The Preparation of {001}TiO2/TiOF2 via a One-Step Hydrothermal Method and Its Degradation Mechanism of Ammonia Nitrogen
Abstract
1. Introduction
2. Methods
2.1. Preparation of Catalysts
2.2. Catalyst Characterization
2.3. Photocatalytic Activity Tests
3. Results and Discussion
3.1. Analysis of Characterization Results
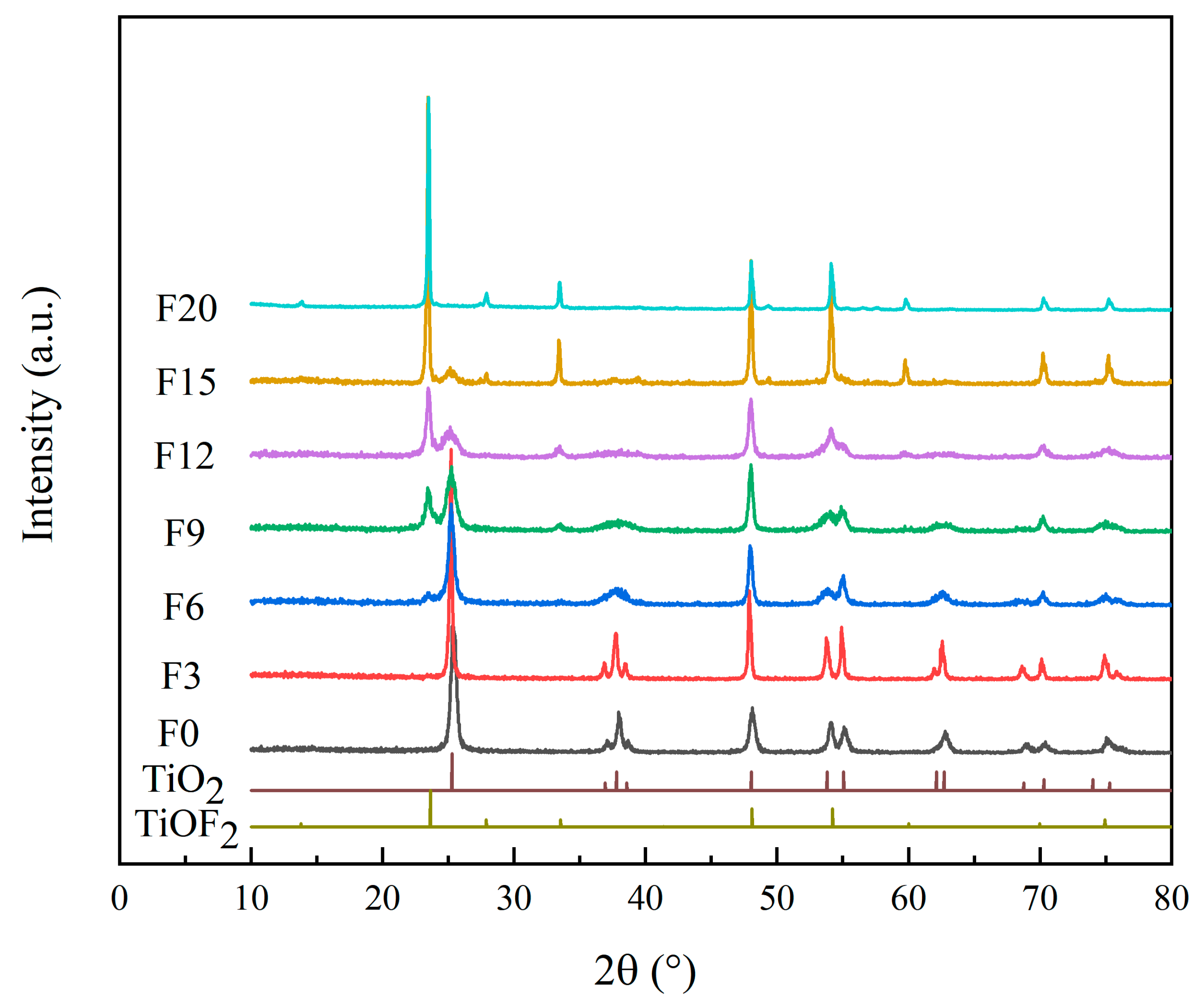
3.1.1. XRD Analysis
3.1.2. Morphology and Lattice Spacing Analysis
3.1.3. Raman Analysis
3.1.4. BET Analysis
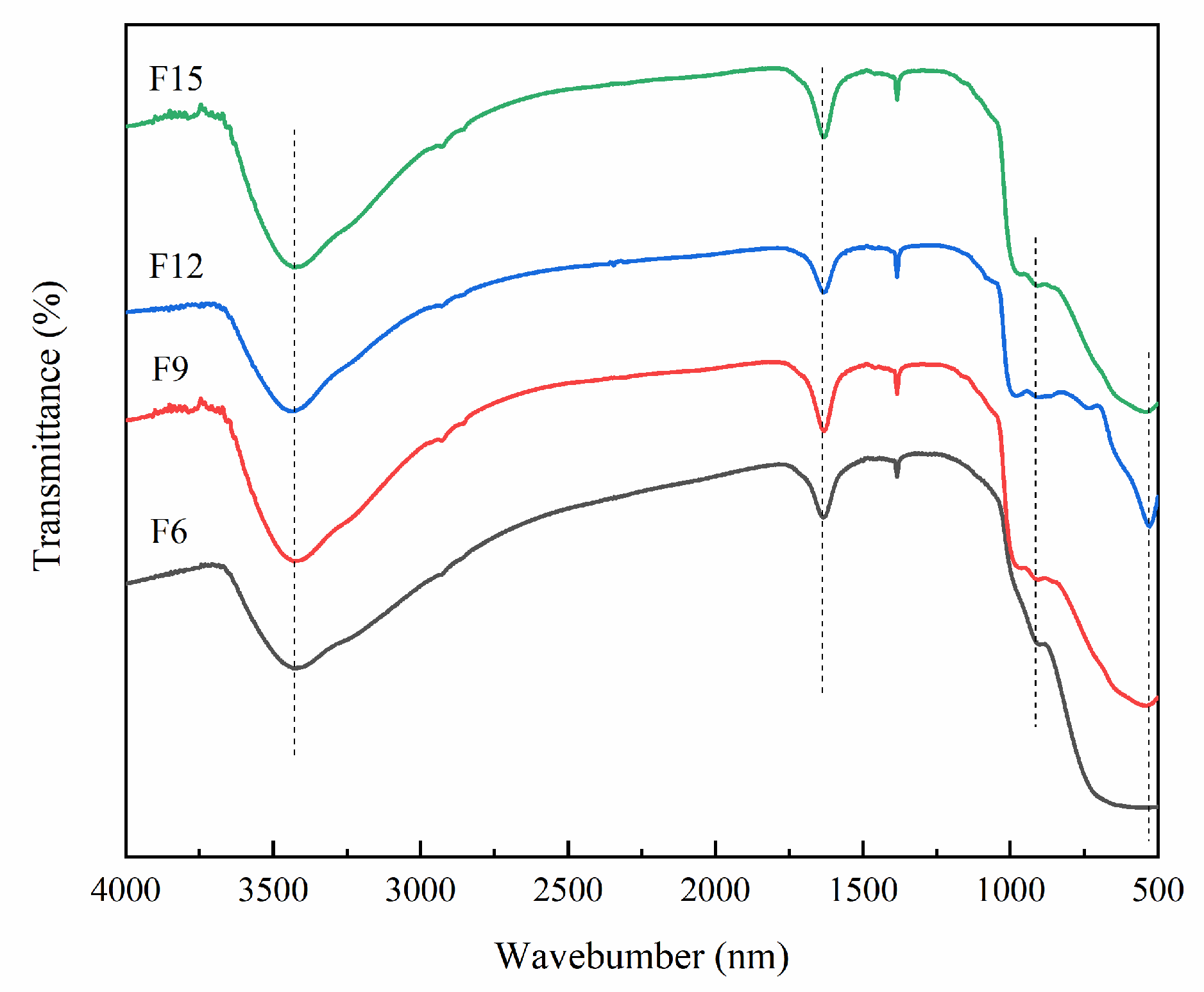
3.1.5. FT-IR Analysis
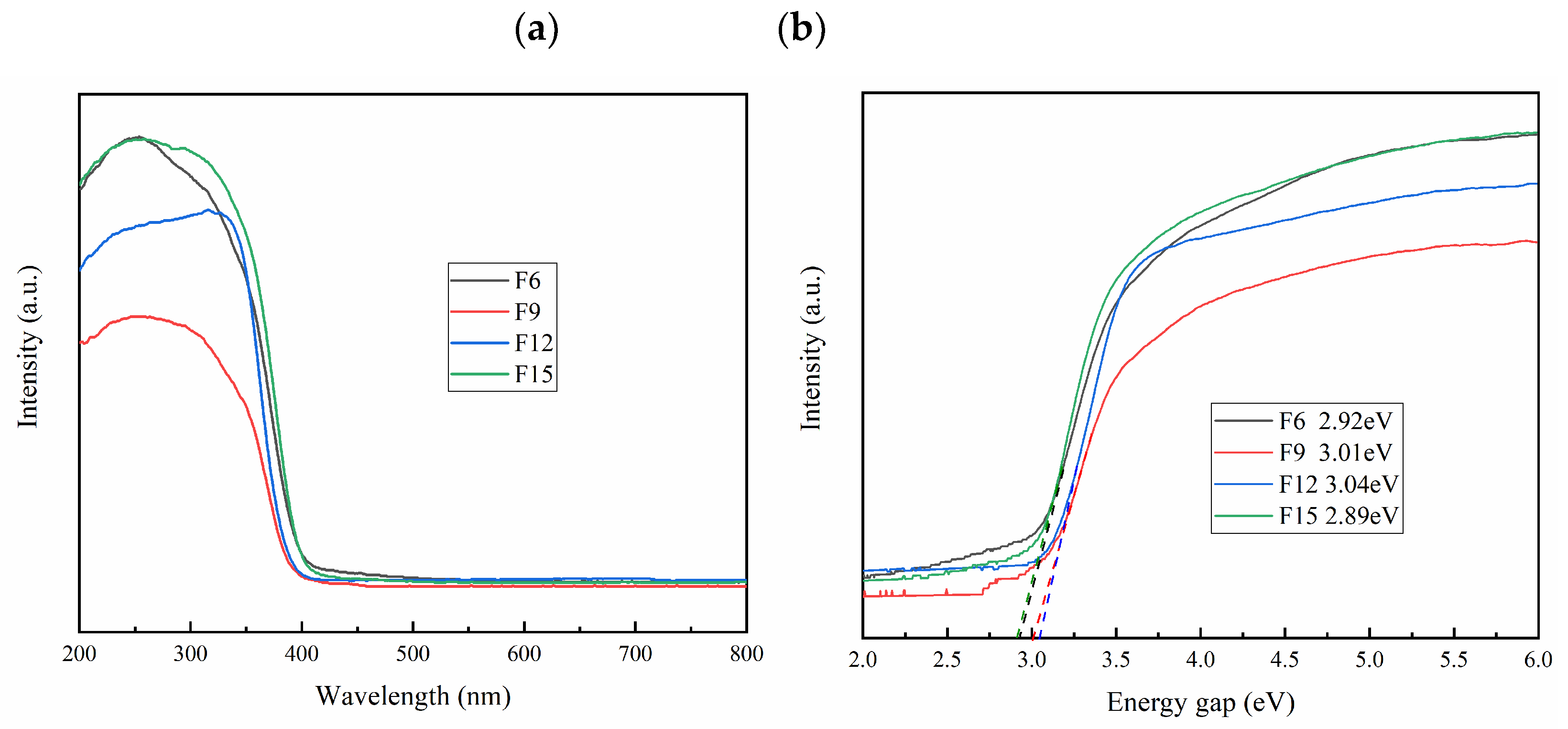
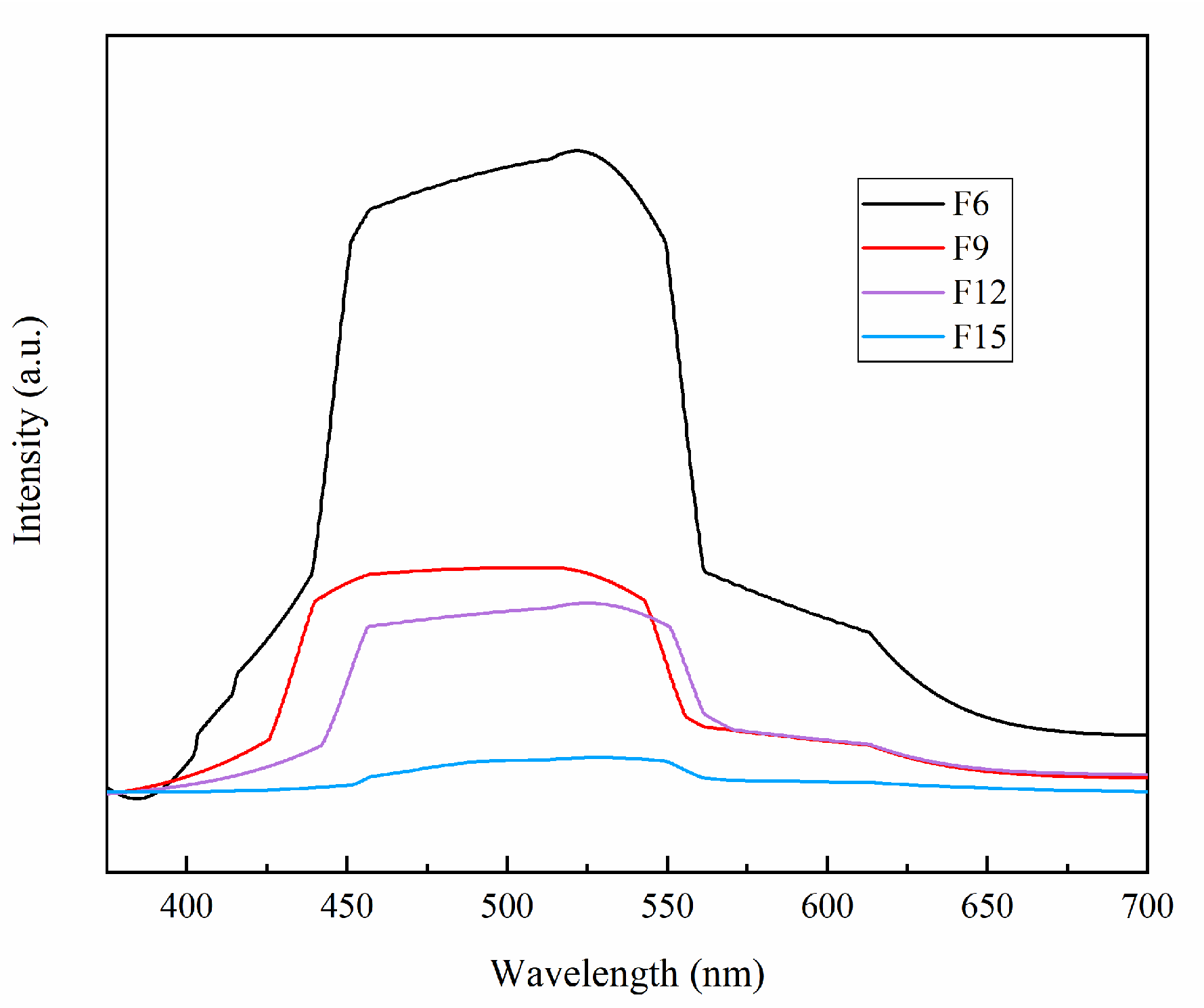
3.1.6. Optical Performance Analysis
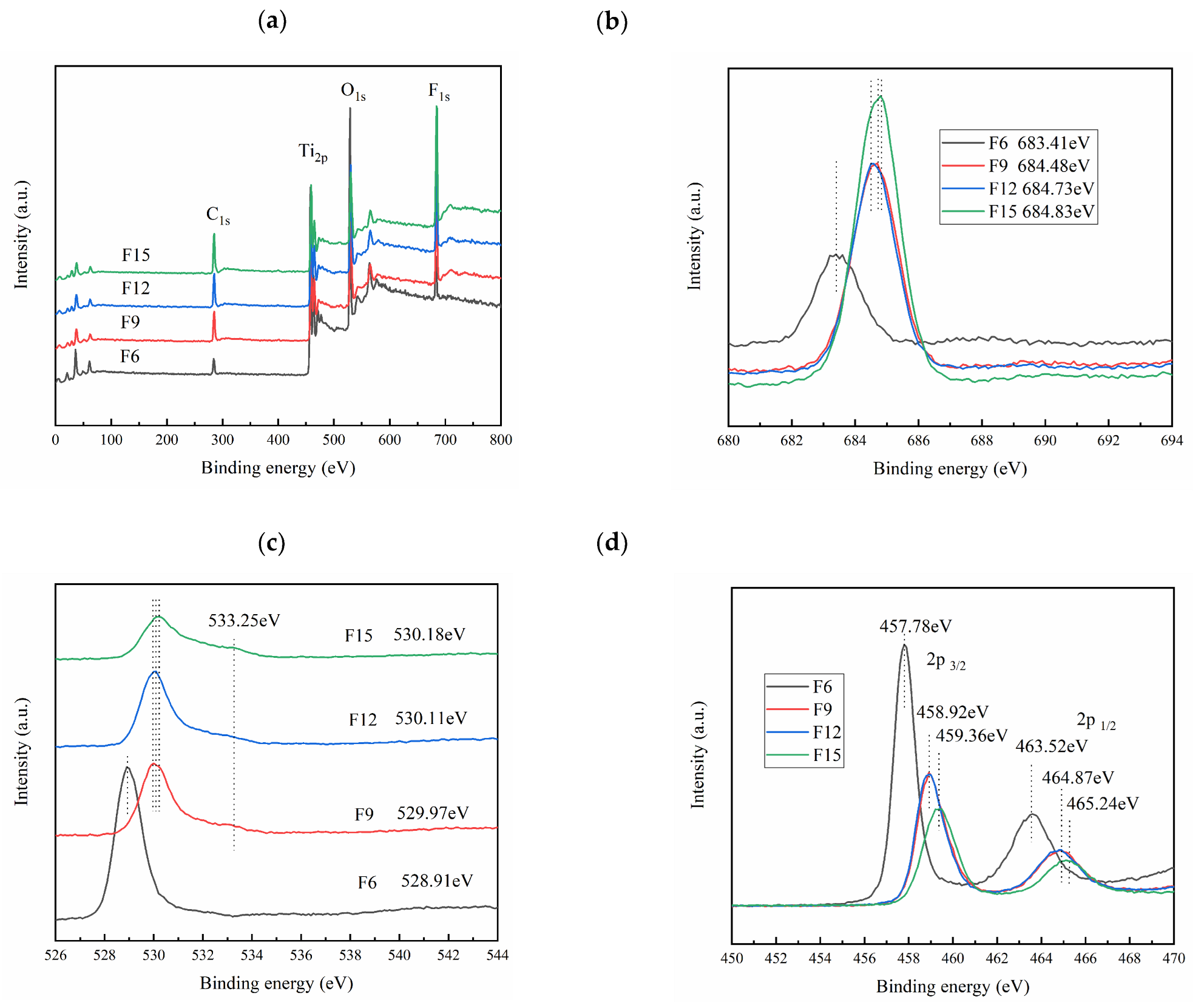
3.1.7. XPS Analysis
3.1.8. PL Analysis
3.1.9. Particle Size Distribution
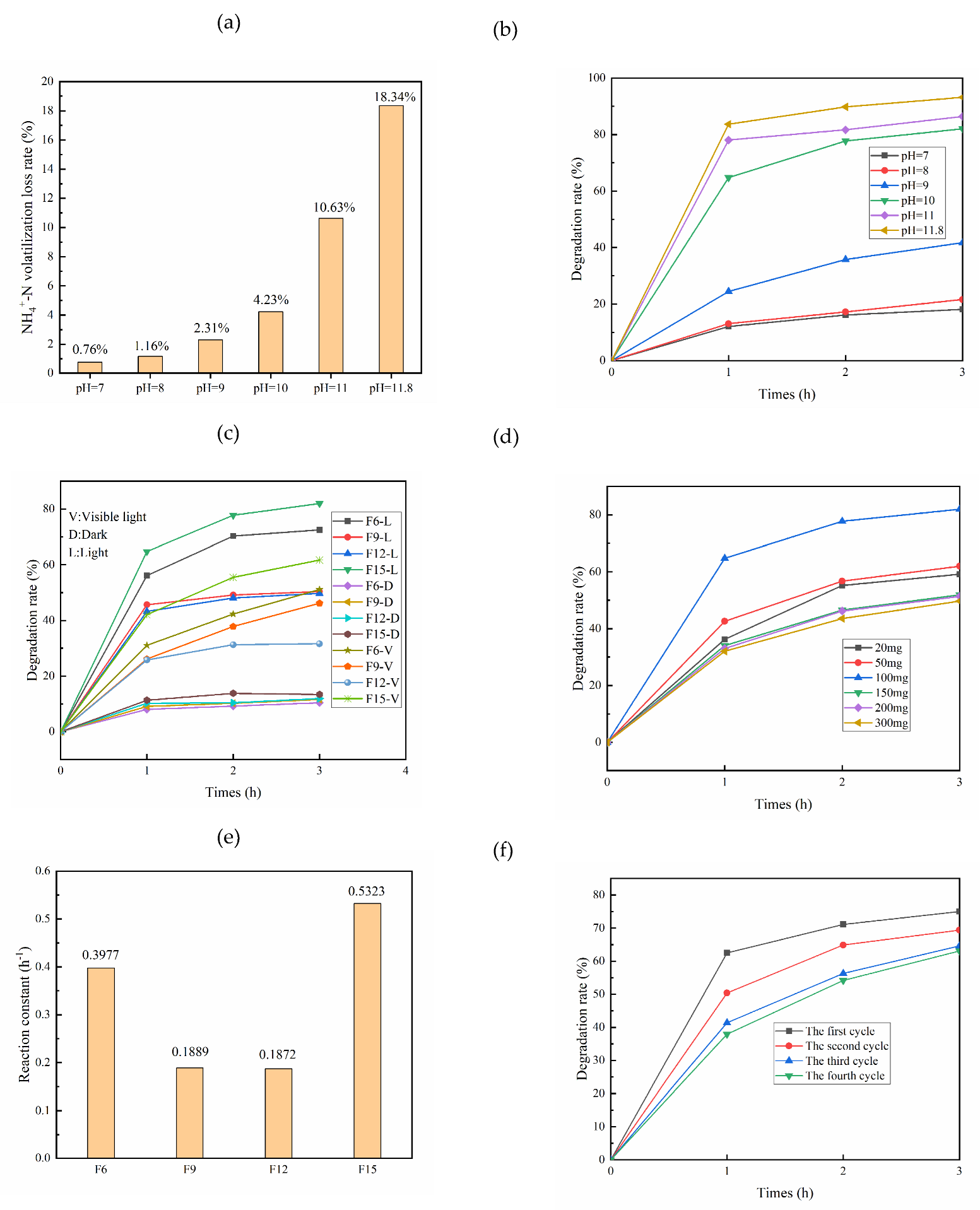
3.2. Analysis of Photocatalytic Degradation of Ammonia Nitrogen
Volatilization of Ammonia Nitrogen
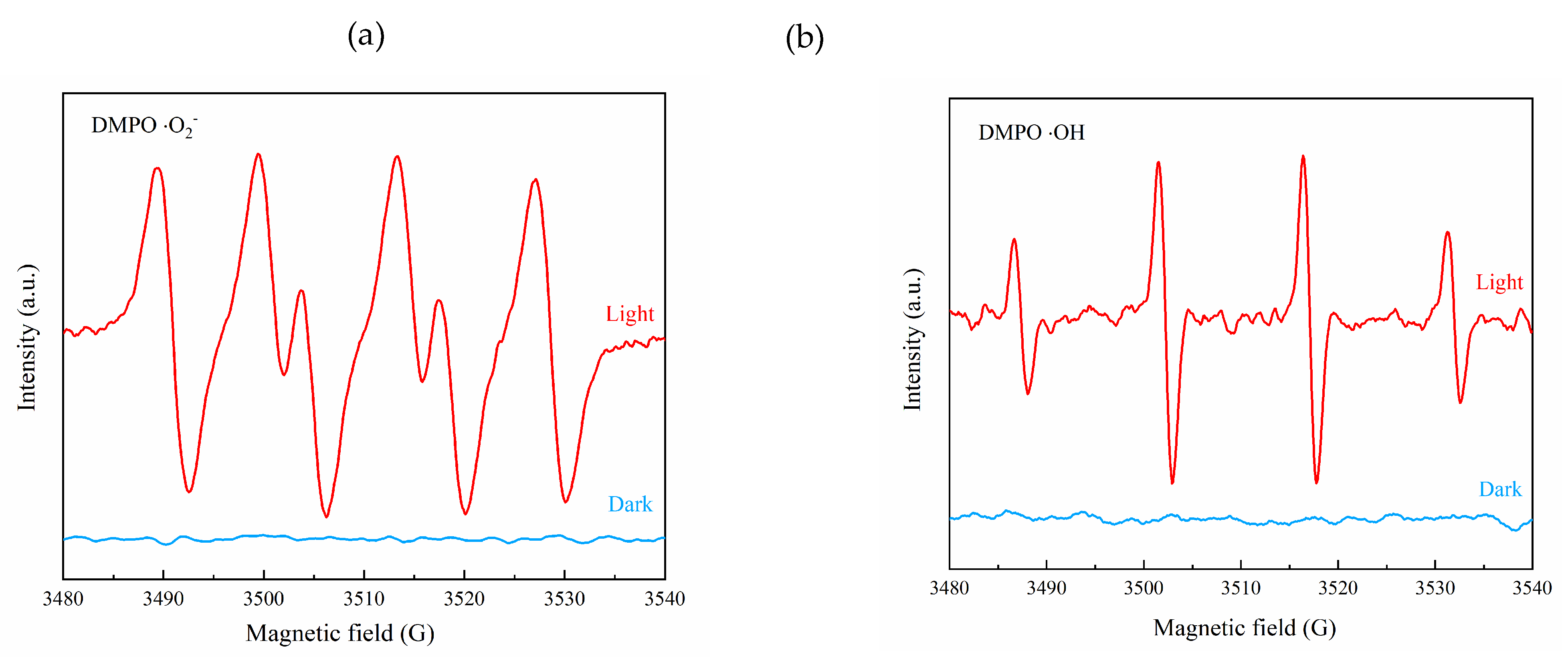
3.3. Mechanism Analysis of Photocatalytic Degradation of Ammonia Nitrogen
4. Summary
- The {001}TiO2/TiOF2 was prepared with 15 mL hydrofluoric acid and had a large specific surface area to provide more active sites for the reaction. It has a 92.25% highly active {001} crystal surface on which hydroxyl oxygen and oxygen vacancy (Ov) in water is adsorbed. It has a strong optical absorption capacity, small bandwidth, and strong photogenerated carrier separation ability. This indicates that the system has good photocatalytic activity;
- The volatilization loss rate of ammonia nitrogen increases with increasing pH. When the initial concentration of ammonia nitrogen is 100 mg/L and pH is 10, the degradation rate of ammonia nitrogen by 100 mg F15 is 93.19%. Throughout the entire reaction process, the catalyst itself has little influence on the change in ammonia nitrogen concentration, and photocatalysis plays a major role;
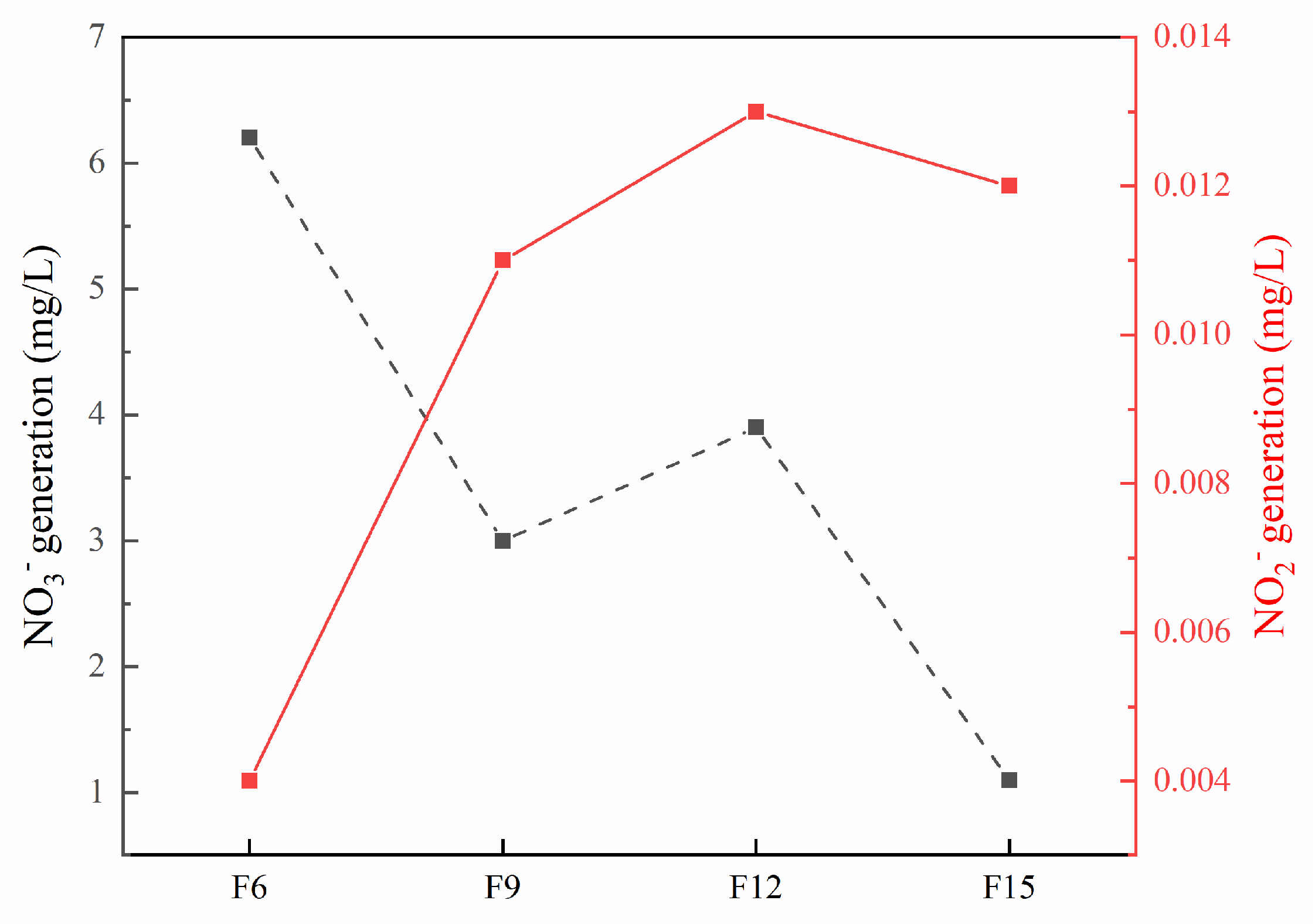
- Hydroxyl and superoxide radicals are produced during F15 degradation of ammonia nitrogen; their products are mainly N2, accompanied by a small amount of NO2− and NO3−.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, S.; Wang, H.; Wang, H.; Jeppesen, E. Effects of nitrate on phosphorus release from lake sediments: A three-month mesocosm experiment. Water Res. 2021, 194, 116894. [Google Scholar] [CrossRef] [PubMed]
- Richards, G.; Gilmore, T.; Mittelstet, A.; Tiffany, L.; Daniel, D. Baseflow nitrate dynamics within nested watersheds of an agricultural stream in Nebraska, USA. Agric. Ecosyst. Environ. 2021, 308, 107223. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, E.; Li, Y.; Liu, H.; Vidal, D.; Doris, E.; John, G. Ecological risks posed by ammonia nitrogen (AN) and un-ionized ammonia (NH3) in seven major river systems of China. Chemosphere 2018, 202, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhang, P.; Zhang, Z.; Liu, J.; Xiao, J.; Gao, F. Simultaneous removal of ammonia nitrogen and recovery of phosphate from swine wastewater by struvite electrochemical precipitation and recycling technology. J. Clean. Prod. 2016, 127, 302. [Google Scholar] [CrossRef]
- Ge, H.; Yu, L.; Chen, Z.; Liu, Z.; Liu, H.; Hu, D.; Wang, H.; Cui, Y.; Zhang, W.; Zou, X. Novel tapered variable diameter biological fluidized bed for treating pesticide wastewater with high nitrogen removal efficiency and a small footprint. Bioresour. Technol. 2021, 330, 12. [Google Scholar] [CrossRef]
- Ren, Y.; Hao, N.; Guo, W.; Wang, D.; Peng, L.; Ni, B.; Wei, W.; Liu, Y. New perspectives on microbial communities and biological nitrogen removal processes in wastewater treatment systems. Bioresour. Technol. 2020, 297, 122491. [Google Scholar] [CrossRef]
- Alshorifi, F.; Alswat, A.; Salama, R. Gold-selenide quantum dots supported onto cesium ferrite nanocomposites for the efficient degradation of rhodamine B. Heliyon 2022, 8, 9652. [Google Scholar] [CrossRef]
- Alshorif, F.; Ali, S.; Salama, R. Promotional Synergistic Effect of Cs–Au NPs on the Performance of Cs-Au/MgFe2O4 Catalysts in Catalysis 3,4-Dihydropyrimidin-2(1H)-Ones and Degradation of RhB Dye. J. Inorg. Organomet. Polym. Mater. 2022, 1–12. [Google Scholar] [CrossRef]
- Alshorifi, F.; Tobbala, D.; El-Bahy, S.; Nassan, M.; Salama, R. The role of phosphotungstic acid in enhancing the catalytic performance of UiO-66 (Zr) and its applications as an efficient solid acid catalyst for coumarins and dihydropyrimidinones synthesis. Catal. Commun. 2022, 169, 106479. [Google Scholar] [CrossRef]
- Zhao, B.; Tian, M.; An, Q.; Ye, J.; Guo, S. Characteristics of a heterotrophic nitrogen removal bacterium and its potential application on treatment of ammonium-rich wastewater. Bioresour. Technol. 2017, 226, 46. [Google Scholar] [CrossRef]
- He, Q.; Song, Q.; Zhan, S.; Zhang, W.; Wang, H. Simultaneous nitrification, denitrification and phosphorus removal in an aerobic granular sequencing batch reactor with mixed carbon sources: Reactor performance, extracellular polymeric substances and microbial successions. Chem. Eng. J. 2018, 331, 841. [Google Scholar] [CrossRef]
- John, H.; John, L.; Helle, M. Photodechlorination of PCB’s in the presence of titanium dioxide in aqueous suspensions. Bull. Environ. Pollut. Toxicol. 1976, 16, 697–701. [Google Scholar]
- Zhu, J.; Jian, Y.; Long, D.; Wang, H.; Zeng, Y.; Li, J.; Xiao, R.; Pu, S. Degradation of ammonia gas by Cu2O/{001}TiO2 and its mechanistic analysis. RSC Adv. 2021, 11, 3695–3702. [Google Scholar] [CrossRef]
- Zhou, Q.; Yin, H.; Wang, A.; Yang, S. Preparation of hollow B—SiO2@TiO2 composites and their photocatalytic performances for degradation of ammonia-nitrogen and green algae in aqueous solution. Chin. J. Chem. Eng. 2019, 27, 2535. [Google Scholar] [CrossRef]
- Yao, F.; Fu, W.; Ge, X.; Wang, L.; Wang, J.; Zhong, W. Preparation and characterization of a copper phosphotungstate/titanium dioxide (Cu-H3PW12O40/TiO2) composite and the photocatalytic oxidation of high-concentration ammonia nitrogen. Sci. Total Environ. 2020, 727, 138425. [Google Scholar] [CrossRef]
- Li, H.; Cao, Y.; Liu, P.; Li, Y.; Zhou, A.; Ye, F.; Xue, S.; Yue, X. Ammonia-nitrogen removal from water with gC3N4-rGO-TiO2 Z-scheme system via photocatalytic nitrification-denitrification process. Environ. Res. 2022, 205, 112434. [Google Scholar] [CrossRef]
- Li, F.; Fu, Z.; Lu, Y. Synthesis of TiOF2 ball-flowers and the phase transitions to TiO2. Adv. Chem. Mater. Metall. Eng. 2013, 634, 2297. [Google Scholar] [CrossRef]
- Huang, Y.; Xia, T.; Shang, M.; Petra, W.; Dakota, K.; Peterson, A.; James, M.; Dong, L.; Chen, X. Structural evolution from TiO2 nanoparticles to nanosheets and their photocatalytic performance in hydrogen generation and environmental pollution removal. RSC Advances 2014, 4, 16146. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, B.; Dai, Y.; Zhu, X.; Liu, Y.; Zhang, X.; Qin, X. The roles of growth conditions on the topotactic transformation from TiOF2 nanocubes to 3D hierarchical TiO2 nanoboxes. CrystEngComm 2013, 15, 3436. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, Z.; Lv, K.; Zheng, Y.; Deng, K. Transformation of TiOF2 cube to a hollow nanobox assembly from anatase TiO2 nanosheets with exposed {001} facets via solvothermal strategy. ACS Appl. Mater. Interfaces 2013, 5, 8663. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, G.; Zhang, H.; Tang, Q.; Xiao, Y.; Wang, Y.; Cao, J. Facile synthesis hierarchical porous structure anatase-rutile TiO2/g-C3N4 composite for efficient photodegradation tetracycline hydrochloride. Appl. Surf. Sci. 2021, 567, 150833. [Google Scholar] [CrossRef]
- Wang, J.; Cao, F.; Bian, Z.; Leung, M.; Li, H. Ultrafine single-crystal TiOF2 nanocubes with mesoporous structure, high activity and durability in visible light driven photocatalysis. Nanoscale 2014, 6, 897. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Zhang, Q.; Liu, J.; Qian, J.; Zhou, X. TiO2 nanoparticles embedded in hollow cube with highly exposed {001} facets: Facile synthesis and photovoltaic applications. J. Alloys Compd. 2016, 656, 863. [Google Scholar] [CrossRef]
- Minero, C.; Mariella, G.; Maurino, V.; Vione, D.; Pelizzetti, E. Photocatalytic Transformation of Organic Compounds in the Presence of Inorganic Ions. 2. Competitive Reactions of Phenol and Alcohols on a Titanium Dioxide-Fluoride System. Langmuir 2000, 16, 8964–8972. [Google Scholar] [CrossRef]
- Lewandowski, M.; Ollis, D. Halide acid pretreatments of photocatalysts for oxidation of aromatic air contaminants: Rate enhancement, rate inhibition, and a thermodynamic rationale. J. Catal. 2003, 217, 38. [Google Scholar] [CrossRef]
- Yang, C.; Mao, G.B.; Tang, Y.W.; Wang, H.; Wang, G.; Zhang, L.; Liu, Q. Synthesis of core-shell nanostructured Cr2O3/C@TiO2 for photocatalytic hydrogen production. Chin. J. Catal. 2021, 42, 225. [Google Scholar] [CrossRef]
- Hou, C.; Hu, B.; Zhu, J. Photocatalytic Degradation of Methylene Blue over TiO2 Pretreated with Varying Concentrations of NaOH. Catalysts 2018, 8, 575. [Google Scholar] [CrossRef]
- Tian, F.; Zhang, Y.P.; Zhang, J.; Pan, C. Raman Spectroscopy: A New Approach to Measure the Percentage of Anatase TiO2 Exposed (001) Facets. J. Phys. Chem. C 2012, 116, 7515. [Google Scholar] [CrossRef]
- Wei, J.X.; Chen, Y.W.; Zhang, H.Y.; Zhuang, Z.; Yu, Y. Hierarchically porous S-scheme CdS/UiO-66 photocatalyst for efficient 4-nitroaniline reduction. Chin. J. Catal. 2021, 42, 78. [Google Scholar] [CrossRef]
- Li, W.; Body, M.; Legein, C.; Dambournetet, D. Identify OH groups in TiOF2 and their impact on the lithium intercalation properties. J. Solid State Chem. 2017, 246, 113. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, X.; Sun, X.; Qian, N.; Wang, M.; Ma, Y. Improved adsorption and degradation performance by S-doping of (001)-TiO2. Beilstein J. Nanotechnol. 2019, 10, 2116. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Li, Z.; Cheng, B.; Zhang, M.; Su, K. Higher UV-shielding ability and lower photocatalytic activity of TiO2@SiO2/APTES and its excellent performance in enhancing the photostability of poly (p-phenylene sulfide). RSC Adv. 2017, 7, 21758. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.H.; Jiang, J.; Wan, Z.; Su, Y.; Tang, H. Insight into charge carrier separation and solar-light utilization: rGO decorated 3D ZnO hollow microspheres for enhanced photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2020, 564, 322. [Google Scholar] [CrossRef]
- Jing, L.; Hodes, G.; Yan, J.Q.; Liu, S. Metal-doped Mo2C (metal = Fe, Co, Ni, Cu) as catalysts on TiO2 for photocatalytic hydrogen evolution in neutral solution. Chin. J. Catal. 2021, 42, 205. [Google Scholar] [CrossRef]
- Oseghe, E.; Ofomaja, A. Facile microwave synthesis of pine cone derived C-doped TiO2 for the photodegradation of tetracycline hydrochloride under visible-LED light. J. Environ. Manag. 2018, 223, 860. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, X.; Lu, Q.; Wang, Q.; Zhen, M. TiOF2/TiO2 composite nanosheets: Effect of hydrothermal synthesis temperature on physicochemical properties and photocatalytic activity. J. Taiwan Inst. Chem. Eng. 2019, 96, 214. [Google Scholar] [CrossRef]
- Wu, J.; Ma, X.; Xu, L.; Zhao, B.; Chen, F. Fluorination promoted photoinduced modulation of Pt clusters on oxygen vacancy enriched TiO2/Pt photocatalyst for superior photocatalytic performance. Appl. Surf. Sci. 2019, 489, 510. [Google Scholar] [CrossRef]
- Lv, C.; Lan, X.; Wang, L.; Dai, X.; Zhang, M.; Cui, J.; Yuan, S.; Wang, S.; Shi, J. Rapidly and highly efficient degradation of tetracycline hydrochloride in wastewater by 3D IO-TiO2-CdS nanocomposite under visible light. Environ. Technol. 2019, 11, 377. [Google Scholar] [CrossRef]
- Zhang, B.; Hu, X.Y.; Liu, E.Z.; Fan, J. Novel S-scheme 2D/2D BiOBr/g-C3N4 heterojunctions with enhanced photocatalytic activity. Chin. J. Catal. 2021, 42, 1519. [Google Scholar] [CrossRef]
- Lv, K.L.; Yua, J.G.; Cui, L.Z.; Chen, S.; Li, M. Preparation of thermally stable anatase TiO2 photocatalyst from TiOF2 precursor and its photocatalytic activity. J. Alloys Compd. 2011, 509, 4557. [Google Scholar] [CrossRef]
- Altomare, M.; Selli, E. Effects of metal nanoparticles deposition on the photocatalytic oxidation of ammonia in TiO2 aqueous suspensions. Catal. Today 2013, 209, 127. [Google Scholar] [CrossRef]

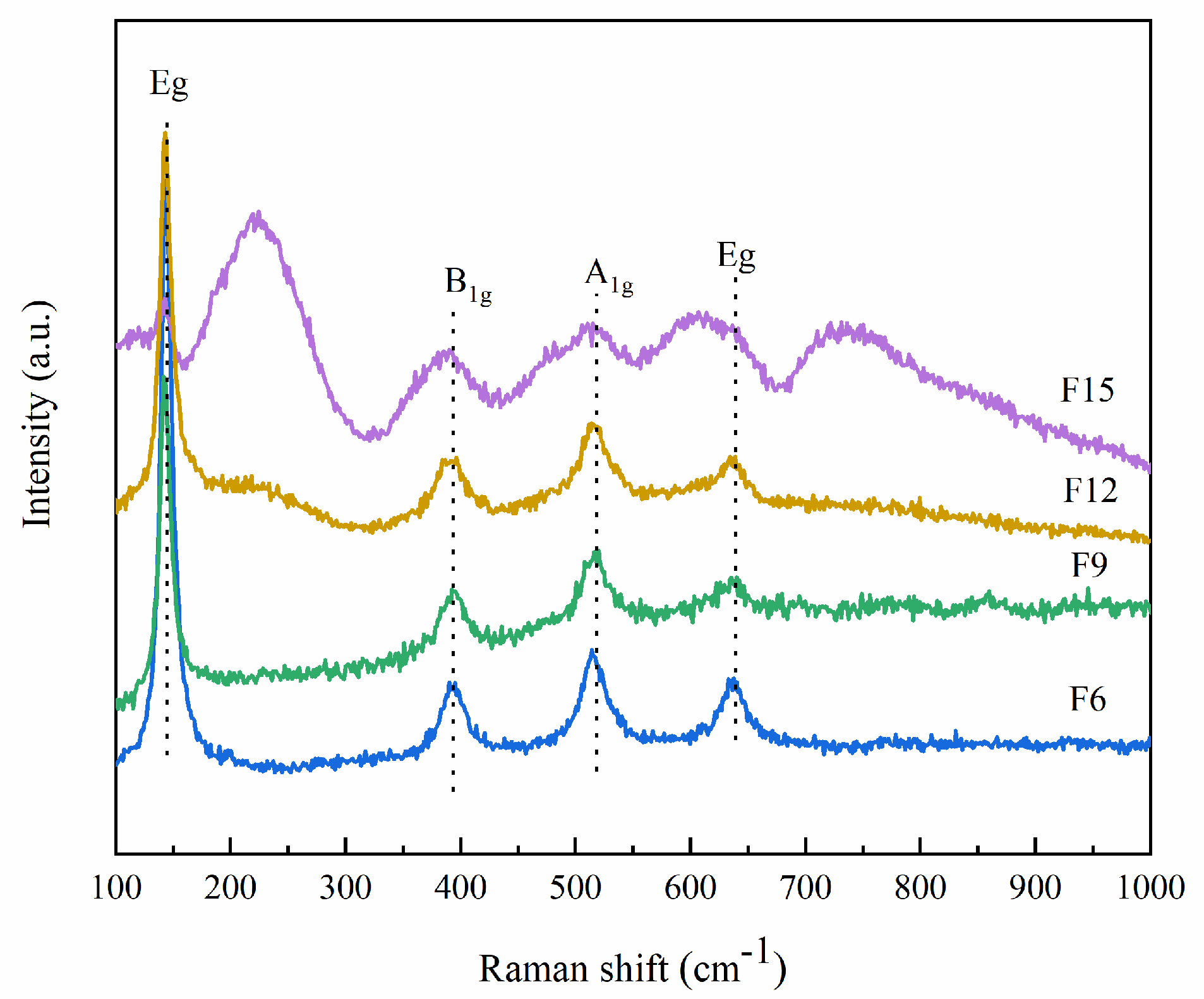


| Samples | Peak Intensity of Eg (514 cm−1) | Peak Intensity of A1g (144 cm−1) | Percentage of {001} |
|---|---|---|---|
| F6 | 1096.91 | 3645.23 | 30.09% |
| F9 | 1129.88 | 2692.69 | 52.09% |
| F12 | 3864.17 | 7018.22 | 55.06% |
| F15 | 1565.51 | 1697.08 | 92.25% |
| Samples | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|
| F6 | 52.54 | 0.14 | 8.96 |
| F9 | 56.76 | 0.16 | 8.95 |
| F12 | 57.86 | 0.15 | 7.95 |
| F15 | 60.89 | 0.14 | 8.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Liu, Z.; Yang, F.; Long, D.; Jian, Y.; Pu, S. The Preparation of {001}TiO2/TiOF2 via a One-Step Hydrothermal Method and Its Degradation Mechanism of Ammonia Nitrogen. Materials 2022, 15, 6465. https://doi.org/10.3390/ma15186465
Zhu J, Liu Z, Yang F, Long D, Jian Y, Pu S. The Preparation of {001}TiO2/TiOF2 via a One-Step Hydrothermal Method and Its Degradation Mechanism of Ammonia Nitrogen. Materials. 2022; 15(18):6465. https://doi.org/10.3390/ma15186465
Chicago/Turabian StyleZhu, Jiaming, Zuohua Liu, Feiyun Yang, Dingbiao Long, Yue Jian, and Shihua Pu. 2022. "The Preparation of {001}TiO2/TiOF2 via a One-Step Hydrothermal Method and Its Degradation Mechanism of Ammonia Nitrogen" Materials 15, no. 18: 6465. https://doi.org/10.3390/ma15186465
APA StyleZhu, J., Liu, Z., Yang, F., Long, D., Jian, Y., & Pu, S. (2022). The Preparation of {001}TiO2/TiOF2 via a One-Step Hydrothermal Method and Its Degradation Mechanism of Ammonia Nitrogen. Materials, 15(18), 6465. https://doi.org/10.3390/ma15186465







