Adsorption Study of Continuous Heavy Metal Ions (Pb2+, Cd2+, Ni2+) Removal Using Cocoa (Theobroma cacao L.) Pod Husks
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Sorbate
2.2. Preparation of Adsorbent
2.3. Chemical Composition of Biomass
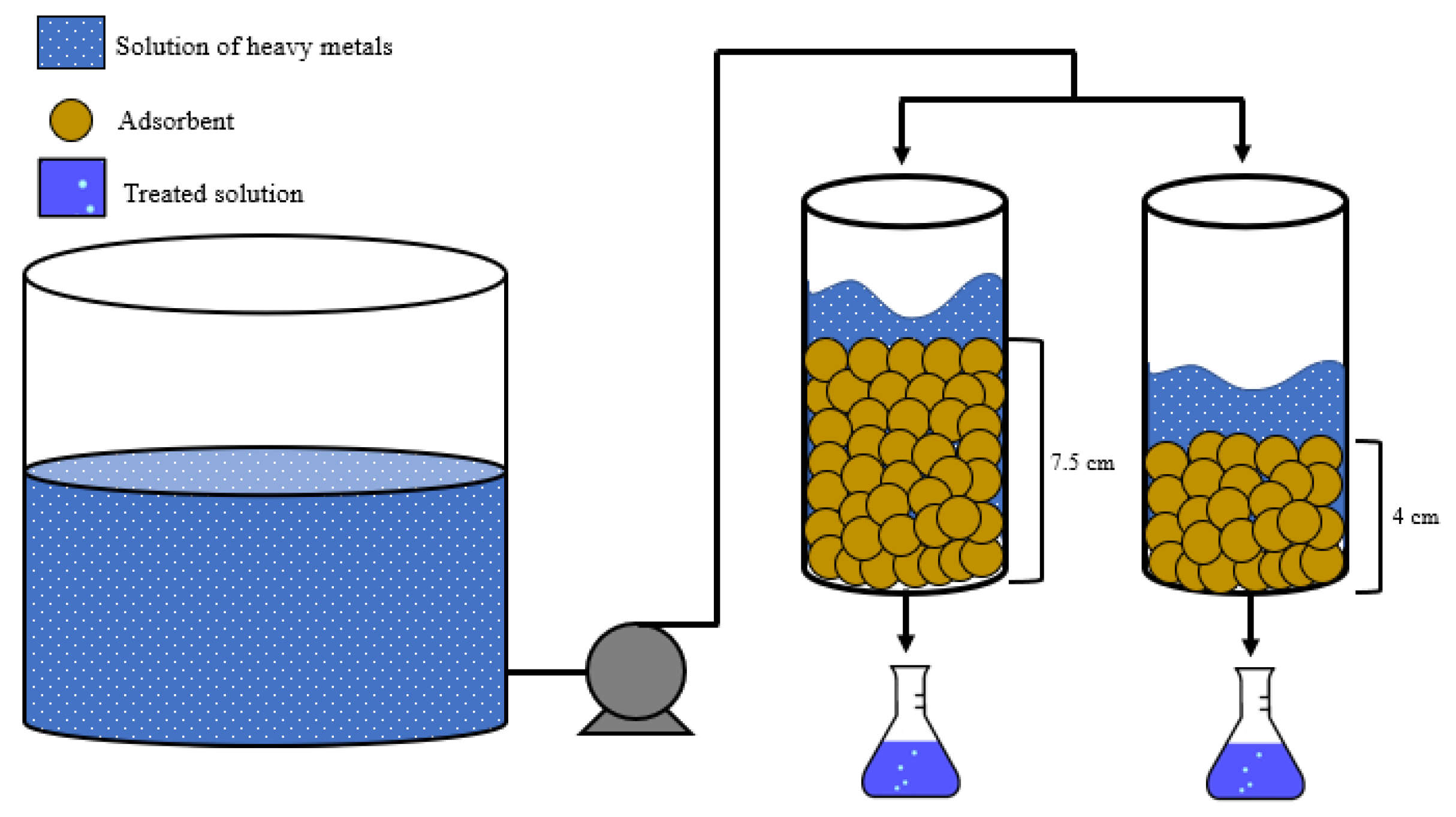
2.4. Continuos Fixed-Bed Column Study
2.5. Adsorption Modeling
3. Results and Discussion
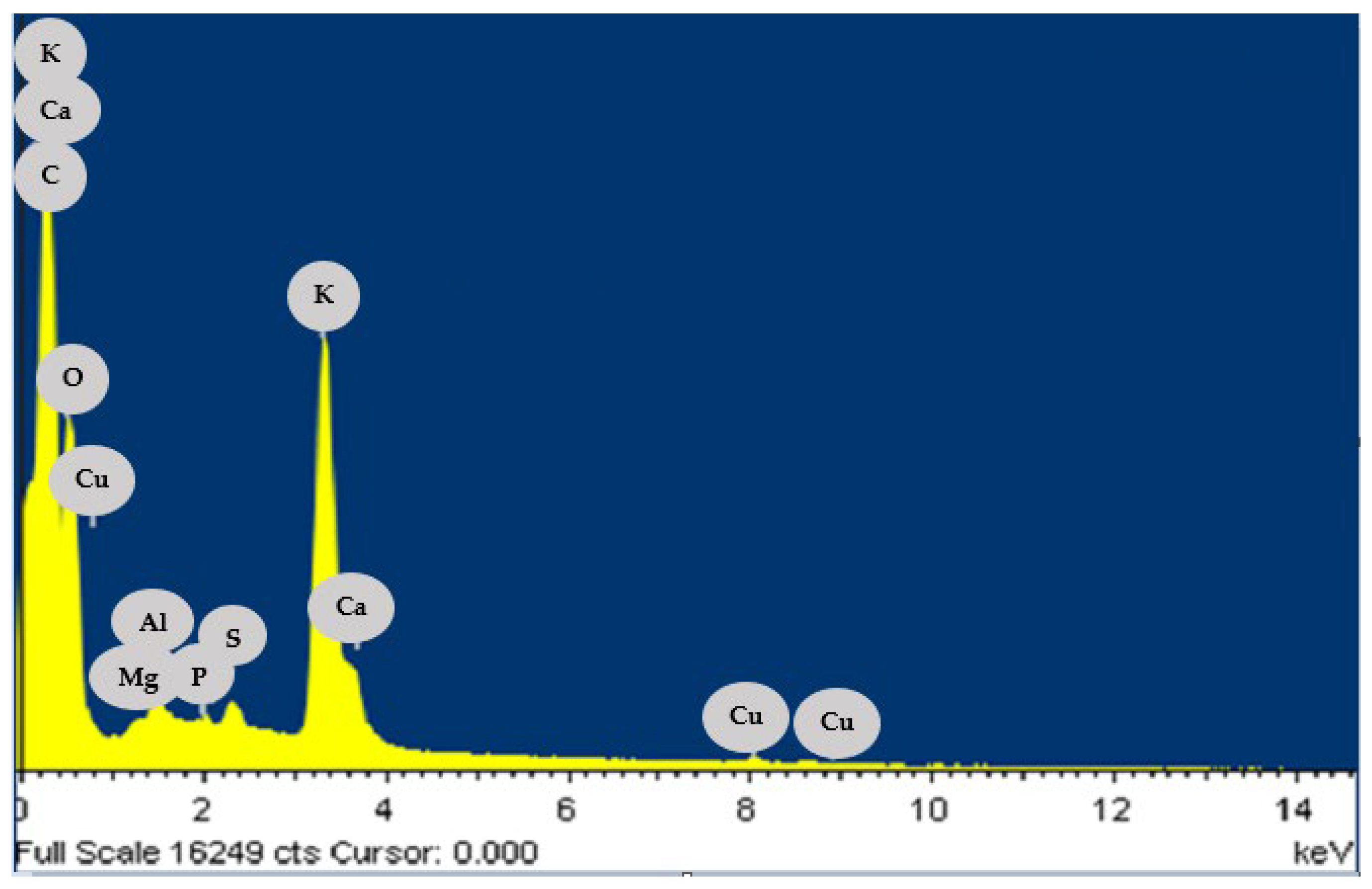
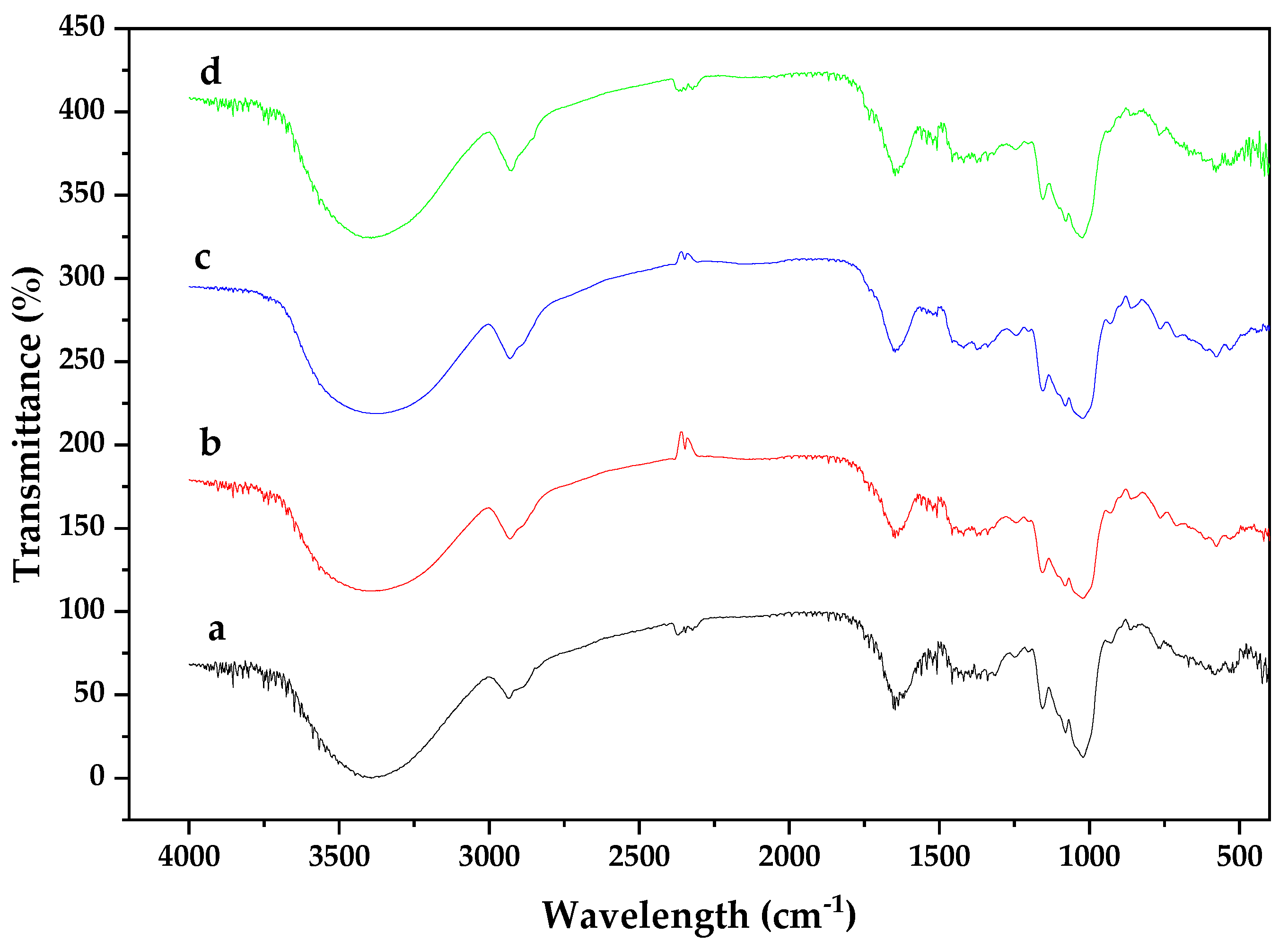
3.1. Chemical Composition of Biomass
3.2. Continuous Fixed-Bed Column Study
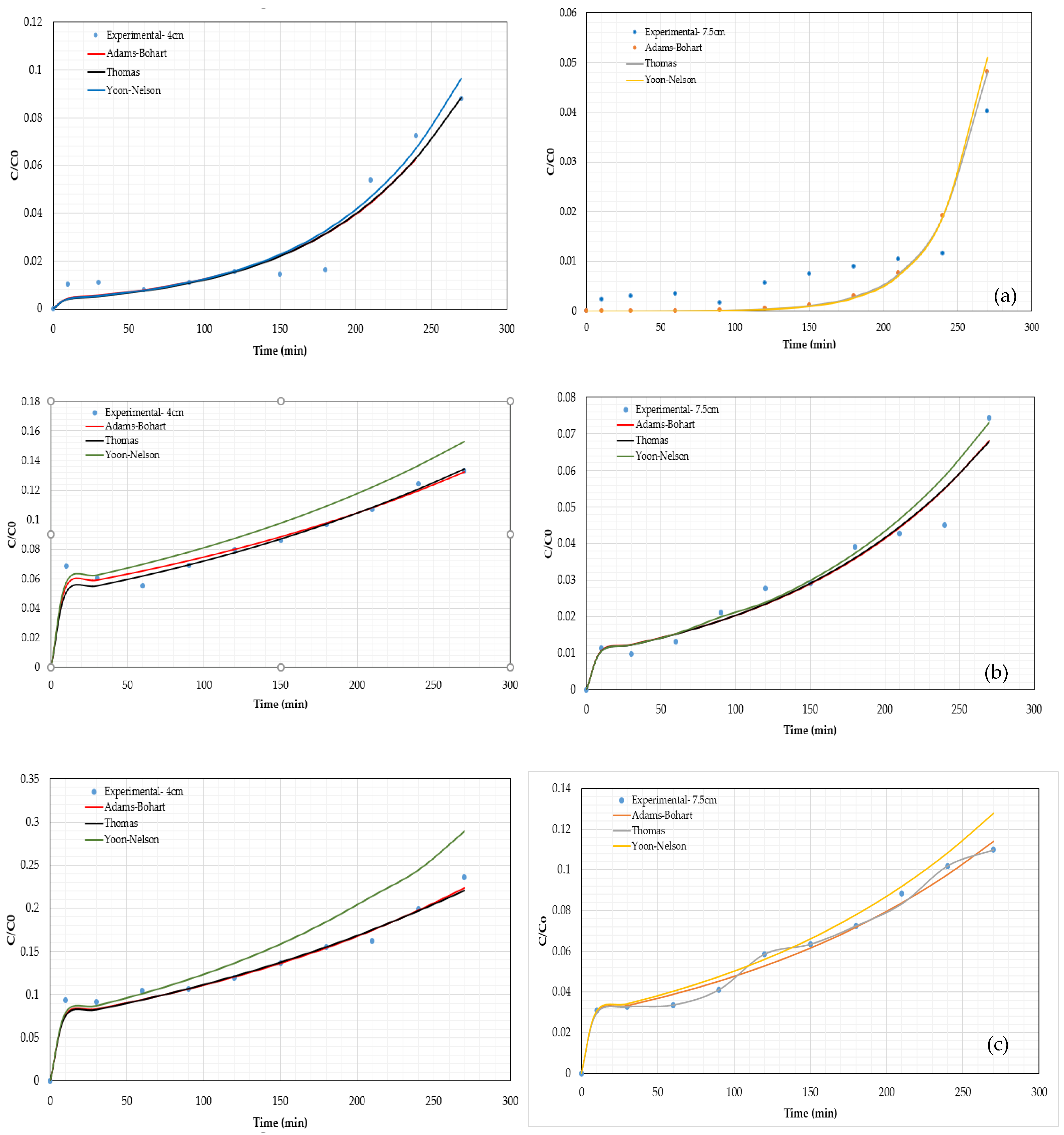
3.3. Adsorption Breaktrough Curves Modeling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, S.; Yun, J.M.; Lee, J.Y.; Hong, G.; Kim, J.S.; Kim, D.; Han, J.G. The Remediation Characteristics of Heavy Metals (Copper and Lead) on Applying Recycled Food Waste Ash and Electrokinetic Remediation Techniques. Appl. Sci. 2021, 11, 7437. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A state-of-the-art review on wastewater treatment techniques: The effectiveness of adsorption method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods. Environ. Technol. Innov. 2021, 22, 101504. [Google Scholar] [CrossRef]
- Charkiewicz, A.E.; Backstrand, J.R. Lead Toxicity and Pollution in Poland. Int. J. Environ. Res. Public Health 2020, 17, 4385. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, X.; Xiong, Z.; Wang, M.; Liu, Q. Environmental Pollution Effect Analysis of Lead Compounds in China Based on Life Cycle. Int. J. Environ. Res. Public Health 2020, 17, 2184. [Google Scholar] [CrossRef] [PubMed]
- Dumat, C.; Shahid, M.; Khalid, S.; Murtaza, B.; Shahid, M.; Khalid, S.; Murtaza, B. Lead Pollution and Human Exposure: Forewarned is Forearmed, and the Question Now Becomes How to Respond to the Threat! In Radionuclides and Heavy Metals in the Environment, 1st ed.; Springer: Cham, Switzerland, 2020; pp. 33–65. [Google Scholar]
- Ezeonuegbu, B.A.; Machido, D.A.; Whong, C.M.Z.; Japhet, W.S.; Alexiou, A.; Elazab, S.T.; Qusty, N.; Yaro, C.A.; Batiha, G.E.S. Agricultural waste of sugarcane bagasse as efficient adsorbent for lead and nickel removal from untreated wastewater: Biosorption, equilibrium isotherms, kinetics and desorption studies. Biotechnol. Rep. 2021, 30, e00614. [Google Scholar] [CrossRef] [PubMed]
- Siregar, A.; Sulistyo, I.; Prayogo, N.A. Heavy metal contamination in water, sediments and Planiliza subviridis tissue in the Donan River, Indonesia. J. Water L. Dev. 2020, 45, 157–164. [Google Scholar] [CrossRef]
- Patel, H.; In, C. Comparison of batch and fixed bed column adsorption: A critical review. Int. J. Environ. Sci. Technol. 2021, 19, 10409–10426. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Kim, H.Y.; Joshi, M.K. Technological trends in heavy metals removal from industrial wastewater: A review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar] [CrossRef]
- Herrera-Barros, A.; Tejada-Tovar, C.; Villabona-Ortíz, A.; Gonzalez-Delgado, Á.; Mejia-Meza, R. Assessment of the Effect of Al2O3 and TiO2 Nanoparticles on Orange Peel Biomass and its Application for Cd (II) and Ni (II) Uptake. Trans. ASABE 2019, 62, 139–147. [Google Scholar] [CrossRef]
- Younas, F.; Mustafa, A.; Farooqi, Z.U.R.; Wang, X.; Younas, S.; Mohy-Ud-din, W.; Hameed, M.A.; Abrar, M.M.; Maitlo, A.A.; Noreen, S.; et al. Current and Emerging Adsorbent Technologies for Wastewater Treatment: Trends, Limitations, and Environmental Implications. Water 2021, 13, 215. [Google Scholar] [CrossRef]
- Torres-Caban, R.; Vega-Olivencia, C.A.; Mina-Camilde, N. Adsorption of Ni2+ and Cd2+ from Water by Calcium Alginate/Spent Coffee Grounds Composite Beads. Appl. Sci. 2019, 9, 4531. [Google Scholar] [CrossRef]
- Suganthi, N.; Srinivasan, K. Phosphorylated tamarind nut carbon for the removal of cadmium ions from aqueous solutions. Indian J. Eng. Mater. Sci. 2010, 17, 382–388. [Google Scholar]
- Jia, Y.; Shi, S.; Liu, J.; Su, S.; Liang, Q.; Zeng, X.; Li, T. Study of the Effect of Pyrolysis Temperature on the Cd2+ Adsorption Characteristics of Biochar. Appl. Sci. 2018, 8, 1019. [Google Scholar] [CrossRef]
- Es-Sahbany, H.; Berradi, M.; Nkhili, S.; Hsissou, R.; Allaoui, M.; Loutfi, M.; Bassir, D.; Belfaquir, M.; El Youbi, M.S. Removal of heavy metals (nickel) contained in wastewater-models by the adsorption technique on natural clay. Mater. Today Proc. 2019, 13, 866–875. [Google Scholar] [CrossRef]
- Aboli, E.; Jafari, D.; Esmaeili, H. Heavy metal ions (lead, cobalt, and nickel) biosorption from aqueous solution onto activated carbon prepared from Citrus limetta leaves. Carbon Lett. 2020, 30, 683–698. [Google Scholar] [CrossRef]
- Jin, Y.; Zeng, C.; Lü, Q.F.; Yu, Y. Efficient adsorption of methylene blue and lead ions in aqueous solutions by 5-sulfosalicylic acid modified lignin. Int. J. Biol. Macromol. 2019, 123, 50–58. [Google Scholar] [CrossRef]
- Chen, H.; Li, W.; Wang, J.; Xu, H.; Liu, Y.; Zhang, Z.; Li, Y.; Zhang, Y. Adsorption of cadmium and lead ions by phosphoric acid-modified biochar generated from chicken feather: Selective adsorption and influence of dissolved organic matter. Bioresour. Technol. 2019, 292, 121948. [Google Scholar] [CrossRef]
- Eletta, O.A.A.; Adeniyi, A.G.; Ighalo, J.O.; Onifade, D.V.; Ayandele, F.O. Valorisation of Cocoa (Theobroma cacao) pod husk as precursors for the production of adsorbents for water treatment. Environ. Technol. Rev. 2020, 9, 20–36. [Google Scholar] [CrossRef]
- Vásquez, Z.S.; De Carvalho, D.P.; Pereira, G.V.M.; Vandenberghe, L.P.S.; De Oliveira, P.Z.; Tiburcio, P.B.; Rogez, H.L.G.; Góes, A.; Soccol, C.R. Biotechnological approaches for cocoa waste management: A review. Waste 2019, 90, 72–83. [Google Scholar] [CrossRef]
- Hernández-Mendoza, A.G.; Saldaña-Trinidad, S.; Martínez-Hernández, S.; Pérez-Sariñana, B.Y.; Láinez, M. Optimization of alkaline pretreatment and enzymatic hydrolysis of cocoa pod husk (Theobroma cacao L.) for ethanol production. Biomass Bioenergy 2021, 154, 106268. [Google Scholar] [CrossRef]
- Soares, T.F.; Oliveira, M.B.P.P. Cocoa by-Products: Characterization of Bioactive Compounds and Beneficial Health Effects. Molecules 2022, 27, 1625. [Google Scholar] [CrossRef]
- Patel, H. Fixed-bed column adsorption study: A comprehensive review. Appl. Water Sci. 2019, 9, 45. [Google Scholar] [CrossRef]
- Cherdchoo, W.; Nithettham, S.; Charoenpanich, J. Removal of Cr(VI) from synthetic wastewater by adsorption onto coffee ground and mixed waste tea. Chemosphere 2019, 221, 758–767. [Google Scholar] [CrossRef]
- Barquilha, C.E.R.; Braga, M.C.B. Adsorption of organic and inorganic pollutants onto biochars: Challenges, operating conditions, and mechanisms. Bioresour. Technol. Rep. 2021, 15, 100728. [Google Scholar] [CrossRef]
- Duarte, E.; Olivero, J.; Jaramillo, B. Studies of chromium removal from tannery wastewaters by quitosan biosorbents obtained shrimp (Litopenaus vanamei). Sci. Tech. 2009, 15, 290–295. [Google Scholar]
- Tejada-Tovar, C.; Villabona-Ortíz, A.; Marimón, W. Biosorption of chromium (VI) in water using modified lignocellulosic material. Rev. Educ. Ing. 2014, 9, 86–97. [Google Scholar]
- Tejada-Tovar, C.; Villabona-Ortiz, A.; Alvarez-Bajaire, G.; Jattin-Torres, L.; Acevedo, D. Evaluation of Theobroma cacao Waste Performance in Nickel Removal (II) in Continuous System. Int. J. Chem. Tech. Res. 2018, 11, 186–194. [Google Scholar]
- Tejada-Tovar, C.; Villabona-Ortíz, A.; Gonzalez-Delgado, Á.; Granados-Conde, C.; Jimenez-Villadiego, M. Kinetics of Mercury and Nickel Adsorption Using Chemically Pretreated Cocoa (Theobroma cacao) Husks. Trans. ASABE 2019, 62, 461–466. [Google Scholar] [CrossRef]
- Biswas, S.; Mishra, U. Continuous Fixed-Bed Column Study and Adsorption Modeling: Removal of Lead Ion from Aqueous Solution by Charcoal Originated from Chemical Carbonization of Rubber Wood Sawdust. J. Chem. 2015, 2015, 907379. [Google Scholar] [CrossRef]
- Tejada-Tovar, C.; Villabona-Ortíz, A.; Alvarez-Bajaire, G.; Jattin-Torres, L.; Granados-Conde, C. Influence of the bed height on the dynamic behavior of a fixed-bed column during mercury biosorption. TecnoLógicas 2017, 20, 71–81. [Google Scholar] [CrossRef][Green Version]
- Tejada-Tovar, C.; Villabona-Ortíz, A.; Caballero-Romero, V.; Paternina-Cuesta, J.; Granados-Conde, C. Parameter optimization for Cr(VI) adsorption breakdown curves onto cocoa shell. Rev. UDCA Actual. Divulg. Cient. 2018, 21, 167–177. [Google Scholar]
- Moreno-Sader, K.; García-Padilla, A.; Realpe, A.; Acevedo-Morantes, M.; Soares, J. Removal of Heavy Metal Water Pollutants ( Co2+ and Ni2+) Using Polyacrylamide/Sodium Montmorillonite (PAM/Na-MMT) Nanocomposites. ACS Omega 2019, 4, 10834–10844. [Google Scholar] [CrossRef] [PubMed]
- Njoku, V.; Ayuk, A.; Ejike, E.; Oguzie, E.; Duru, C.; Bello, O. Cocoa Pod Husk as a Low Cost Biosorbent for the Removal of Pb(II) and Cu(II) from Aqueous Solutions. Aust. J. Basic Appl. Sci. 2011, 5, 101–110. [Google Scholar]
- Kalaivani, S.; Vidhyadevi, T.; Murugesan, A.; Baskaralingam, P.; Anuradha, C.; Ravikumar, L.; Sivanesan, S. Equilibrium and kinetic studies on the adsorption of Ni(II) ion from an aqueous solution using activated carbon prepared from Theobroma cacao (cocoa) shell. Desalin. Water Treat. 2015, 54, 1629–1641. [Google Scholar] [CrossRef]
- Kede, C.; Ndibewu, P.; Kalumba, M.; Panichev, N.; Ngomo, H.; Ketcha, J. Adsorption of Mercury(II) onto activated carbons derived from Theobroma cacao pod husk. S. Afr. J. Chem. Eng. 2015, 68, 226–235. [Google Scholar]
- Valencia-Ríos, J.; Castellar-Ortega, G. Prediction of breakthrough curves for the removal of lead (II) in aqueous solution onto activated carbon in a packed column. Rev. Fac. Ing. Univ. Antioq. 2013, 66, 141–158. [Google Scholar]
- Onwordi, C.; Uche, C.; Ameh, A.; Petrik, L. Comparative study of the adsorption capacity of lead (II) ions onto bean husk and fish scale from aqueous solution. Water Reuse Desalin. 2019, 9, 249–262. [Google Scholar] [CrossRef]
- Rojas, H.; Guerrero, D.; Vásquez, O.; Valencia, J. Aplicación del Modelo de Bohart y Adams en la Remoción de Mercurio de Drenajes de Minería por Adsorción con Carbón Activado. Inf. Tecnol. 2012, 23, 21–32. [Google Scholar] [CrossRef]
- Han, R.; Ding, D.; Xu, Y.; Zou, W.; Wang, Y.; Li, Y.; Zou, L. Use of rice husk for the adsorption of congo red from aqueous solution in column mode. Bioreseource Technol. 2008, 99, 2938–2946. [Google Scholar] [CrossRef]
- Calero, M.; Blázquez, G.; Hernáinz, F.; Ronda, A.; Martín, M. Biosorción de cobre con corteza de pino en columna de lecho de fijo: Optimización de las variables del proceso. Afinidad 2012, 69, 175–184. [Google Scholar]
- Ghribi, A.; Chlendi, M. Modeling of Fixed Bed Adsorption: Application to the Adsorption of an Organic Dye. Asian J. Text. 2011, 1, 161–171. [Google Scholar] [CrossRef]
- Forero-Nuñez, C.; Jochum, J.; Sierra, F. Effect of particle size and addition of cocoa pod husk on the properties of sawdust and coal pellets. Ing. Investig. 2015, 35, 17–23. [Google Scholar] [CrossRef]
- Lu, F.; Rodriguez-Garcia, J.; Van Damme, I.; Westwood, N.J.; Shaw, L.; Robinson, J.S.; Warren, G.; Chatzifragkou, A.; Mason, S.M.; Gomez, L.; et al. Valorisation strategies for cocoa pod husk and its fractions. Curr. Opin. Green Sustain. Chem. 2018, 14, 80–88. [Google Scholar] [CrossRef]
- Yuan, W.; Cheng, J.; Huang, H.; Xiong, S.; Gao, J.; Zhang, J.; Feng, S. Optimization of cadmium biosorption by Shewanella putrefaciens using a Box-Behnken design. Ecotoxicol. Environ. Saf. 2019, 175, 138–147. [Google Scholar] [CrossRef]
- Asuquo, E.D.; Martin, A.D. Sorption of cadmium (II) ion from aqueous solution onto sweet potato (Ipomoea batatas L.) peel adsorbent: Characterisation, kinetic and isotherm studies. J. Environ. Chem. Eng. 2016, 4, 54215298. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, S.; Dong, C.; Meng, Z.; Wang, X. Competitive adsorption behaviour and mechanisms of cadmium, nickel and ammonium from aqueous solution by fresh and ageing rice straw biochars. Bioresour. Technol. 2020, 303, 122853. [Google Scholar] [CrossRef]
- Pino, G.; Souza de Mesquita, L.; Torem, M.; Saavedra Pinto, G. Biosorption of cadmium by green coconut shell powder. Miner. Eng. 2006, 19, 380–387. [Google Scholar] [CrossRef]
- Okoya, A.; Akinyele, A.; Ofoezie, I.; Amuda, O.; Alayande, O.; Makinde, O. Adsorption of heavy metal ions onto chitosan grafted cocoa husk char. Afr. J. Pure Appl. Chem. 2014, 8, 147–161. [Google Scholar]
- Xia, L.; Xu, X.; Zhu, W.; Huang, Q.; Chen, W. A comparative study on the biosorption of Cd2+ onto Paecilomyces lilacinus XLA and Mucoromycote sp. XLC. Int. J. Mol. Sci. 2015, 16, 15670–15687. [Google Scholar] [CrossRef]
- Basu, M.; Guha, A.K.; Ray, L. Adsorption Behavior of Cadmium on Husk of Lentil. Process Saf. Environ. Prot. 2017, 106, 11–22. [Google Scholar] [CrossRef]
- Huang, X.; Chen, T.; Zou, X.; Zhu, M.; Chen, D.; Pan, M. The adsorption of Cd(II) on manganese oxide investigated by batch and modeling techniques. Int. J. Environ. Res. Public Health 2017, 14, 1145. [Google Scholar] [CrossRef]
- Mahdi, Z.; Yu, Q.J.; El Hanandeh, A. Investigation of the kinetics and mechanisms of nickel and copper ions adsorption from aqueous solutions by date seed derived biochar. J. Environ. Chem. Eng. 2018, 6, 1171–1181. [Google Scholar] [CrossRef]
- Tejeda-Benítez, L.; Tejada-Tovar, C.; Marimón-Bolívar, W.; Villabona-Ortiz, A. Estudio de modificación química y física de biomasa (Citrus sinensis y Musa paradisiaca) para la adsorción de metales pesados en solución. Rev. Luna Azul 2014, 39, 124–142. [Google Scholar] [CrossRef]
- Castillo-Borobio, S. Reutilización de Raspo Procedente de la Industria Vinícola Para la Extracción de Metales en Efluentes Líquidos. Master’s Thesis, Universitat Politècnica de Catalunya Barcelona Tech, Barcelona, Spain, 2004. [Google Scholar]
- Vera, L.M.; Bermejo, D.; Uguña, M.F.; Garcia, N.; Flores, M.; Gonzalez, E. Fixed bed column modeling of lead (II) and cadmium (II) ions biosorption on sugarcane bagasse. Environ. Eng. Res. 2019, 24, 31–37. [Google Scholar] [CrossRef]
- Assaad, E.; Azzouz, A.; Nistor, D.; Ursu, A.V.; Sajin, T.; Miron, D.N.; Monette, F.; Niquette, P.; Hausler, R. Metal removal through synergic coagulation-flocculation using an optimized chitosan-montmorillonite system. Appl. Clay Sci. 2007, 37, 258–274. [Google Scholar] [CrossRef]
- Yahya, M.D.; Ihejirika, C.V.; Iyaka, Y.A.; Garba, U.; Olugbenga, A.G. Continuous Sorption of Chromium Ions from Simulated Effluents using Citric Acid Modified Sweet Potato Peels. Niger. J. Technol. Dev. 2020, 17, 47–54. [Google Scholar] [CrossRef]
- Khalfa, L.; Sdiri, A.; Bagane, M.; Cervera, M.L. A calcined clay fixed bed adsorption studies for the removal of heavy metals from aqueous solutions. J. Clean. Prod. 2021, 278, 123935. [Google Scholar] [CrossRef]
- Cortés, R. Efecto de la Modificación de Una Zeolita Natural Mexicana en la Sorción de Cadmio y 4-Clorofenol. Ph.D. Thesis, Universities Allied for Essential Medicines, Toluca, Mexico, 2007. [Google Scholar]
- Rafati, L.; Ehrampoush, M.H.; Rafati, A.A.; Mokhtari, M.; Mahvi, A.H. Fixed bed adsorption column studies and models for removal of ibuprofen from aqueous solution by strong adsorbent Nano-clay composite. J. Environ. Health Sci. Eng. 2019, 17, 753–765. [Google Scholar] [CrossRef]
- Dávila-Guzmán, N. Caracterización del Proceso de Biosorción de Metales Pesados Mediante Residuos Sólidos de Café. Doctorado Thesis, Universidad Autónoma de Nuevo León, San Nicolás de los Garza, Mexico, 2012. [Google Scholar]
- Abdolali, A.; Ngo, H.H.; Guo, W.; Zhou, J.L.; Zhang, J.; Liang, S.; Chang, S.W.; Nguyen, D.D.; Liu, Y. Application of a breakthrough biosorbent for removing heavy metals from synthetic and real wastewaters in a lab-scale continuous fixed-bed column. Bioresour. Technol. 2017, 229, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Blancas, V.; Aguilar-Madera, C.G.; Flores-Cano, J.V.; Leyva-Ramos, R.; Padilla-Ortega, E.; Ocampo-Pérez, R. Evaluation of mass transfer mechanisms involved during the adsorption of metronidazole on granular activated carbon in fixed bed column. J. Water Process Eng. 2020, 36, 101303. [Google Scholar] [CrossRef]
- Tyagi, U. Enhanced adsorption of metal ions onto Vetiveria zizanioides biochar via batch and fixed bed studies. Bioresour. Technol. 2022, 345, 126475. [Google Scholar] [CrossRef] [PubMed]
- Madeira, R.; Camps, I. First-principles calculations of nickel, cadmium, and lead nanoclusters adsorption on single-wall (10,0) boron-nitride nanotube. Appl. Surf. Sci. 2022, 573, 151547. [Google Scholar] [CrossRef]
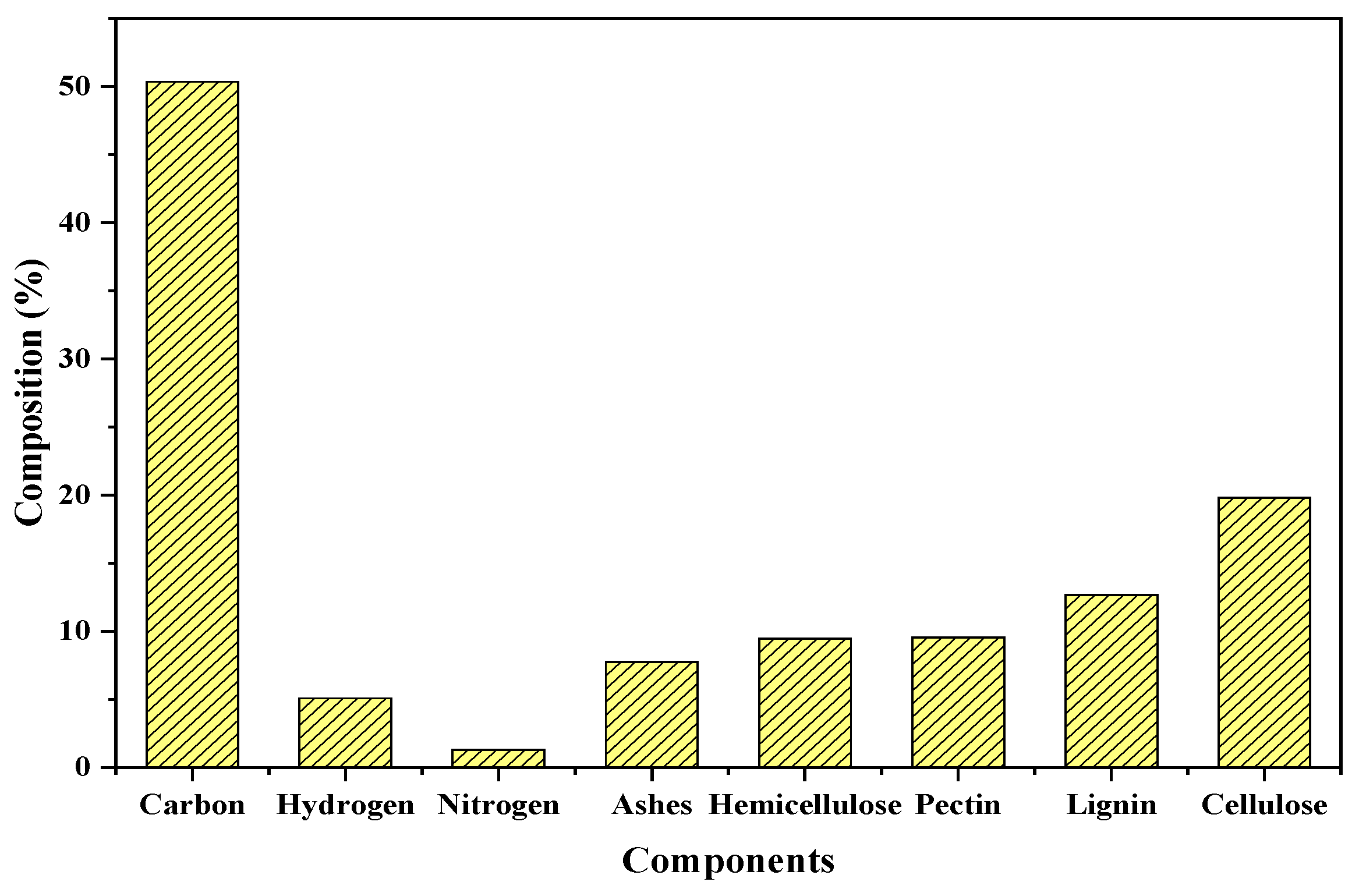


| Parameter | Method |
|---|---|
| Carbon (%) | AOAC 949.14 |
| Hydrogen (%) | AOAC 949.14 |
| Nitrogen (%) | Total Kjeldahl nitrogen |
| Ashes (%) | Thermogravimetry |
| Pectin (%) | Digestion-thermogravimetry |
| Lignin (%) | Photocalorimetry |
| Cellulose (%) | Digestion-thermogravimetry |
| Hemicellulose (%) | Digestion-thermogravimetry |
| Elemental composition | EDS analysis |
| Functional groups | FT-IR analysis |
| Carbon (%) | AOAC 949.14 |
| Heavy Metal Ion | Optimum Operating Conditions | Reference |
|---|---|---|
| Ni2+, Co2+ | Co = 60–100 ppm | [35] |
| Pb2+, Cu2+ | pH = 6 | [36] |
| Ni2+ | Co = 25–150 ppm | [37] |
| Hg2+ | pH = 6–8 | [38] |
| Ni2+ | pH = 6 | [37] |
| Ni2+, Co2+ | Co = 60–100 ppm | [35] |
| Metal | Bed Depth (cm) | Tb (min) | Qb (mg/g) | Maximum Removal Yield (%) |
|---|---|---|---|---|
| Pb2+ | 4 | 210 | 18 | 98.99 |
| 7.5 | 270 | 25.2 | 99.76 | |
| Ni2+ | 4 | 30 | 6.98 | 93.27 |
| 7.5 | 240 | 14.31 | 98.80 | |
| Cd2+ | 4 | - | - | 90.60 |
| 7.5 | 120 | 7.2 | 96.90 |
| Model | Parameter | Pb2+ | Ni2+ | Cd2+ | |||
|---|---|---|---|---|---|---|---|
| 7.5 cm | 4 cm | 7.5 cm | 4 cm | 7.5 cm | 4 cm | ||
| Adams–Bohart | KAB (L mg−1 min−1) | 2.01 × 10−4 | 1.15 × 10−4 | 7.48 × 10−5 | 3.73 × 10−5 | 5.13 × 10−5 | 4.13 × 10−5 |
| N0 (mg L−1) | 1903.88 | 1121.24 | 1561.73 | 3837.16 | 1621.07 | 2775.80 | |
| SE | 2.08 × 10−5 | 5.79 × 10−5 | 1.88 × 10−5 | 3.31 × 10−5 | 1.20 × 10−5 | 8.43 × 10−5 | |
| R2 | 0.95 | 0.96 | 0.97 | 0.97 | 0.99 | 0.98 | |
| Thomas | KTH (mL mg−1 min−1) | 0.207 | 0.122 | 0.079 | 0.043 | 0.055 | 0.048 |
| q0 (mg g−1) | 25.66 | 55.36 | 38.01 | 89.34 | 38.52 | 64.03 | |
| SS | 2.07 × 10−5 | 5.75 × 10−5 | 1.92 × 10−5 | 4.14 × 10−5 | 1.11 × 10−5 | 1.05 × 10−4 | |
| R2 | 0.95 | 0.96 | 0.97 | 0.97 | 0.99 | 0.98 | |
| Yoon–Nelson | KYN (mL mg−1 min−1) | 0.021 | 0.012 | 7.57 × 10−3 | 3.82 × 10−3 | 5.49 × 10−3 | 5.02×103 |
| SE | 2.19 × 10−5 | 6.62 × 10−5 | 2.35 × 10−5 | 4.57 × 10−5 | 1.45 × 10−5 | 1.95 × 10−4 | |
| R2 | 0.95 | 0.96 | 0.97 | 0.97 | 0.98 | 0.99 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tejada-Tovar, C.; Villabona-Ortíz, A.; González-Delgado, Á. Adsorption Study of Continuous Heavy Metal Ions (Pb2+, Cd2+, Ni2+) Removal Using Cocoa (Theobroma cacao L.) Pod Husks. Materials 2022, 15, 6937. https://doi.org/10.3390/ma15196937
Tejada-Tovar C, Villabona-Ortíz A, González-Delgado Á. Adsorption Study of Continuous Heavy Metal Ions (Pb2+, Cd2+, Ni2+) Removal Using Cocoa (Theobroma cacao L.) Pod Husks. Materials. 2022; 15(19):6937. https://doi.org/10.3390/ma15196937
Chicago/Turabian StyleTejada-Tovar, Candelaria, Angel Villabona-Ortíz, and Ángel González-Delgado. 2022. "Adsorption Study of Continuous Heavy Metal Ions (Pb2+, Cd2+, Ni2+) Removal Using Cocoa (Theobroma cacao L.) Pod Husks" Materials 15, no. 19: 6937. https://doi.org/10.3390/ma15196937
APA StyleTejada-Tovar, C., Villabona-Ortíz, A., & González-Delgado, Á. (2022). Adsorption Study of Continuous Heavy Metal Ions (Pb2+, Cd2+, Ni2+) Removal Using Cocoa (Theobroma cacao L.) Pod Husks. Materials, 15(19), 6937. https://doi.org/10.3390/ma15196937









