Mechanobiology Platform Realized Using Photomechanical Mxene Nanocomposites: Bilayer Photoactuator Design and In Vitro Mechanical Forces Stimulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of the Bilayer Structure
2.2. Observation and Characterization of Photomechanical Actuation
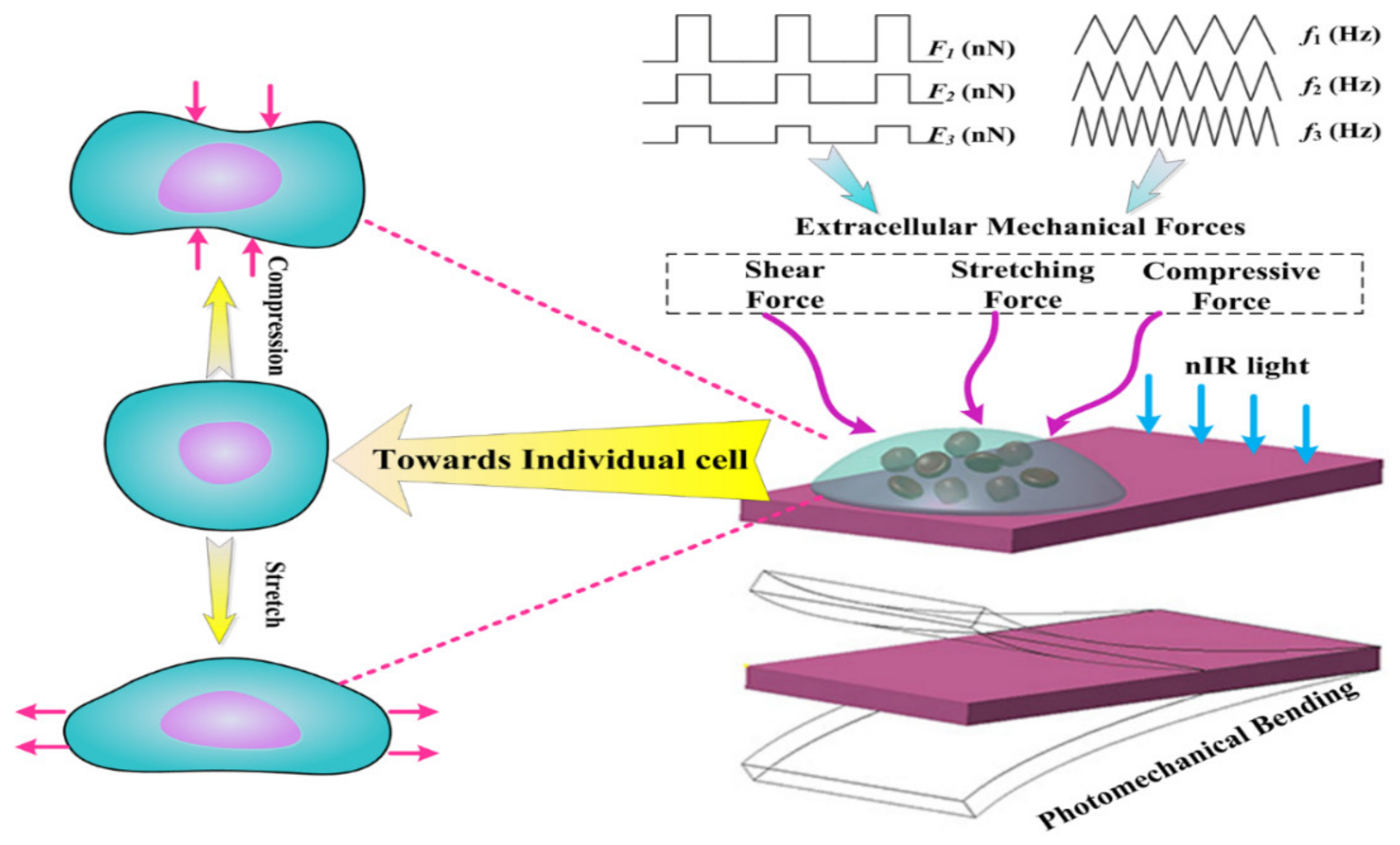
2.3. In Vitro Cell Mechanical Force Loading
2.4. Cell Counting and Cell Apoptosis Evaluation
3. Results and Discussion
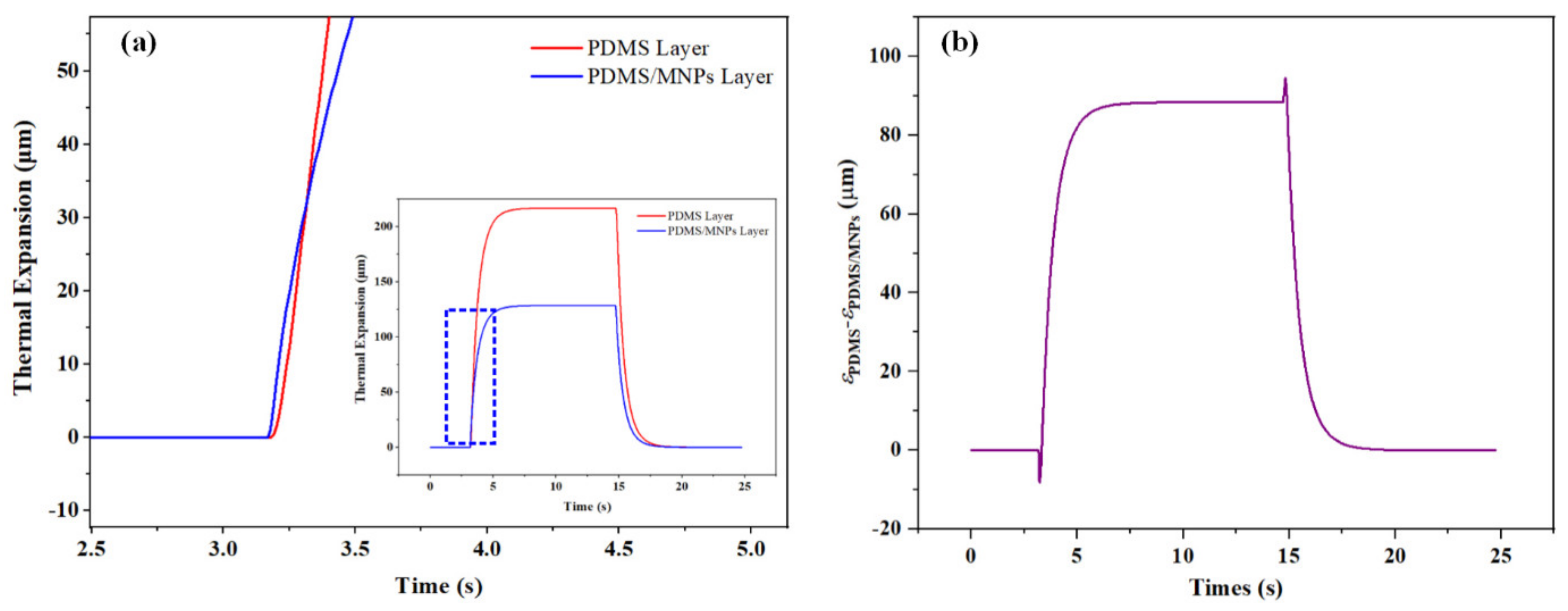
3.1. Two-Step Photomechanical Bending Process
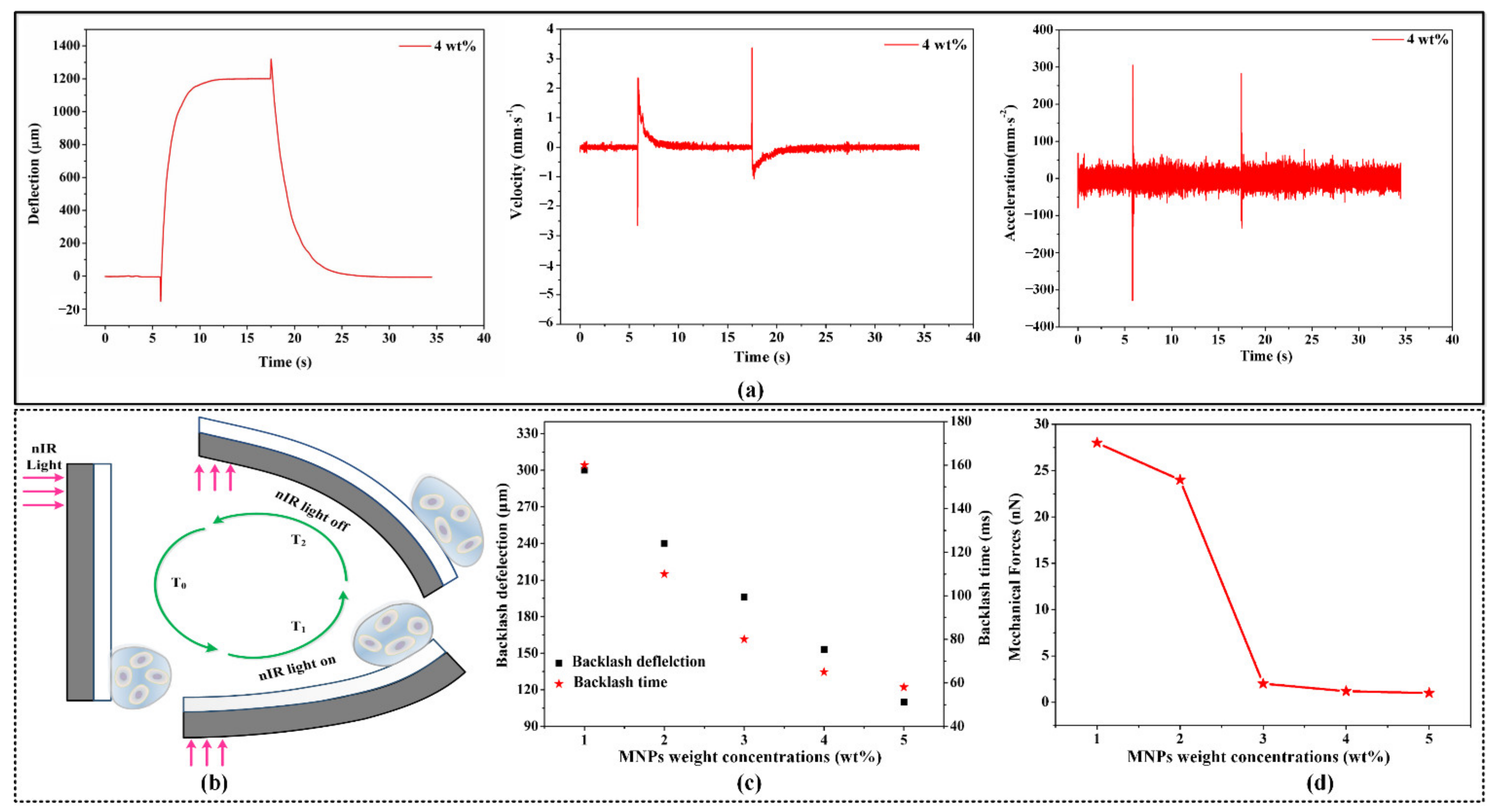
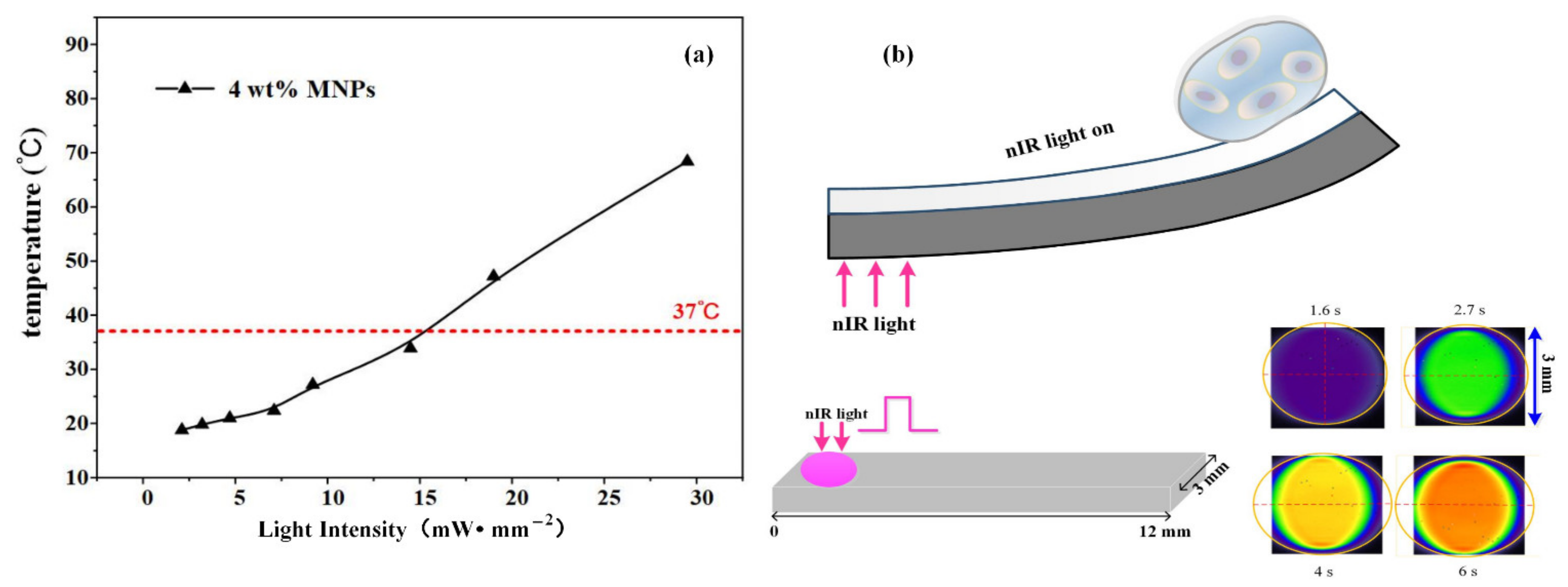
3.2. Mechanical Forces Loading Analysis
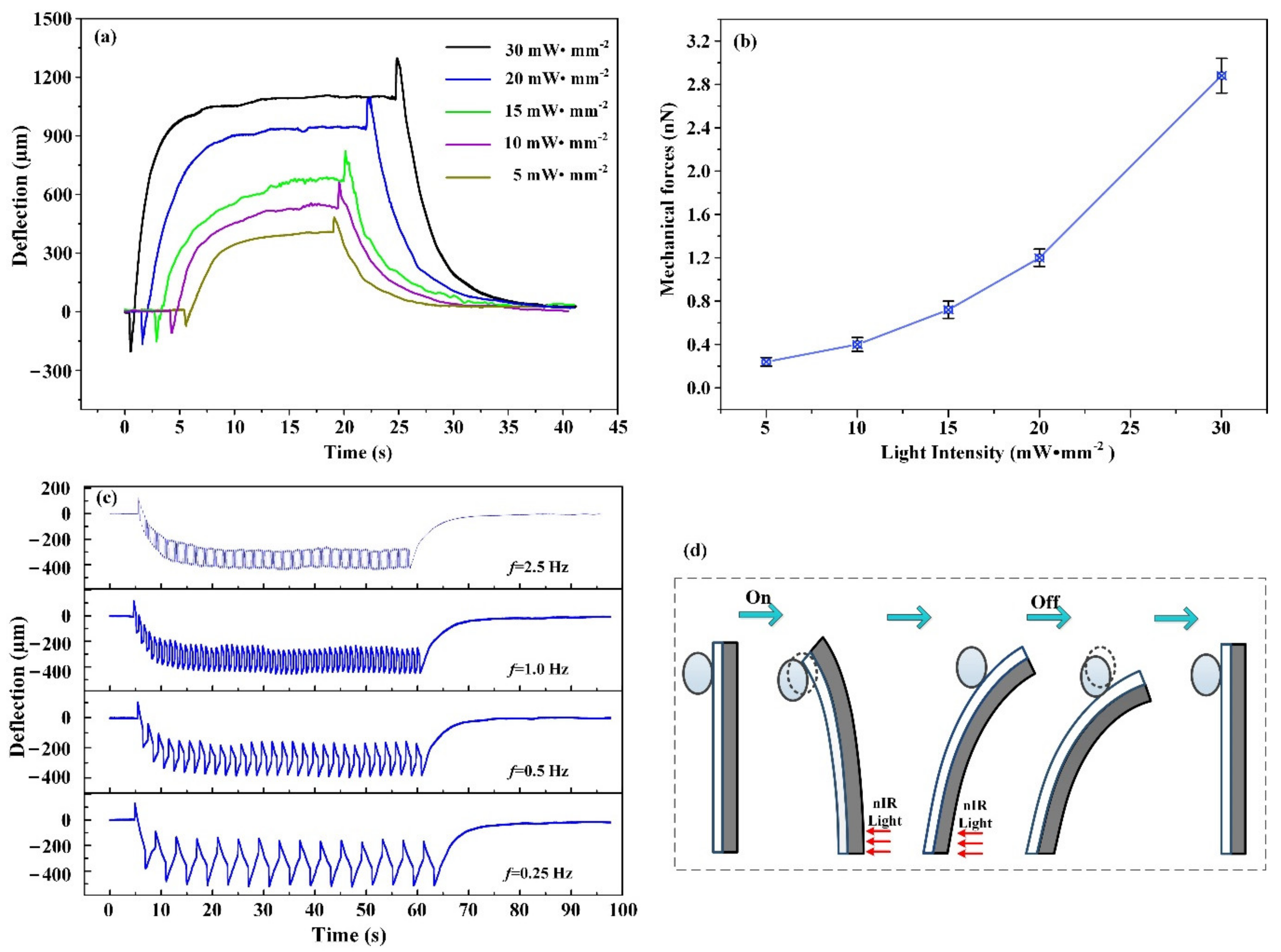
3.3. Mechanical Forces Adjustment with nIR Light
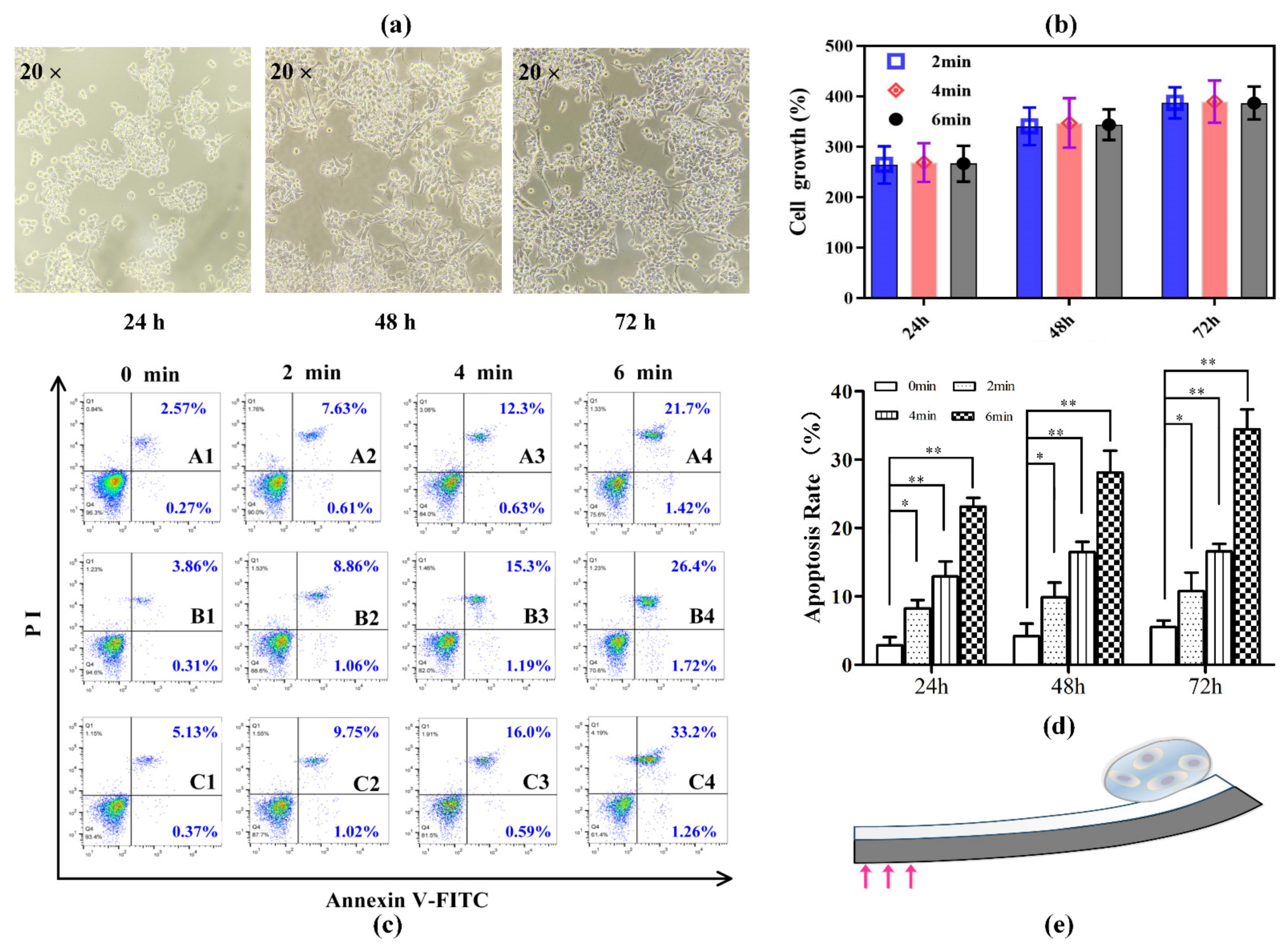
3.4. Cell Viability under Photomechanical Actuations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Martino, F.; Perestrelo, A.R.; Vinarský, V.; Pagliari, S.; Forte, G. Cellular mechanotransduction: From tension to function. Front. Physiol. 2018, 9, 824. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, D.; Versaevel, M.; Bruyère, C.; Alaimo, L.; Luciano, M.; Vercruysse, E.; Procès, A.; Gabriele, S. Innovative tools for mechanobiology: Unraveling outside-in and inside-out mechanotransduction. Front. Bioeng. Biotechnol. 2019, 7, 162. [Google Scholar] [CrossRef] [PubMed]
- Puech, P.-H.; Bongrand, P. Mechanotransduction as a major driver of cell behaviour: Mechanisms, and relevance to cell organization and future research. Open Biol. 2021, 11, 210256. [Google Scholar] [CrossRef] [PubMed]
- Jaalouk, D.E.; Lammerding, J. Mechanotransduction gone awry. Nat. Rev. Mol. Cell Biol. 2009, 10, 63–73. [Google Scholar] [CrossRef]
- Uhler, C.; Shivashankar, G. Regulation of genome organization and gene expression by nuclear mechanotransduction. Nat. Rev. Mol. Cell Biol. 2017, 18, 717–727. [Google Scholar] [CrossRef]
- Nims, R.J.; Pferdehirt, L.; Guilak, F. Mechanogenetics: Harnessing mechanobiology for cellular engineering. Curr. Opin. Biotechnol. 2022, 73, 374–379. [Google Scholar] [CrossRef]
- Benayahu, D.; Wiesenfeld, Y.; Sapir-Koren, R. How is mechanobiology involved in mesenchymal stem cell differentiation toward the osteoblastic or adipogenic fate? J. Cell. Physiol. 2019, 234, 12133–12141. [Google Scholar] [CrossRef]
- Wolfenson, H.; Yang, B.; Sheetz, M.P. Steps in mechanotransduction pathways that control cell morphology. Annu. Rev. Physiol. 2019, 81, 585. [Google Scholar] [CrossRef]
- Miller, A.E.; Hu, P.; Barker, T.H. Feeling things out: Bidirectional signaling of the cell–ECM interface, implications in the mechanobiology of cell spreading, migration, proliferation, and differentiation. Adv. Healthc. Mater. 2020, 9, 1901445. [Google Scholar] [CrossRef]
- Kurniawan, N.A.; Chaudhuri, P.K.; Lim, C.T. Mechanobiology of cell migration in the context of dynamic two-way cell–matrix interactions. J. Biomech. 2016, 49, 1355–1368. [Google Scholar] [CrossRef]
- Romani, P.; Valcarcel-Jimenez, L.; Frezza, C.; Dupont, S. Crosstalk between mechanotransduction and metabolism. Nat. Rev. Mol. Cell Biol. 2021, 22, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Salvi, A.M.; DeMali, K.A. Mechanisms linking mechanotransduction and cell metabolism. Curr. Opin. Cell Biol. 2018, 54, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Wevers, N.R.; van Vught, R.; Wilschut, K.J.; Nicolas, A.; Chiang, C.; Lanz, H.L.; Trietsch, S.J.; Joore, J.; Vulto, P. High-throughput compound evaluation on 3D networks of neurons and glia in a microfluidic platform. Sci. Rep. 2016, 6, 38856. [Google Scholar] [CrossRef] [PubMed]
- Poon, C. Factors implicating the validity and interpretation of mechanobiology studies in simulated microgravity environments. Eng. Rep. 2020, 2, e12242. [Google Scholar] [CrossRef]
- Alfieri, R.; Vassalli, M.; Viti, F. Flow-induced mechanotransduction in skeletal cells. Biophys. Rev. 2019, 11, 729–743. [Google Scholar] [CrossRef]
- Huang, Q.; Hu, X.; He, W.; Zhao, Y.; Hao, S.; Wu, Q.; Li, S.; Zhang, S.; Shi, M. Fluid shear stress and tumor metastasis. Am. J. Cancer Res. 2018, 8, 763. [Google Scholar]
- Xin, Y.; Li, K.; Yang, M.; Tan, Y. Fluid shear stress induces EMT of circulating tumor cells via JNK signaling in favor of their survival during hematogenous dissemination. Int. J. Mol. Sci. 2020, 21, 8115. [Google Scholar] [CrossRef]
- Solis, A.G.; Bielecki, P.; Steach, H.R.; Sharma, L.; Harman, C.C.; Yun, S.; de Zoete, M.R.; Warnock, J.N.; To, S.; York, A.G. Mechanosensation of cyclical force by PIEZO1 is essential for innate immunity. Nature 2019, 573, 69–74. [Google Scholar] [CrossRef]
- Douville, N.J.; Zamankhan, P.; Tung, Y.-C.; Li, R.; Vaughan, B.L.; Tai, C.-F.; White, J.; Christensen, P.J.; Grotberg, J.B.; Takayama, S. Combination of fluid and solid mechanical stresses contribute to cell death and detachment in a microfluidic alveolar model. Lab Chip 2011, 11, 609–619. [Google Scholar] [CrossRef]
- Friedrich, O.; Merten, A.-L.; Schneidereit, D.; Guo, Y.; Schürmann, S.; Martinac, B. Stretch in focus: 2D inplane cell stretch systems for studies of cardiac mechano-signaling. Front. Bioeng. Biotechnol. 2019, 7, 55. [Google Scholar] [CrossRef]
- Main, R.P.; Shefelbine, S.J.; Meakin, L.B.; Silva, M.J.; van der Meulen, M.C.; Willie, B.M. Murine axial compression tibial loading model to study bone mechanobiology: Implementing the model and reporting results. J. Orthop. Res. 2020, 38, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Warboys, C.M.; Ghim, M.; Weinberg, P.D. Understanding mechanobiology in cultured endothelium: A review of the orbital shaker method. Atherosclerosis 2019, 285, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Fritton, S.P.; Weinbaum, S. Fluid and solute transport in bone: Flow-induced mechanotransduction. Annu. Rev. Fluid Mech. 2009, 41, 347. [Google Scholar] [CrossRef] [PubMed]
- Farge, E. Mechanotransduction in development. Curr. Top. Dev. Biol. 2011, 95, 243–265. [Google Scholar] [PubMed]
- Uslu, F.E.; Davidson, C.D.; Mailand, E.; Bouklas, N.; Baker, B.M.; Sakar, M.S. Engineered extracellular matrices with integrated wireless microactuators to study mechanobiology. Adv. Mater. 2021, 33, 2102641. [Google Scholar] [CrossRef]
- Stucki, J.D.; Hobi, N.; Galimov, A.; Stucki, A.O.; Schneider-Daum, N.; Lehr, C.-M.; Huwer, H.; Frick, M.; Funke-Chambour, M.; Geiser, T. Medium throughput breathing human primary cell alveolus-on-chip model. Sci. Rep. 2018, 8, 14359. [Google Scholar] [CrossRef]
- Kaarj, K.; Yoon, J.-Y. Methods of delivering mechanical stimuli to organ-on-a-chip. Micromachines 2019, 10, 700. [Google Scholar] [CrossRef]
- Zhang, Y.; Ramasundara, S.D.Z.; Preketes-Tardiani, R.E.; Cheng, V.; Lu, H.; Ju, L.A. Emerging microfluidic approaches for platelet mechanobiology and interplay with circulatory systems. Front. Cardiovasc. Med. 2021, 8, 766513. [Google Scholar] [CrossRef]
- Millet, M.; Ben Messaoud, R.; Luthold, C.; Bordeleau, F. Coupling microfluidic platforms, microfabrication, and tissue engineered scaffolds to investigate tumor cells mechanobiology. Micromachines 2019, 10, 418. [Google Scholar] [CrossRef]
- Polacheck, W.J.; Li, R.; Uzel, S.G.; Kamm, R.D. Microfluidic platforms for mechanobiology. Lab Chip 2013, 13, 2252–2267. [Google Scholar] [CrossRef]
- Kim, D.-H.; Wong, P.K.; Park, J.; Levchenko, A.; Sun, Y. Microengineered platforms for cell mechanobiology. Annu. Rev. Biomed. Eng. 2009, 11, 203–233. [Google Scholar] [CrossRef] [PubMed]
- Tay, A.; Schweizer, F.E.; Di Carlo, D. Micro-and nano-technologies to probe the mechano-biology of the brain. Lab Chip 2016, 16, 1962–1977. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-E.; Song, K.H.; Doh, J. Microfabricated platforms for the analysis of immune cell migration under complex microenvironments. JMST Adv. 2021, 3, 1–9. [Google Scholar] [CrossRef]
- Ugolini, G.S.; Cruz-Moreira, D.; Visone, R.; Redaelli, A.; Rasponi, M. Microfabricated physiological models for in vitro drug screening applications. Micromachines 2016, 7, 233. [Google Scholar] [CrossRef]
- Panina, L.V.; Gurevich, A.; Beklemisheva, A.; Omelyanchik, A.; Levada, K.; Rodionova, V. Spatial Manipulation of Particles and Cells at Micro-and Nanoscale via Magnetic Forces. Cells 2022, 11, 950. [Google Scholar] [CrossRef] [PubMed]
- Modena, M.M.; Chawla, K.; Misun, P.M.; Hierlemann, A. Smart cell culture systems: Integration of sensors and actuators into microphysiological systems. ACS Chem. Biol. 2018, 13, 1767–1784. [Google Scholar] [CrossRef]
- Thomée, E. Actuation Concepts for In Vivo-Like Mechanical Strain. Elveflow. 2020. Available online: https://www.elveflow.com/microfluidic-reviews/organs-on-chip-3d-cell-culture/actuation-mechanical-strain/ (accessed on 29 June 2022).
- Ebara, M. Shape-memory surfaces for cell mechanobiology. Sci. Technol. Adv. Mater. 2015, 16, 014804. [Google Scholar] [CrossRef]
- Chen, J.; Hamilton, L.E.; Mather, P.T.; Henderson, J.H. Cell-Responsive Shape Memory Polymers. ACS Biomater. Sci. Eng. 2022, 8, 2960–2969. [Google Scholar] [CrossRef]
- Zako, T.; Matsushita, S.; Hoshi, T.; Aoyagi, T. Direct Surface Modification of Polycaprolactone-Based Shape Memory Materials to Introduce Positive Charge Aiming to Enhance Cell Affinity. Materials 2021, 14, 5797. [Google Scholar] [CrossRef]
- Escalera-López, D.; Garcia-Amorós, J.; Velasco, D. Smectic-B Liquid Single Crystal Elastomers as Efficient Optical Mechanotransducers. Macromol. Chem. Phys. 2018, 219, 1700550. [Google Scholar] [CrossRef]
- Doostmohammadi, A.; Ladoux, B. Physics of liquid crystals in cell biology. Trends Cell Biol. 2021, 32, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Sutton, A.; Shirman, T.; Timonen, J.V.; England, G.T.; Kim, P.; Kolle, M.; Ferrante, T.; Zarzar, L.D.; Strong, E.; Aizenberg, J. Photothermally triggered actuation of hybrid materials as a new platform for in vitro cell manipulation. Nat. Commun. 2017, 8, 14700. [Google Scholar] [CrossRef]
- Zheng, Y.; Han, M.K.L.; Jiang, Q.; Li, B.; Feng, J.; del Campo, A. 4D hydrogel for dynamic cell culture with orthogonal, wavelength-dependent mechanical and biochemical cues. Mater. Horiz. 2020, 7, 111–116. [Google Scholar] [CrossRef]
- Chandorkar, Y.; Castro Nava, A.; Schweizerhof, S.; Van Dongen, M.; Haraszti, T.; Köhler, J.; Zhang, H.; Windoffer, R.; Mourran, A.; Möller, M. Cellular responses to beating hydrogels to investigate mechanotransduction. Nat. Commun. 2019, 10, 4027. [Google Scholar] [CrossRef] [PubMed]
- Poulin, A.; Rosset, S.; Shea, H. Fabrication and Characterization of Silicone-Based Dielectric Elastomer Actuators for Mechanical Stimulation of Living Cells, Electroactive Polymer Actuators and Devices (EAPAD) XX. In Proceedings of the SPIE Smart Structures and Materials + Nondestructive Evaluation and Health Monitoring, Denver, CO, USA, 4–8 March 2018; pp. 133–141. [Google Scholar]
- Kim, D.-U.; Lee, S.; Chang, S.-H. Dynamic cell culture device using electroactive polymer actuators with composite electrodes to transfer in-plane mechanical strain to cells. Int. J. Precis. Eng. Manuf. Green Technol. 2021, 8, 969–980. [Google Scholar] [CrossRef]
- Liu, Z.; Wan, X.; Wang, Z.L.; Li, L. Electroactive biomaterials and systems for cell fate determination and tissue regeneration: Design and applications. Adv. Mater. 2021, 33, 2007429. [Google Scholar] [CrossRef]
- Yao, X.; Qian, Y.; Fan, C. Electroactive nanomaterials in the peripheral nerve regeneration. J. Mater. Chem. B 2021, 9, 6958–6972. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Melaku, A.Z.; Ilhami, F.B.; Chiu, C.-W.; Cheng, C.-C. Conductive Supramolecular Polymer Nanocomposites with Tunable Properties to Manipulate Cell Growth and Functions. Int. J. Mol. Sci. 2022, 23, 4332. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, D.; Zhang, Y.; Chen, J.; Li, D.; He, C.; Liu, H. Mechanobiology Platform Realized Using Photomechanical Mxene Nanocomposites: Bilayer Photoactuator Design and In Vitro Mechanical Forces Stimulation. Materials 2022, 15, 6869. https://doi.org/10.3390/ma15196869
Niu D, Zhang Y, Chen J, Li D, He C, Liu H. Mechanobiology Platform Realized Using Photomechanical Mxene Nanocomposites: Bilayer Photoactuator Design and In Vitro Mechanical Forces Stimulation. Materials. 2022; 15(19):6869. https://doi.org/10.3390/ma15196869
Chicago/Turabian StyleNiu, Dong, Yanli Zhang, Jinlan Chen, Dachao Li, Chunmeng He, and Hongzhong Liu. 2022. "Mechanobiology Platform Realized Using Photomechanical Mxene Nanocomposites: Bilayer Photoactuator Design and In Vitro Mechanical Forces Stimulation" Materials 15, no. 19: 6869. https://doi.org/10.3390/ma15196869
APA StyleNiu, D., Zhang, Y., Chen, J., Li, D., He, C., & Liu, H. (2022). Mechanobiology Platform Realized Using Photomechanical Mxene Nanocomposites: Bilayer Photoactuator Design and In Vitro Mechanical Forces Stimulation. Materials, 15(19), 6869. https://doi.org/10.3390/ma15196869




