] [CrossRef]
- Gerstenfeld, L.C.; Cullinane, D.M.; Barnes, G.L.; Graves, D.T.; Einhorn, T.A. Fracture healing as a post-natal developmental process: Molecular, spatial, and temporal aspects of its regulation. J. Cell. Biochem. 2003, 88, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Kon, T.; Cho, T.J.; Aizawa, T.; Yamazaki, M.; Nooh, N.; Graves, D.; Gerstenfeld, L.C.; Einhorn, T.A. Expression of osteoprotegerin, receptor activator of NF-kappaB ligand (Osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J. Bone Miner. Res. 2001, 16, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.C.; Estes, B.T.; Awad, H.A.; Guilak, F. Chondrogenic Differentiation of Adipose-Derived Adult Stem Cells by a Porous Scaffold Derived from Native Articular Cartilage Extracellular Matrix. Tissue Eng. Part A 2009, 15, 231–241. [Google Scholar] [CrossRef]
- Horwood, N.J. Macrophage Polarization and Bone Formation: A review. Clin. Rev. Allerg. Immunol. 2016, 51, 79–86. [Google Scholar] [CrossRef]
- Zhang, Y.; Bose, T.; Unger, R.E.; Jansen, J.A.; Kirkpatrick, C.J.; van den Beucken, J.J.J.P. Macrophage type modulates osteogenic differentiation of adipose tissue MSCs. Cell Tissue Res. 2017, 369, 273–286. [Google Scholar] [CrossRef]
- Gong, L.; Zhao, Y.; Zhang, Y.; Ruan, Z. The Macrophage Polarization Regulates MSC Osteoblast Differentiation in vitro. Ann. Clin. Lab. Sci. 2016, 46, 65–71. [Google Scholar]
- Cai, Y.; Sukhova, G.K.; Wong, H.K.; Xu, A.; Tergaonkar, V.; Vanhoutte, P.M.; Tang, E.H. Rap1 induces cytokine production in pro-inflammatory macrophages through NFkappaB signaling and is highly expressed in human atherosclerotic lesions. Cell Cycle 2015, 14, 3580–3592. [Google Scholar] [CrossRef]
- Gomez-Cambronero, J.; Kantonen, S. A river runs through it: How autophagy, senescence, and phagocytosis could be linked to phospholipase D by Wnt signaling. J. Leukocyte Biol. 2014, 96, 779–784. [Google Scholar] [CrossRef]
- Thabet, N.A.; El-Guendy, N.; Mohamed, M.M.; Shouman, S.A. Suppression of macrophages—Induced inflammation via targeting RAS and PAR-4 signaling in breast cancer cell lines. Toxicol. Appl. Pharm. 2019, 385, 114773. [Google Scholar] [CrossRef]
- Santoni, G.; Morelli, M.B.; Amantini, C.; Santoni, M.; Nabissi, M.; Marinelli, O.; Santoni, A. “Immuno-Transient Receptor Potential Ion Channels”: The Role in Monocyte- and Macrophage-Mediated Inflammatory Responses. Front. Immunol. 2018, 9, 1273. [Google Scholar] [CrossRef]
- Lin, T.; Pajarinen, J.; Nabeshima, A.; Lu, L.; Nathan, K.; Jamsen, E.; Yao, Z.; Goodman, S.B. Preconditioning of murine mesenchymal stem cells synergistically enhanced immunomodulation and osteogenesis. Stem Cell Res. Ther. 2017, 8, 277. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Zou, J.; Wang, W.; Yang, A. BMP2 induces hMSC osteogenesis and matrix remodeling. Mol. Med. Rep. 2021, 23, 125. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.-W.; Fan, X.-C.; Zhao, Y.-X.; Zhao, W.; Zhang, Y.-Q.; Zhang, R.-H.; Cheng, L. Biocompatible Nano-Hydroxyapatites Regulate Macrophage Polarization. Materials 2022, 15, 6986. https://doi.org/10.3390/ma15196986
Zhao D-W, Fan X-C, Zhao Y-X, Zhao W, Zhang Y-Q, Zhang R-H, Cheng L. Biocompatible Nano-Hydroxyapatites Regulate Macrophage Polarization. Materials. 2022; 15(19):6986. https://doi.org/10.3390/ma15196986
Chicago/Turabian StyleZhao, Da-Wang, Xin-Cheng Fan, Yi-Xiang Zhao, Wei Zhao, Yuan-Qiang Zhang, Ren-Hua Zhang, and Lei Cheng. 2022. "Biocompatible Nano-Hydroxyapatites Regulate Macrophage Polarization" Materials 15, no. 19: 6986. https://doi.org/10.3390/ma15196986
APA StyleZhao, D.-W., Fan, X.-C., Zhao, Y.-X., Zhao, W., Zhang, Y.-Q., Zhang, R.-H., & Cheng, L. (2022). Biocompatible Nano-Hydroxyapatites Regulate Macrophage Polarization. Materials, 15(19), 6986. https://doi.org/10.3390/ma15196986






