An Electroconductive, Thermosensitive, and Injectable Chitosan/Pluronic/Gold-Decorated Cellulose Nanofiber Hydrogel as an Efficient Carrier for Regeneration of Cardiac Tissue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
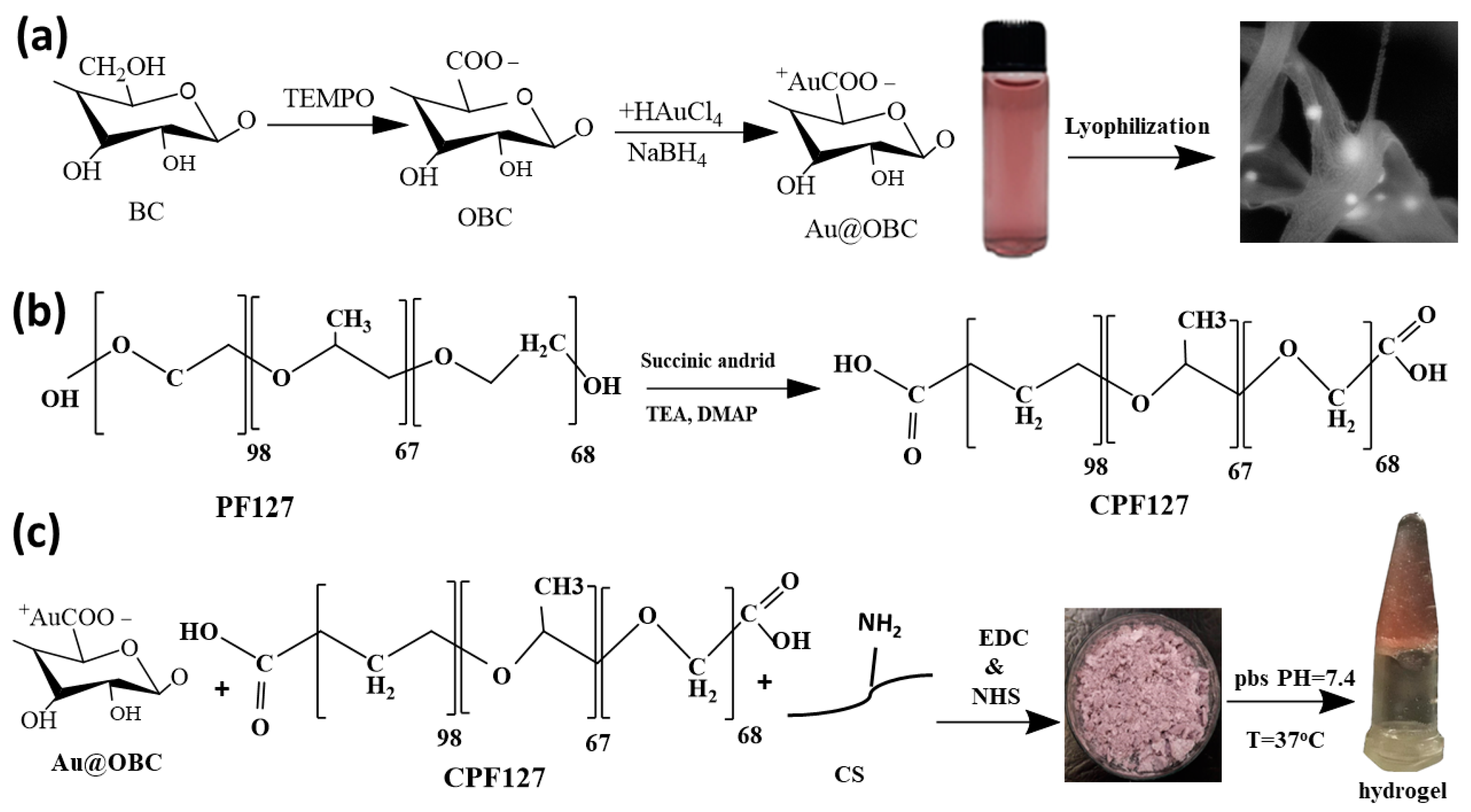
2.2. In Situ Synthesis of Gold Nanoparticles on Bacterial Cellulose Nanofibers
2.3. Preparation of Injectable Nanocomposite Hydrogels
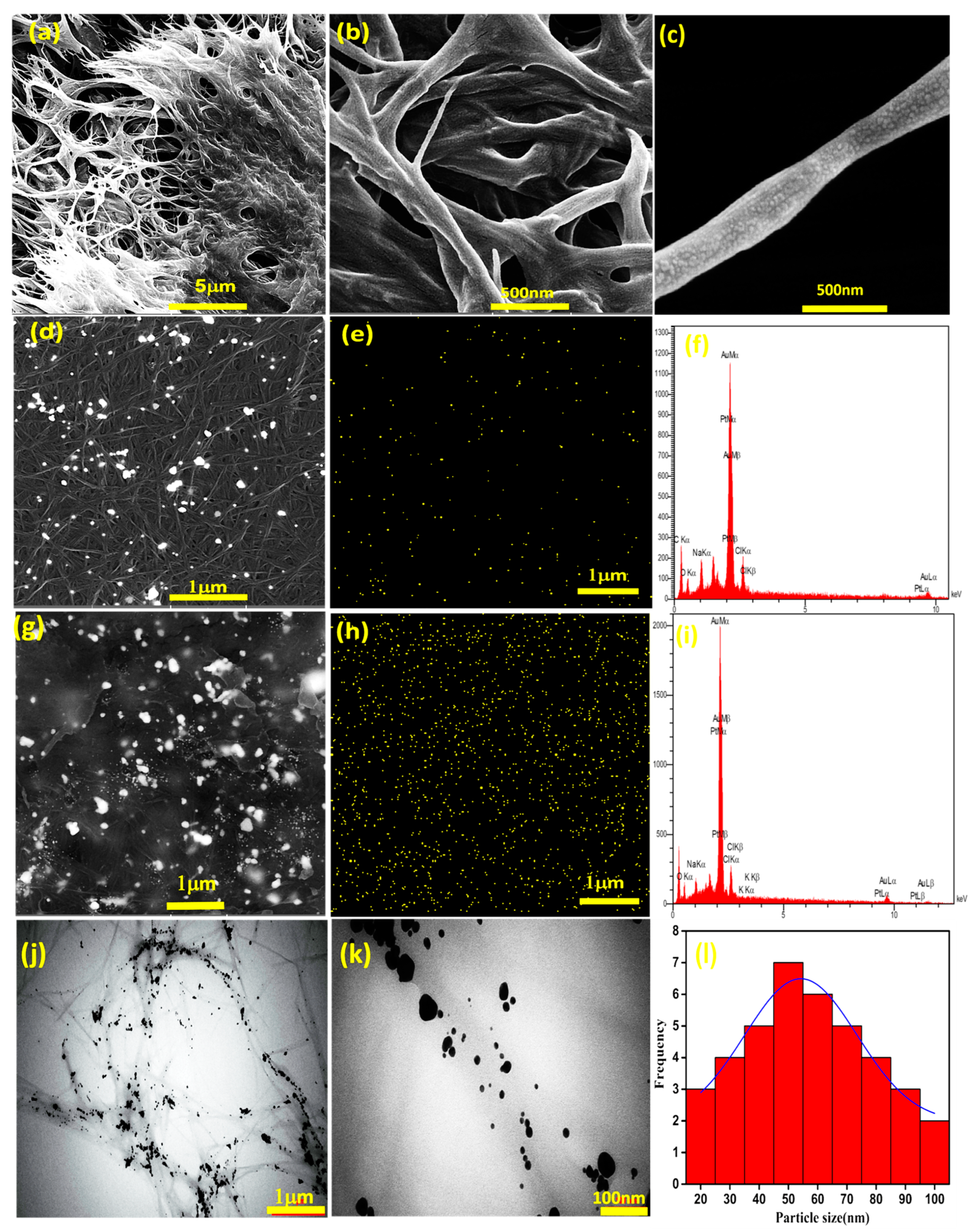
2.4. Material Characterizations
2.5. Rheological Analysis
2.6. Electrical Conductance
2.7. Determination of the Swelling Ratio
2.8. In Vitro Degradation
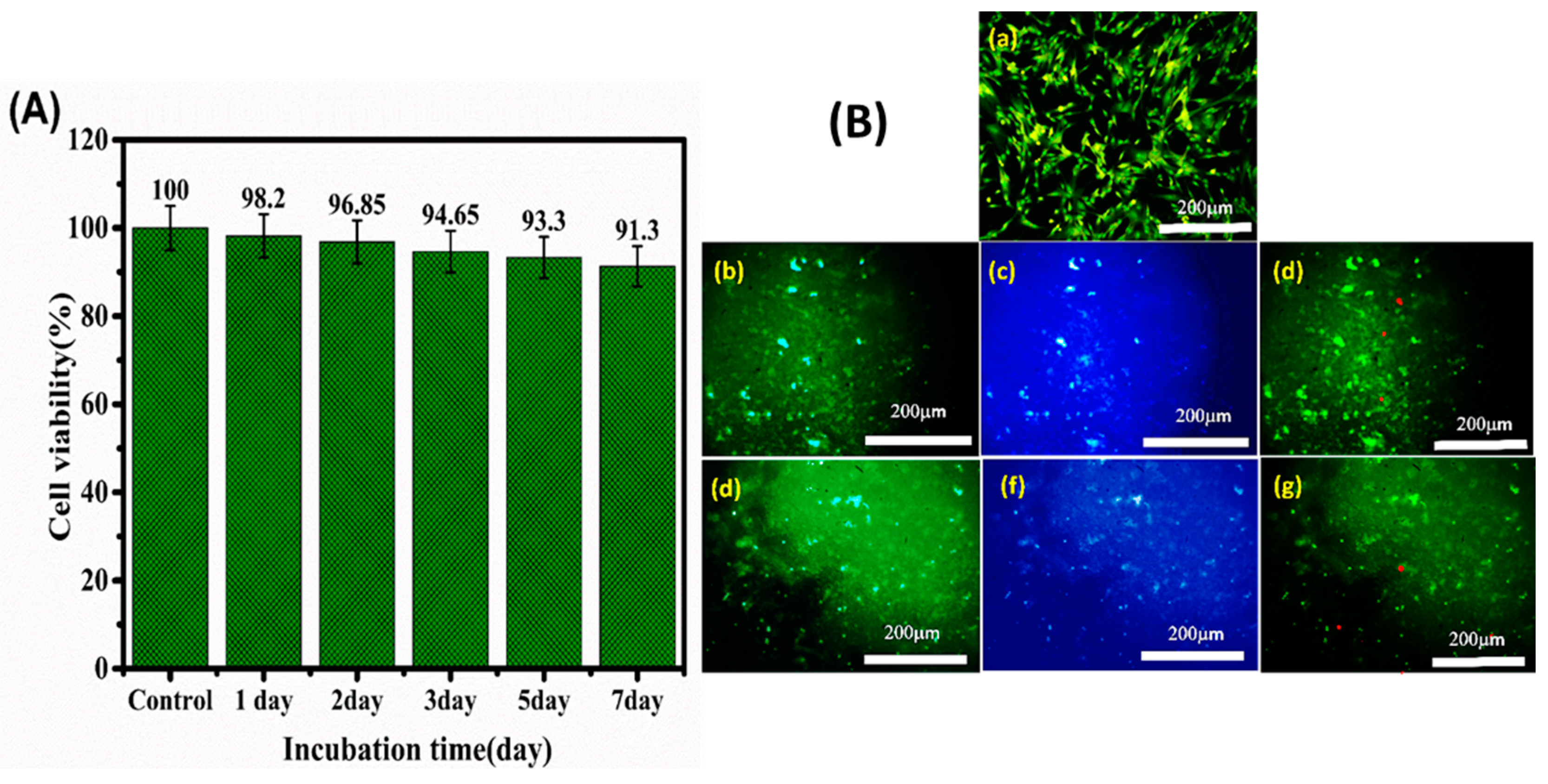
2.9. Cytocompatibility and Cell Adhesion
3. Results
3.1. Characterizations of Thermosensitive and Electroconductive Hydrogels
3.2. Rheological Analysis
3.3. Electrical Conductance
3.4. Swelling and Biodegradation of Hydrogels
3.5. Cell Viability and Cardiac Cell Function
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, U.; Haverich, A. Engineering cardiac muscle: New ways to refurbish old hearts? Eur. J. Cardio-Thoracic Surg. 2014, 45, 216–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, A.; Khattab, A.; Islam, M.A.; Hweij, K.A.; Zeitouny, J.; Waters, R.; Sayegh, M.; Hossain, M.M.; Paul, A. Injectable Hydrogels for Cardiac Tissue Repair after Myocardial Infarction. Adv. Sci. 2015, 2, 1500122. [Google Scholar] [CrossRef] [PubMed]
- Li, R.K.; Jia, Z.Q.; Weisel, R.D.; Mickle, D.A.G.; Choi, A.; Yau, T.M. Survival and function of bioengineered cardiac grafts. Circulation 1999, 100, II-63. [Google Scholar] [CrossRef]
- Barker, R.A.; Carpenter, M.K.; Forbes, S.; Goldman, S.A.; Jamieson, C.; Murry, C.E.; Takahashi, J.; Weir, G. The Challenges of First-in-Human Stem Cell Clinical Trials: What Does This Mean for Ethics and Institutional Review Boards? Stem Cell Rep. 2018, 10, 1429–1431. [Google Scholar] [CrossRef]
- Richardson, W.J.; Clarke, S.A.; Alexander Quinn, T.; Holmes, J.W. Physiological implications of myocardial scar structure. Compr. Physiol. 2015, 5, 1877–1909. [Google Scholar] [CrossRef] [Green Version]
- Rog-Zielinska, E.A.; Norris, R.A.; Kohl, P.; Markwald, R. The Living scar-cardiac fibroblasts and the injured heart. Trends Mol. Med. 2016, 22, 99–114. [Google Scholar] [CrossRef] [Green Version]
- Geng, X.; Liu, B.; Liu, J.; Liu, D.; Lu, Y.; Sun, X.; Liang, K.; Kong, B. Interfacial tissue engineering of heart regenerative medicine based on soft cell-porous scaffolds. J. Thorac. Dis. 2018, 10, S2333–S2345. [Google Scholar] [CrossRef]
- Montero, P.; Flandes-Iparraguirre, M.; Musquiz, S.; Pérez Araluce, M.; Plano, D.; Sanmartín, C.; Orive, G.; Gavira, J.J.; Prosper, F.; Mazo, M.M. Cells, Materials, and Fabrication Processes for Cardiac Tissue Engineering. Front. Bioeng. Biotechnol. 2020, 8, 955. [Google Scholar] [CrossRef]
- Nguyen, M.M.; Gianneschi, N.C.; Christman, K.L. Developing injectable nanomaterials to repair the heart. Curr. Opin. Biotechnol. 2015, 34, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Nicodemus, G.D.; Bryant, S.J. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng.-Part B Rev. 2008, 14, 149–165. [Google Scholar] [CrossRef]
- Wang, Y.; Tu, S.; Pinchuk, A.N.; Xiong, M.P. Active drug encapsulation and release kinetics from hydrogel-in-liposome nanoparticles. J. Colloid Interface Sci. 2013, 406, 247–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vo, T.N.; Kasper, F.K.; Mikos, A.G. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv. Drug Deliv. Rev. 2012, 64, 1292–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Luna, V.H.; González-Reynoso, O. Encapsulation of biological agents in hydrogels for therapeutic applications. Gels 2018, 4, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, W.; Wold, L.E.; Dow, J.S.; Kloner, R.A. Thickening of the infarcted wall by collagen injection improves left ventricular function in rats: A novel approach to preserve cardiac function after myocardial infarction. J. Am. Coll. Cardiol. 2005, 46, 714–719. [Google Scholar] [CrossRef] [Green Version]
- Ifkovits, J.L.; Tous, E.; Minakawa, M.; Morita, M.; Robb, J.D.; Koomalsingh, K.J.; Gorman, J.H.; Gorman, R.C.; Burdick, J.A. Injectable hydrogel properties influence infarct expansion and extent of postinfarction left ventricular remodeling in an ovine model. Proc. Natl. Acad. Sci. USA 2010, 107, 11507–11512. [Google Scholar] [CrossRef] [Green Version]
- So, J.Y.; Yong, H.F.; Choon, H.L.; Bum, S.K.; Ho, S.S.; Park, Y.; Sun, K. Regeneration of ischemic heart using hyaluronic acid-based injectable hydrogel. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2009, 91, 163–171. [Google Scholar] [CrossRef]
- Landa, N.; Miller, L.; Feinberg, M.S.; Holbova, R.; Shachar, M.; Freeman, I.; Cohen, S.; Leor, J. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation 2008, 117, 1388–1396. [Google Scholar] [CrossRef] [Green Version]
- Kofidis, T.; De Bruin, J.L.; Hoyt, G.; Lebl, D.R.; Tanaka, M.; Yamane, T.; Chang, C.P.; Robbins, R.C. Injectable bioartificial myocardial tissue for large-scale intramural cell transfer and functional recovery of injured heart muscle. J. Thorac. Cardiovasc. Surg. 2004, 128, 571–578. [Google Scholar] [CrossRef] [Green Version]
- Seif-Naraghi, S.B.; Singelyn, J.M.; Salvatore, M.A.; Osborn, K.G.; Wang, J.J.; Sampat, U.; Kwan, O.L.; Strachan, G.M.; Wong, J.; Schup-Magoffin, P.J.; et al. Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction. Sci. Transl. Med. 2013, 5, 173ra25. [Google Scholar] [CrossRef] [Green Version]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A comparative review of natural and synthetic biopolymer composite scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Place, E.S.; George, J.H.; Williams, C.K.; Stevens, M.M. Synthetic polymer scaffolds for tissue engineering. Chem. Soc. Rev. 2009, 38, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, M.; Salavati-Niasari, M.; Mohandes, F. Injectable hydrogels based on oxidized alginate-gelatin reinforced by carbon nitride quantum dots for tissue engineering. Int. J. Pharm. 2021, 602, 120660. [Google Scholar] [CrossRef]
- Eslahi, N.; Simchi, A.; Mehrjoo, M.; Shokrgozar, M.A.; Bonakdar, S. Hybrid cross-linked hydrogels based on fibrous protein/block copolymers and layered silicate nanoparticles: Tunable thermosensitivity, biodegradability and mechanical durability. RSC Adv. 2016, 6, 62944–62957. [Google Scholar] [CrossRef]
- Lu, W.N.; Lü, S.H.; Wang, H.B.; Li, D.X.; Duan, C.M.; Liu, Z.Q.; Hao, T.; He, W.J.; Xu, B.; Fu, Q.; et al. Functional improvement of infarcted heart by co-injection of embryonic stem cells with temperature-responsive chitosan hydrogel. Tissue Eng.-Part A 2009, 15, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Lee, S.Y.; Joung, Y.K.; Na, J.S.; Lee, M.C.; Park, K.D. Thermosensitive chitosan-Pluronic hydrogel as an injectable cell delivery carrier for cartilage regeneration. Acta Biomater. 2009, 5, 1956–1965. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.C.; Washington, C.; Davies, M.C.; Hadgraft, J. The behaviour of Pluronic F127 in aqueous solution studied using fluorescent probes. Int. J. Pharm. 1987, 40, 93–99. [Google Scholar] [CrossRef]
- Youn, J.; Choi, J.H.; Lee, S.; Lee, S.W.; Moon, B.K.; Song, J.E.; Khang, G. Pluronic f-127/silk fibroin for enhanced mechanical property and sustained release drug for tissue engineering biomaterial. Materials 2021, 14, 1287. [Google Scholar] [CrossRef]
- Gratieri, T.; Gelfuso, G.M.; Rocha, E.M.; Sarmento, V.H.; de Freitas, O.; Lopez, R.F.V. A poloxamer/chitosan in situ forming gel with prolonged retention time for ocular delivery. Eur. J. Pharm. Biopharm. 2010, 75, 186–193. [Google Scholar] [CrossRef]
- Mayol, L.; Biondi, M.; Quaglia, F.; La Rotonda, M.I.; Fusco, S.; Borzacchiello, A.; Ambrosio, L. Injectable thermally responsive mucoadhesive gel for sustained protein delivery. Biomacromolecules 2011, 12, 28–33. [Google Scholar] [CrossRef]
- Jung, Y.S.; Park, W.; Park, H.; Lee, D.K.; Na, K. Thermo-sensitive injectable hydrogel based on the physical mixing of hyaluronic acid and Pluronic F-127 for sustained NSAID delivery. Carbohydr. Polym. 2017, 156, 403–408. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K. Recent progress in biomedical applications of pluronic (PF127): Pharmaceutical perspectives. J. Control. Release 2015, 209, 120–138. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Raub, C.B.; Choy, J.S.; Luo, X. Modulating the properties of flow-assembled chitosan membranes in microfluidics with glutaraldehyde crosslinking. J. Mater. Chem. B 2020, 8, 2519–2529. [Google Scholar] [CrossRef] [PubMed]
- Karvandian, F.M.; Shafiei, N.; Mohandes, F.; Dolatyar, B.; Zandi, N.; Zeynali, B.; Simchi, A. Glucose cross-linked hydrogels conjugate HA nanorods as bone scaffolds: Green synthesis, characterization and in vitro studies. Mater. Chem. Phys. 2020, 242, 122515. [Google Scholar] [CrossRef]
- Ghanbari, M.; Salavati-Niasari, M.; Mohandes, F. Thermosensitive alginate-gelatin-nitrogen-doped carbon dots scaffolds as potential injectable hydrogels for cartilage tissue engineering applications. RSC Adv. 2021, 11, 18423–18431. [Google Scholar] [CrossRef] [PubMed]
- Min, J.H.; Patel, M.; Koh, W.G. Incorporation of conductive materials into hydrogels for tissue engineering applications. Polymers 2018, 10, 1078. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Shi, X.; Tian, L.; Sun, H.; Wu, Y.; Li, X.; Li, J.; Wei, Y.; Han, X.; Zhang, J.; et al. AuNP–Collagen Matrix with Localized Stiffness for Cardiac-Tissue Engineering: Enhancing the Assembly of Intercalated Discs by β1-Integrin-Mediated Signaling. Adv. Mater. 2016, 28, 10230–10235. [Google Scholar] [CrossRef]
- Navaei, A.; Rahmani Eliato, K.; Ros, R.; Migrino, R.Q.; Willis, B.C.; Nikkhah, M. The influence of electrically conductive and non-conductive nanocomposite scaffolds on the maturation and excitability of engineered cardiac tissues. Biomater. Sci. 2019, 7, 585–595. [Google Scholar] [CrossRef]
- Navaei, A.; Saini, H.; Christenson, W.; Sullivan, R.T.; Ros, R.; Nikkhah, M. Gold nanorod-incorporated gelatin-based conductive hydrogels for engineering cardiac tissue constructs. Acta Biomater. 2016, 41, 133–146. [Google Scholar] [CrossRef]
- Chemie, A. Süd-Chemie Milestones 1857 to 2005, Developments in 2006. Angew. Chemie Int. Ed. 2007, 46. [Google Scholar] [CrossRef]
- Buonerba, A.; Grassi, A. Trends in sustainable synthesis of organics by gold nanoparticles embedded in polymer matrices. Catalysts 2021, 11, 714. [Google Scholar] [CrossRef]
- Manuscript, A. Gold nanoparticles confined in imidazolium-based porous organic polymers to assemble a microfluidic reactor: Controllable growth and enhanced catalytic activity. Mater. Chem. A 2017. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, J.; Zhu, D.; Li, T. One-pot synthesized porphyrin-based polymer supported gold nanoparticles as efficient catalysts for alkyne hydration and alcohol oxidation in water. Gold Bull. 2019, 52, 19–26. [Google Scholar] [CrossRef]
- Liu, W.; Du, H.; Zhang, M.; Liu, K.; Liu, H.; Xie, H.; Zhang, X.; Si, C. Bacterial Cellulose-Based Composite Sca ff olds for Biomedical Applications: A Review. ACS Sustain. Chem. Eng. 2020, 8, 7536–7562. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, S.; Wang, B.; Yao, J.; Wang, H. TEMPO-oxidized bacterial cellulose nanofibers-supported gold nanoparticles with superior catalytic properties. Carbohydr. Polym. 2017, 160, 34–42. [Google Scholar] [CrossRef]
- Everaerts, F.; Torrianni, M.; Hendriks, M.; Feijen, J. Biomechanical properties of carbodiimide crosslinked collagen: Influence of the formation of ester crosslinks. J. Biomed. Mater. Res. Part A 2007, 85, 547–555. [Google Scholar] [CrossRef]
- Khalil, H.P.S.A.; Jummaat, F.; Yahya, E.B.; Olaiya, N.G.; Kumar, U.S.U.; Bairwan, R.; Suriani, A.B. A Review on Micro- to Nanocellulose Biopolymer Scaffold Forming for Tissue. Polymers 2020, 12, 2043. [Google Scholar] [CrossRef]
- Novotna, K.; Havelka, P.; Sopuch, T.; Kolarova, K.; Vosmanska, V.; Lisa, V.; Svorcik, V.; Bacakova, L. Cellulose-based materials as scaffolds for tissue engineering. Cellulose 2013, 20, 2263–2278. [Google Scholar] [CrossRef] [Green Version]
- Rezaei, A.; Karimizade, A.; Takallu, S. Collagen/cellulose nanofiber hydrogel scaffold: Physical, mechanical and cell biocompatibility properties. Cellulose 2019, 27, 927–940. [Google Scholar] [CrossRef]
- Barra, A.; Alves, Z.; Ferreira, N.M.; Martins, M.A.; Oliveira, H.; Ferreira, L.P.; Cruz, M.M.; Carvalho, M.D.D.; Neumayer, S.M.; Rodriguez, B.J.; et al. Biocompatible chitosan-based composites with properties suitable for hyperthermia therapy. J. Mater. Chem. B 2020, 8, 1256–1265. [Google Scholar] [CrossRef]
- Fiorati, A.; Linciano, C.; Galante, C.; Raucci, M.G.; Altomare, L. Bioactive hydrogels: Design and characterization of cellulose-derived injectable composites. Materials 2021, 14, 4511. [Google Scholar] [CrossRef]
- Rodrigues, C.; Fernanda, C.; Camilo, F. Evaluation of catalytic activity of cellulose films decorated with gold nanoparticles in the reduction of 4-nitrophenol. Cellulose 2020, 1, 3919–3929. [Google Scholar] [CrossRef]
- Yom-Tov, O.; Frisman, I.; Seliktar, D.; Bianco-Peled, H. A novel method for hydrogel nanostructuring. Eur. Polym. J. 2014, 52, 137–145. [Google Scholar] [CrossRef]
- Ahsan, A.; Farooq, M.A.; Parveen, A. Thermosensitive chitosan-based injectable hydrogel as an efficient anticancer drug carrier. ACS Omega 2020, 5, 20450–20460. [Google Scholar] [CrossRef]
- Grosskopf, A.K.; Roth, G.A.; Smith, A.A.A.; Gale, E.C.; Hernandez, H.L.; Appel, E.A. Injectable supramolecular polymer–nanoparticle hydrogels enhance human mesenchymal stem cell delivery. Bioeng. Transl. Med. 2020, 5, e10147. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.A.; Momin, A.; Strano, M.; Rane, K. Implementation of viscosity and density models for improved numerical analysis of melt flow dynamics in the nozzle during extrusion-based additive manufacturing. Prog. Addit. Manuf. 2022, 7, 41–54. [Google Scholar] [CrossRef]
- Rosti, M.E.; Takagi, S. Shear-thinning and shear-thickening emulsions in shear flows. Phys. Fluids 2021, 33, 083319. [Google Scholar] [CrossRef]
- Malkin, A.Y.; Isayev, A. Rheology: Concepts, Methods, and Applications, 3rd ed.; ChemTec Publishing: Scarborough, ON, Canada, 2017. [Google Scholar]
- Huang, B.; Liu, M.; Long, Z.; Shen, Y.; Zhou, C. Effects of halloysite nanotubes on physical properties and cytocompatibility of alginate composite hydrogels. Mater. Sci. Eng. C 2017, 70, 303–310. [Google Scholar] [CrossRef]
- Glukhova, S.A.; Molchanov, V.S.; Lokshin, B.V.; Rogachev, A.V.; Tsarenko, A.A.; Patsaev, T.D.; Kamyshinsky, R.A.; Philippova, O.E. Printable alginate hydrogels with embedded network of halloysite nanotubes: Effect of polymer cross-linking on rheological properties and microstructure. Polymers 2021, 13, 4130. [Google Scholar] [CrossRef]
- Rył, A.; Owczarz, P. Injectability of thermosensitive, low-concentrated chitosan colloids as flow phenomenon through the capillary under high shear rate conditions. Polymers 2020, 12, 2260. [Google Scholar] [CrossRef]
- Omar, H.; Smales, G.J.; Henning, S.; Li, Z.; Wang, D.Y.; Schönhals, A.; Szymoniak, P. Calorimetric and dielectric investigations of epoxy-based nanocomposites with halloysite nanotubes as nanofillers. Polymers 2021, 13, 1634. [Google Scholar] [CrossRef]
- Moura, M.J.; Figueiredo, M.M.; Gil, M.H. Rheological study of genipin cross-linked chitosan hydrogels. Biomacromolecules 2007, 8, 3823–3829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, S.; Cheng, G.; Yang, T.; Ren, X.; Gao, G. Mechanical and Rheological Behavior of Hybrid Cross-Linked Polyacrylamide/Cationic Micelle Hydrogels. Macromol. Mater. Eng. 2017, 302, 1700402. [Google Scholar] [CrossRef]
- Meyers, K.; Lee, B.P.; Rajachar, R.M. Electroactive polymeric composites to mimic the electromechanical properties of myocardium in cardiac tissue repair. Gels 2021, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Engineering, M. Dynamic Viscoelasticity and Surface Properties of Porcine Left Anterior Descending Coronary Arteries. Cardiovasc. Eng. Technol. 2017, 8, 41–56. [Google Scholar] [CrossRef] [Green Version]
- Jamburidze, A.; De Corato, M.; Huerre, A.; Pommella, A.; Garbin, V. High-frequency linear rheology of hydrogels probed by ultrasound-driven microbubble dynamics. Soft Matter 2017, 13, 3946–3953. [Google Scholar] [CrossRef]
- Potse, M.; Dubé, B.; Vinet, A. Cardiac anisotropy in boundary-element models for the electrocardiogram. Med. Biol. Eng. Comput. 2009, 47, 719–729. [Google Scholar] [CrossRef] [Green Version]
- Yeganeh, H.; Ai, J.; Gharibi, R.; Azami, M.; Faghihi, F. Synthesis, characterization and antioxidant activity of a novel electroactive and biodegradable polyurethane for cardiac tissue engineering application. Mater. Sci. Eng. C 2014, 44, 24–37. [Google Scholar] [CrossRef]
- Lee, J.; Serna, F.; Nickels, J.; Schmidt, C.E. Carboxylic Acid-Functionalized Conductive Polypyrrole as a Bioactive Platform for Cell Adhesion. Biomacromolecules 2006, 7, 1692–1695. [Google Scholar] [CrossRef] [Green Version]
- Shevach, M.; Maoz, B.M.; Feiner, R.; Shapira, A.; Dvir, T. Nanoengineering gold particle composite fibers for cardiac tissue engineering. J. Mater. Chem. B 2013, 1, 5210–5217. [Google Scholar] [CrossRef]
- Caccavo, D.; Cascone, S.; Lamberti, G.; Barba, A.A.; Larsson, A. Swellable Hydrogel-based Systems for Controlled Drug Delivery. Smart Drug Deliv. Syst. 2016, 237–303. [Google Scholar] [CrossRef] [Green Version]
- Chitosan, F.; Zhang, W.; Gilstrap, K.; Wu, L.; KC, R.B.; Moss, M.A.; Wang, Q.; Lu, X.; He, X. Synthesis and Characterization of Thermally Responsive Pluronic Controlled Release and Intracellular Delivery of Small Molecules. ACS Nano 2010, 4, 6747–6759. [Google Scholar]
- Hoch, E.; Tovar, G.E.M.; Borchers, K. Biopolymer-based hydrogels for cartilage tissue engineering. Bioinspired Biomim. Nanobiomater. 2016, 5, 51–66. [Google Scholar] [CrossRef]
- Liu, Y.; Nguyen, A.; Allen, A.; Zoldan, J.; Huang, Y.; Chen, J.Y. Regenerated cellulose micro-nano fiber matrices for transdermal drug release. Mater. Sci. Eng. C 2017, 74, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Alkilany, A.M.; Murphy, C.J. Toxicity and cellular uptake of gold nanoparticles: What we have learned so far? J. Nanopart. Res. 2010, 12, 2313–2333. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Au Concentration (mM) | TG (°C) | Shear-Thinning Index (m) | Consistency Index (Pa) | ||
|---|---|---|---|---|---|
| 20 | 21.4 | 3300 | 1460 | 0.02 | 2.66 |
| 75 | 15 | 22,800 | 2460 | 0.21 | 1.67 |
| Method | CS–CPF-Au20@OBC | CS–CPF-Au75@OBC |
|---|---|---|
| Ionic conductivity | 1.4 × 10−4 S/m | 2.7 × 10−3 S/m |
| Four-point probe device | 2 × 10−3 S/m | 6 × 10−2 S/m |
| Two-point probe device | 1 × 10−3 S/m | 10−2 S/m |
| I–V | 5 × 10−4 S/m | 7 × 10−3 S/m |
| TEER device with HESC-CM | - | 0.086 S/m |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tohidi, H.; Maleki-Jirsaraei, N.; Simchi, A.; Mohandes, F.; Emami, Z.; Fassina, L.; Naro, F.; Conti, B.; Barbagallo, F. An Electroconductive, Thermosensitive, and Injectable Chitosan/Pluronic/Gold-Decorated Cellulose Nanofiber Hydrogel as an Efficient Carrier for Regeneration of Cardiac Tissue. Materials 2022, 15, 5122. https://doi.org/10.3390/ma15155122
Tohidi H, Maleki-Jirsaraei N, Simchi A, Mohandes F, Emami Z, Fassina L, Naro F, Conti B, Barbagallo F. An Electroconductive, Thermosensitive, and Injectable Chitosan/Pluronic/Gold-Decorated Cellulose Nanofiber Hydrogel as an Efficient Carrier for Regeneration of Cardiac Tissue. Materials. 2022; 15(15):5122. https://doi.org/10.3390/ma15155122
Chicago/Turabian StyleTohidi, Hajar, Nahid Maleki-Jirsaraei, Abdolreza Simchi, Fatemeh Mohandes, Zahra Emami, Lorenzo Fassina, Fabio Naro, Bice Conti, and Federica Barbagallo. 2022. "An Electroconductive, Thermosensitive, and Injectable Chitosan/Pluronic/Gold-Decorated Cellulose Nanofiber Hydrogel as an Efficient Carrier for Regeneration of Cardiac Tissue" Materials 15, no. 15: 5122. https://doi.org/10.3390/ma15155122
APA StyleTohidi, H., Maleki-Jirsaraei, N., Simchi, A., Mohandes, F., Emami, Z., Fassina, L., Naro, F., Conti, B., & Barbagallo, F. (2022). An Electroconductive, Thermosensitive, and Injectable Chitosan/Pluronic/Gold-Decorated Cellulose Nanofiber Hydrogel as an Efficient Carrier for Regeneration of Cardiac Tissue. Materials, 15(15), 5122. https://doi.org/10.3390/ma15155122








