Novel Osteogenic and Easily Handled Endodontic Calcium Silicate Cement Using Pluronic F127 Hydrogel
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of F127 Hydrogel-Based CSC
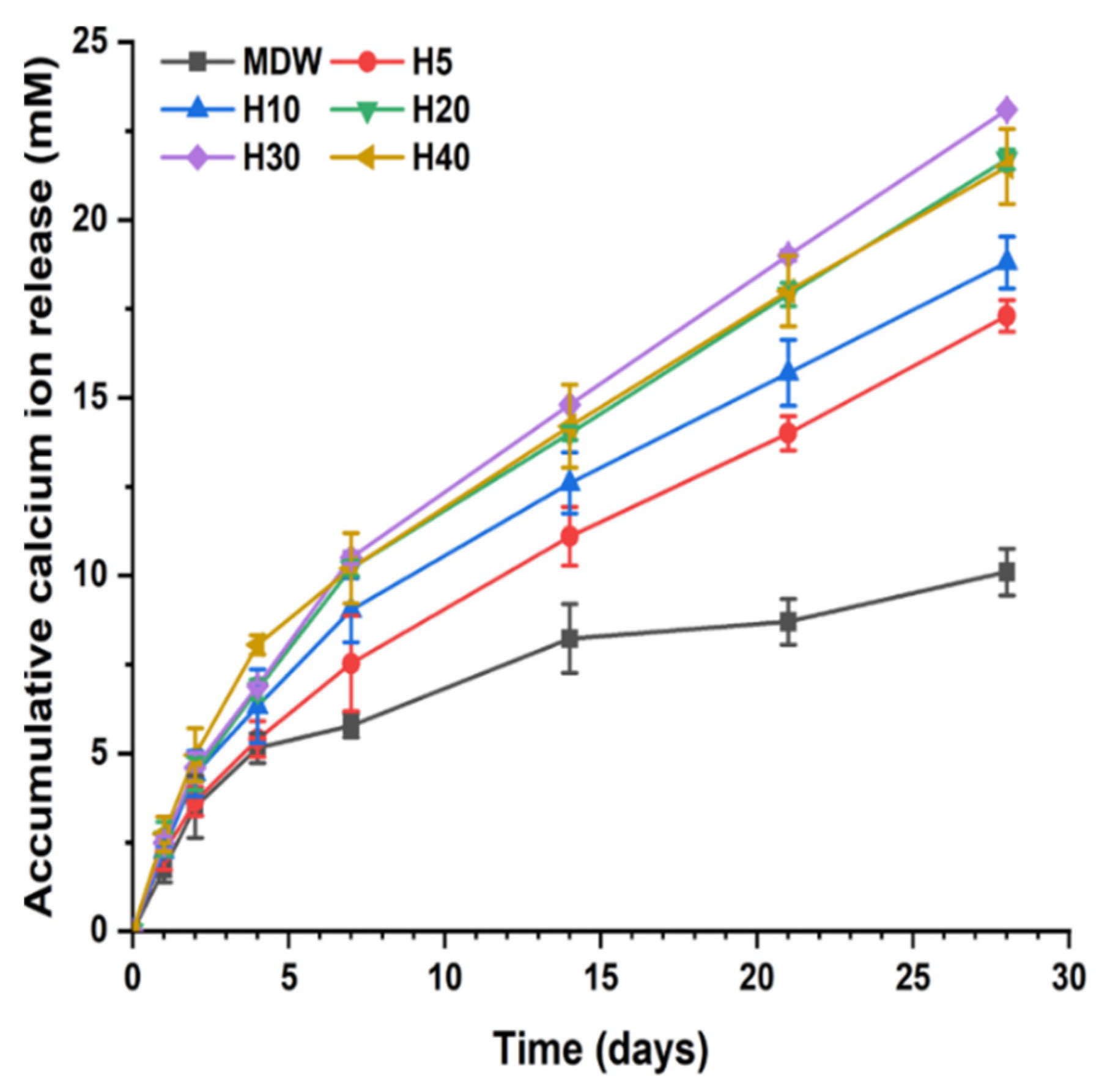
2.3. Calcium Ion Release
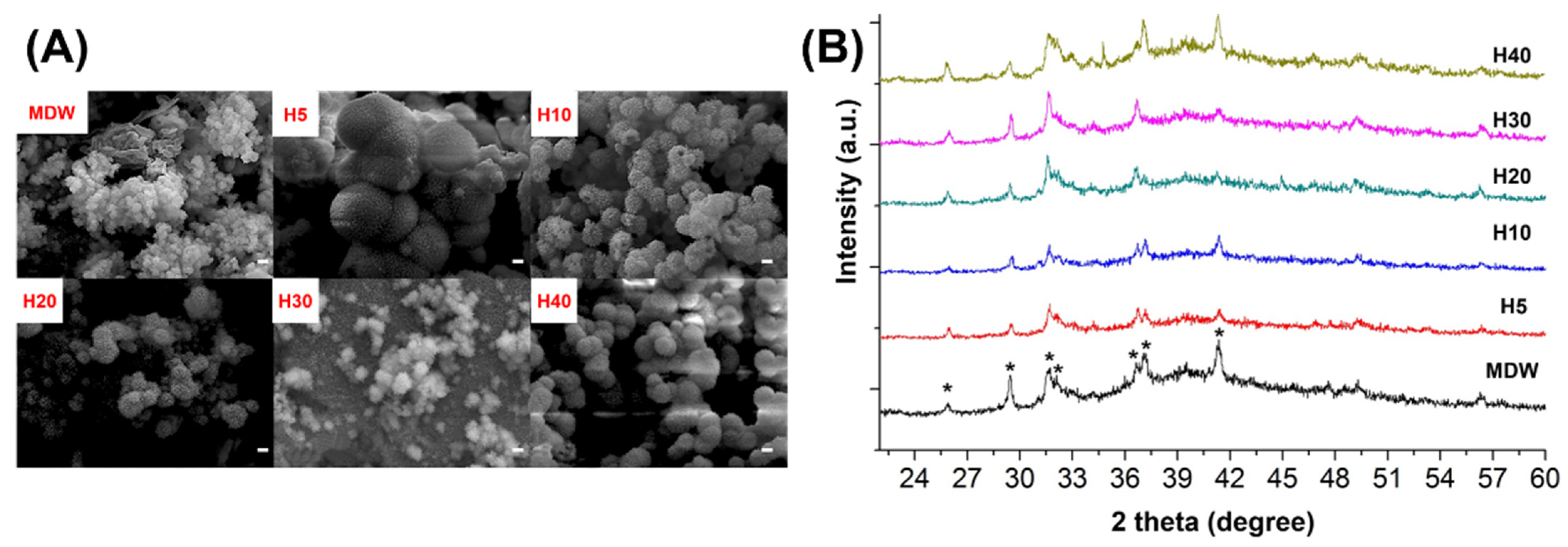
2.4. Apatite Mineralization Assessment
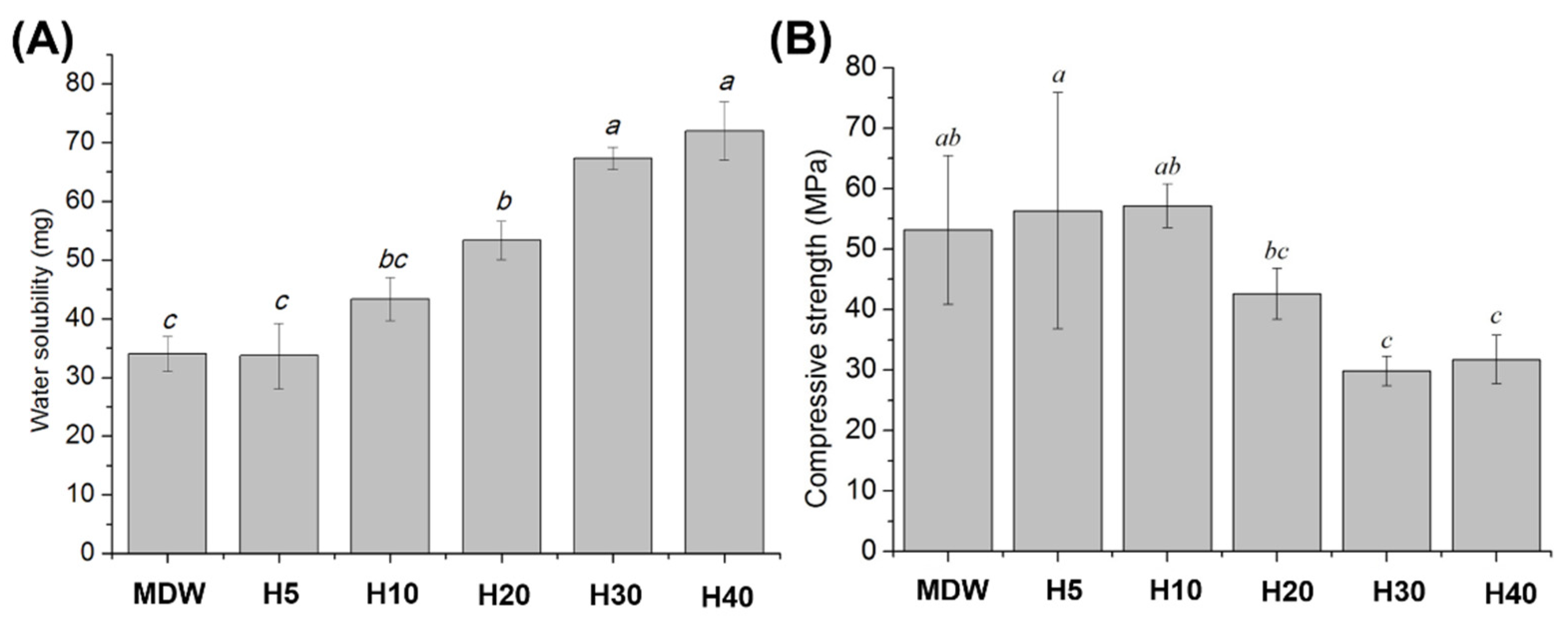
2.5. Water Solubility
2.6. Compressive Strength
2.7. Cell Culture
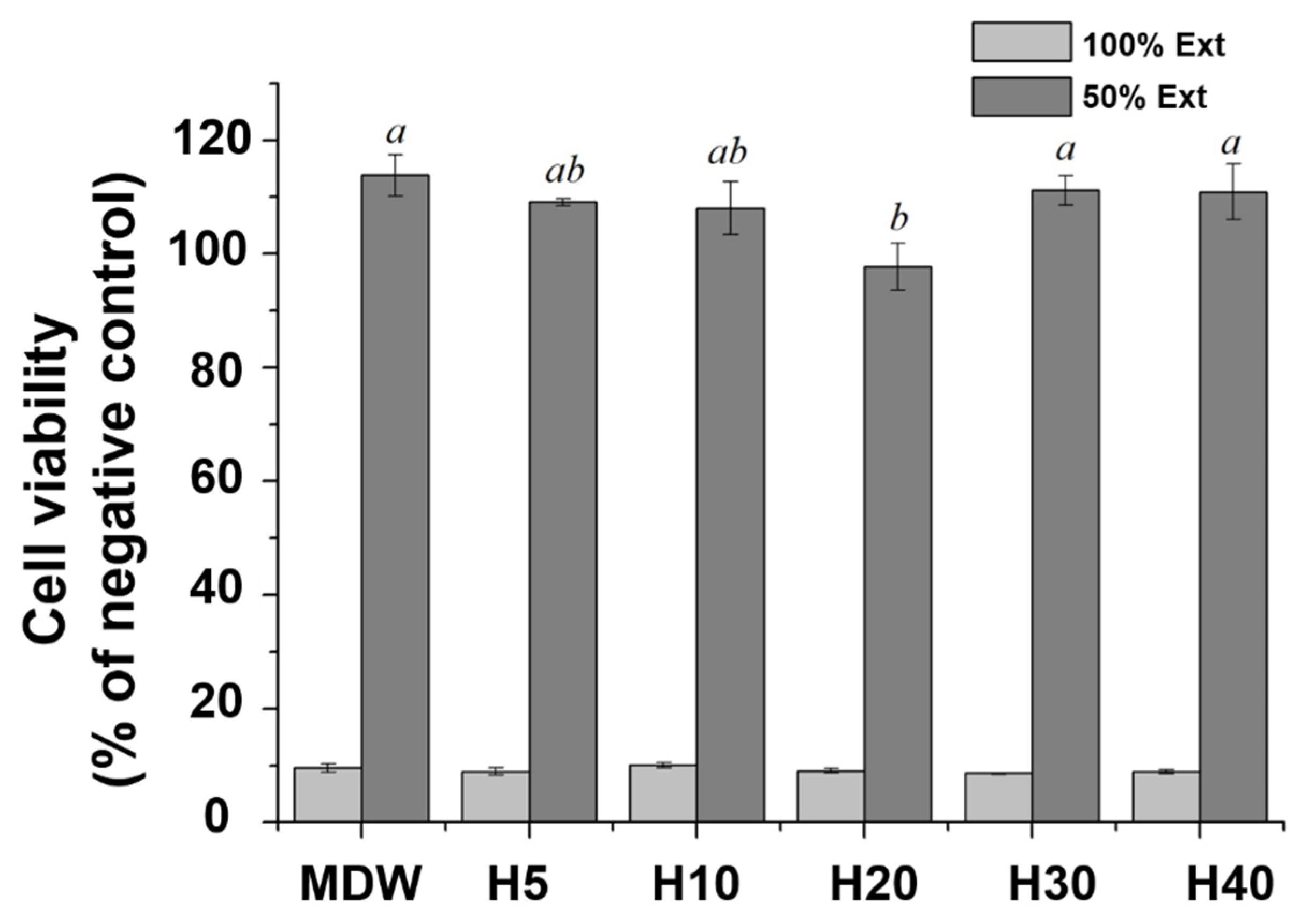
2.8. Cell Viability
2.9. In Vitro Osteogenic Properties
2.10. Statistical Analysis
3. Results
3.1. In Vitro Characterization of F127-Based Hydrogel with CSC
3.2. Physical and Mechanical Properties of F127-Based Hydrogel with CSC
3.3. Cell Viability
3.4. Osteogenic Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yadav, R.; Kumar, M. Dental restorative composite materials: A review. J. Oral Biosci. 2019, 61, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Kumar, M. Investigation of the physical, mechanical and thermal properties of nano and microsized particulate-filled dental composite material. J. Compos. Mater. 2020, 54, 2623–2633. [Google Scholar] [CrossRef]
- Yadav, R. Analytic hierarchy process-technique for order preference by similarity to ideal solution: A multi criteria decision-making technique to select the best dental restorative composite materials. Polym. Compos. 2021, 42, 6867–6877. [Google Scholar] [CrossRef]
- Torabinejad, M.; Chivian, N. Clinical applications of mineral trioxide aggregate. J. Endod. 1999, 25, 197–205. [Google Scholar] [CrossRef]
- Ford, T.R.P.; Torabinejad, M.; McKendry, D.J.; Hong, C.-U.; Kariyawasam, S.P. Use of mineral trioxide aggregate for repair of furcal perforations. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1995, 79, 756–763. [Google Scholar] [CrossRef]
- Holland, R.; de Souza, V.; Nery, M.J.; Otoboni Filho, J.A.; Bernabé, P.F.E.; Dezan, E., Jr. Reaction of dogs‘ teeth to root canal filling with mineral trioxide aggregate or a glass ionomer sealer. J. Endod. 1999, 25, 728–730. [Google Scholar] [CrossRef]
- Bogen, G.; Kim, J.S.; Bakland, L.K. Direct pulp capping with mineral trioxide aggregate: An observational study. J. Am. Dent. Assoc. 2008, 139, 305–315. [Google Scholar] [CrossRef]
- Yan, M.; Wu, J.; Yu, Y.; Wang, Y.; Xie, L.; Zhang, G.; Yu, J.; Zhang, C. Mineral Trioxide Aggregate Promotes the Odonto/Osteogenic Differentiation and Dentinogenesis of Stem Cells from Apical Papilla via Nuclear Factor Kappa B Signaling Pathway. J. Endod. 2014, 40, 640–647. [Google Scholar] [CrossRef]
- Saghiri, M.A.; Lotfi, M.; Saghiri, A.M.; Vosoughhosseini, S.; Fatemi, A.; Shiezadeh, V.; Ranjkesh, B. Effect of pH on Sealing Ability of White Mineral Trioxide Aggregate as a Root-end Filling Material. J. Endod. 2008, 34, 1226–1229. [Google Scholar] [CrossRef] [PubMed]
- Parirokh, M.; Torabinejad, M. Mineral trioxide aggregate: A comprehensive literature review—Part III: Clinical applications, drawbacks, and mechanism of action. J. Endod. 2010, 36, 400–413. [Google Scholar] [CrossRef]
- Siboni, F.; Taddei, P.; Zamparini, F.; Prati, C.; Gandolfi, M.G. Properties of BioRoot RCS, a tricalcium silicate endodontic sealer modified with povidone and polycarboxylate. Int. Endod. J. 2017, 50, e120–e136. [Google Scholar] [CrossRef] [PubMed]
- Parirokh, M.; Asgary, S.; Eghbal, M.J.; Kakoei, S.; Samiee, M. A Comparative Study of Using a Combination of Calcium Chloride and Mineral Trioxide Aggregate as the Pulp-capping Agent on Dogs’ Teeth. J. Endod. 2011, 37, 786–788. [Google Scholar] [CrossRef] [PubMed]
- Vitti, R.P.; Prati, C.; Sinhoreti, M.; Zanchi, C.H.; e Silva, M.G.S.; Ogliari, F.A.; Piva, E.; Gandolfi, M.G. Chemical–physical properties of experimental root canal sealers based on butyl ethylene glycol disalicylate and MTA. Dent. Mater. 2013, 29, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.-C.; Chen, C.-H.; Wang, C.-Z.; Wang, Y.-H.; Chang, J.-K.; Wang, G.-J.; Ho, M.-L.; Wang, C.-K. Preparation of porous bioceramics using reverse thermo-responsive hydrogels in combination with rhBMP-2 carriers: In vitro and in vivo evaluation. J. Mech. Behav. Biomed. Mater. 2013, 27, 64–76. [Google Scholar] [CrossRef]
- Sharpe, A.L.; Daily, A.M.; Horava, S.D.; Peppas, A.N. Therapeutic applications of hydrogels in oral drug delivery. Expert Opin. Drug Deliv. 2014, 11, 901–915. [Google Scholar] [CrossRef]
- Bray, L.J.; Binner, M.; Holzheu, A.; Friedrichs, J.; Freudenberg, U.; Hutmacher, D.W.; Werner, C. Multi-parametric hydrogels support 3D in vitro bioengineered microenvironment models of tumour angiogenesis. Biomaterials 2015, 53, 609–620. [Google Scholar] [CrossRef]
- Lavanya, K.; Chandran, S.V.; Balagangadharan, K.; Selvamurugan, N. Temperature-and pH-responsive chitosan-based injectable hydrogels for bone tissue engineering. Mater. Sci. Eng. C 2020, 111, 110862. [Google Scholar] [CrossRef]
- Shang, J.; Theato, P. Smart composite hydrogel with pH-, ionic strength-and temperature-induced actuation. Soft Matter 2018, 14, 8401–8407. [Google Scholar] [CrossRef]
- Ciofani, G.; Raffa, V.; Pensabene, V.; Menciassi, A.; Dario, P. Dispersion of Multi-walled Carbon Nanotubes in Aqueous Pluronic F127 Solutions for Biological Applications. Full- Nanotub. Carbon Nanostruct. 2009, 17, 11–25. [Google Scholar] [CrossRef]
- Wenzel, J.G.W.; Balaji, K.S.S.; Koushik, K.; Navarre, C.; Duran, S.H.; Rahe, C.H.; Kompella, U.B. Pluronic F127 gel formulations of deslorelin and GnRH reduce drug degradation and sustain drug release and effect in cattle. J. Control. Release 2002, 85, 51–59. [Google Scholar] [CrossRef]
- Moore, T.; Croy, S.; Mallapragada, S.; Pandit, N. Experimental investigation and mathematical modeling of Pluronic® F127 gel dissolution: Drug release in stirred systems. J. Control. Release 2000, 67, 191–202. [Google Scholar] [CrossRef]
- Dantas Lopes dos Santos, D.; Besegato, J.F.; de Melo, P.B.G.; Oshiro Junior, J.A.; Chorilli, M.; Deng, D.; Bagnato, V.S.; Rastelli, A.N.d.S. Curcumin-loaded Pluronic® F-127 Micelles as a Drug Delivery System for Curcumin-mediated Photodynamic Therapy for Oral Application. Photochem. Photobiol. 2021, 97, 1072–1088. [Google Scholar] [CrossRef] [PubMed]
- Shriky, B.; Kelly, A.; Isreb, M.; Babenko, M.; Mahmoudi, N.; Rogers, S.; Shebanova, O.; Snow, T.; Gough, T. Pluronic F127 thermosensitive injectable smart hydrogels for controlled drug delivery system development. J. Colloid Interface Sci. 2020, 565, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Gu, Z.; Xu, J.; Zhao, M.; Liu, G.; Wu, J. Acid-responsive composite hydrogel platform with space-controllable stiffness and calcium supply for enhanced bone regeneration. Chem. Eng. J. 2020, 396, 125353. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Z.; Pan, D.; Li, H.; Shen, J. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomes Combined Pluronic F127 Hydrogel Promote Chronic Diabetic Wound Healing and Complete Skin Regeneration. Int. J. Nanomed. 2020, 15, 5911–5926. [Google Scholar] [CrossRef]
- Nie, S.; Hsiao, W.W.; Pan, W.; Yang, Z. Thermoreversible Pluronic® F127-based hydrogel containing liposomes for the controlled delivery of paclitaxel: In vitro drug release, cell cytotoxicity, and uptake studies. Int. J. Nanomed. 2011, 6, 151–166. [Google Scholar]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- ISO-6876; Dental Root Canal Sealing Materials. International Standards Organization: Geneva, Switzerland, 2012.
- ISO 9917-1; Dentistry-Water-based Cements—Part 1: Powder/Liquid Acid-Base Cements. ISO: Geneva, Switzerland, 2007.
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for Cytotoxicity: In Vitro Methods. International Standards Organization: Geneva, Switzerland, 2009.
- ISO 10993-12; Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. International Standard Organization: Geneva, Switzerland, 2012.
- Ryu, J.-H.; Kang, T.-Y.; Shin, H.; Kim, K.-M.; Hong, M.-H.; Kwon, J.-S. Osteogenic Properties of Novel Methylsulfonylmethane-Coated Hydroxyapatite Scaffold. Int. J. Mol. Sci. 2020, 21, 8501. [Google Scholar] [CrossRef]
- Ber, B.S.; Hatton, J.F.; Stewart, G.P. Chemical Modification of ProRoot MTA to Improve Handling Characteristics and Decrease Setting Time. J. Endod. 2007, 33, 1231–1234. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Taddei, P.; Siboni, F.; Modena, E.; Ciapetti, G.; Prati, C. Development of the foremost light-curable calcium-silicate MTA cement as root-end in oral surgery. Chemical–physical properties, bioactivity and biological behavior. Dent. Mater. 2011, 27, e134–e157. [Google Scholar] [CrossRef]
- Borges, R.; Kai, K.C.; Lima, C.A.; Zezell, D.M.; de Araujo, D.R.; Marchi, J. Bioactive glass/poloxamer 407 hydrogel composite as a drug delivery system: The interplay between glass dissolution and drug release kinetics. Colloids Surfaces B: Biointerfaces 2021, 206, 111934. [Google Scholar] [CrossRef] [PubMed]
- Diniz, I.M.A.; Chen, C.; Xu, X.; Ansari, S.; Zadeh, H.H.; Marques, M.M.; Shi, S.; Moshaverinia, A. Pluronic F-127 hydrogel as a promising scaffold for encapsulation of dental-derived mesenchymal stem cells. J. Mater. Sci. Mater. Med. 2015, 26, 153. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Wang, J.; Sun, E.; Yang, L.; Yan, H.-M.; Jia, X.-B.; Zhang, Z.-H. Preparation and evaluation of icariside II-loaded binary mixed micelles using Solutol HS15 and Pluronic F127 as carriers. Drug Deliv. 2016, 23, 3248–3256. [Google Scholar] [CrossRef] [PubMed]
- Tay, F.R.; Pashley, D.H.; Rueggeberg, F.A.; Loushine, R.J.; Weller, R.N. Calcium Phosphate Phase Transformation Produced by the Interaction of the Portland Cement Component of White Mineral Trioxide Aggregate with a Phosphate-containing Fluid. J. Endod. 2007, 33, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, N.; Caicedo, R.; Ritwik, P.; Moiseyeva, R.; Kawashima, I. Physicochemical Basis of the Biologic Properties of Mineral Trioxide Aggregate. J. Endod. 2005, 31, 97–100. [Google Scholar] [CrossRef]
- Gwon, K.; Jo, E.-J.; Sahu, A.; Lee, J.Y.; Kim, M.-G.; Tae, G. Improved near infrared-mediated hydrogel formation using diacrylated Pluronic F127-coated upconversion nanoparticles. Mater. Sci. Eng. C 2018, 90, 77–84. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K. Recent progress in biomedical applications of Pluronic (PF127): Pharmaceutical perspectives. J. Control. Release 2015, 209, 120–138. [Google Scholar] [CrossRef]
- Nabavizadeh, M.R.; Moazzami, F.; Gholami, A.; Mehrabi, V.; Ghahramani, Y. Cytotoxic Effect of Nano Fast Cement and ProRoot Mineral Trioxide Aggregate on L-929 Fibroblast Cells: An in vitro Study. J. Dent. 2022, 23, 13. [Google Scholar]
- Braet, H.; Rahimi-Gorji, M.; Debbaut, C.; Ghorbaniasl, G.; Van Walleghem, T.; Cornelis, S.; Cosyns, S.; Vervaet, C.; Willaert, W.; Ceelen, W.; et al. Exploring high pressure nebulization of Pluronic F127 hydrogels for intraperitoneal drug delivery. Eur. J. Pharm. Biopharm. 2021, 169, 134–143. [Google Scholar] [CrossRef]
- Ye, F.; Yaghmur, A.; Jensen, H.; Larsen, S.; Larsen, C.; Østergaard, J. Real-time UV imaging of drug diffusion and release from Pluronic F127 hydrogels. Eur. J. Pharm. Sci. 2011, 43, 236–243. [Google Scholar] [CrossRef]
- Zhou, P.; Xia, D.; Ni, Z.; Ou, T.; Wang, Y.; Zhang, H.; Mao, L.; Lin, K.; Xu, S.; Liu, J. Calcium silicate bioactive ceramics induce osteogenesis through oncostatin M. Bioact. Mater. 2021, 6, 810–822. [Google Scholar] [CrossRef] [PubMed]
- Golub, E.; Boesze-Battaglia, K. The role of alkaline phosphatase in mineralization. Curr. Opin. Orthop. 2007, 18, 444–448. [Google Scholar] [CrossRef]
- An, S.; Gao, Y.; Ling, J.; Wei, X.; Xiao, Y. Calcium ions promote osteogenic differentiation and mineralization of human dental pulp cells: Implications for pulp capping materials. J. Mater. Sci. Mater. Med. 2012, 23, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Santiago, M.C.; Gomes-Cornélio, A.L.; de Oliveira, L.A.; Tanomaru-Filho, M.; Salles, L.P. Calcium silicate-based cements cause environmental stiffness and show diverse potential to induce osteogenesis in human osteoblastic cells. Sci. Rep. 2021, 11, 16784. [Google Scholar] [CrossRef] [PubMed]
- Vimalraj, S. Alkaline phosphatase: Structure, expression and its function in bone mineralization. Gene 2020, 754, 144855. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, J.-H.; Roh, J.; Mangal, U.; Kim, K.-M.; Choi, S.-H.; Kwon, J.-S. Novel Osteogenic and Easily Handled Endodontic Calcium Silicate Cement Using Pluronic F127 Hydrogel. Materials 2022, 15, 6919. https://doi.org/10.3390/ma15196919
Ryu J-H, Roh J, Mangal U, Kim K-M, Choi S-H, Kwon J-S. Novel Osteogenic and Easily Handled Endodontic Calcium Silicate Cement Using Pluronic F127 Hydrogel. Materials. 2022; 15(19):6919. https://doi.org/10.3390/ma15196919
Chicago/Turabian StyleRyu, Jeong-Hyun, Jiyeon Roh, Utkarsh Mangal, Kwang-Mahn Kim, Sung-Hwan Choi, and Jae-Sung Kwon. 2022. "Novel Osteogenic and Easily Handled Endodontic Calcium Silicate Cement Using Pluronic F127 Hydrogel" Materials 15, no. 19: 6919. https://doi.org/10.3390/ma15196919
APA StyleRyu, J.-H., Roh, J., Mangal, U., Kim, K.-M., Choi, S.-H., & Kwon, J.-S. (2022). Novel Osteogenic and Easily Handled Endodontic Calcium Silicate Cement Using Pluronic F127 Hydrogel. Materials, 15(19), 6919. https://doi.org/10.3390/ma15196919







