Functionalization of TiO2 for Better Performance as Orthopedic Implants
Abstract
1. Introduction
2. Important Facts about Orthopedic Implant
2.1. Implant Failure
2.2. Fundamental Requirements of Orthopedic Implants
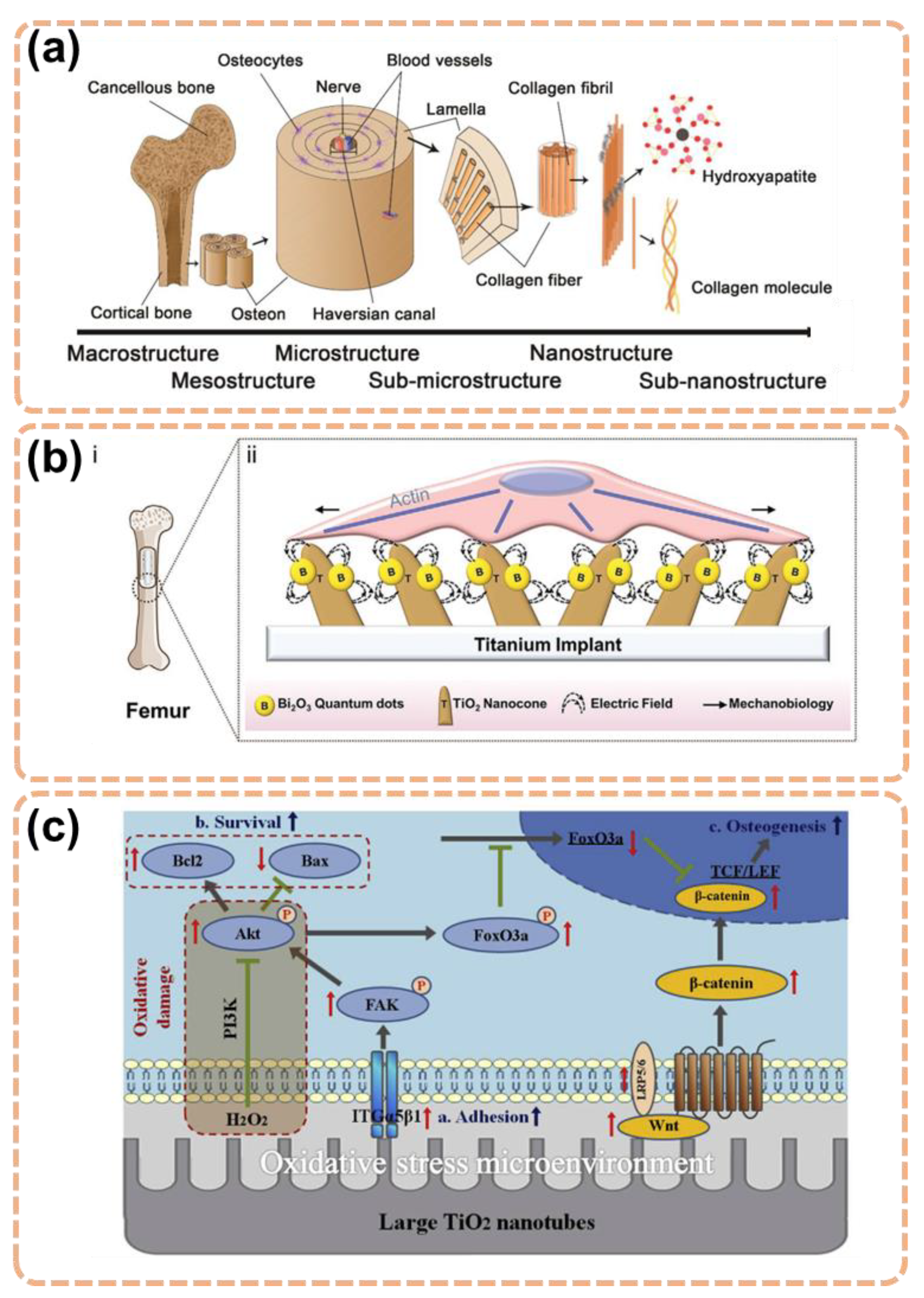
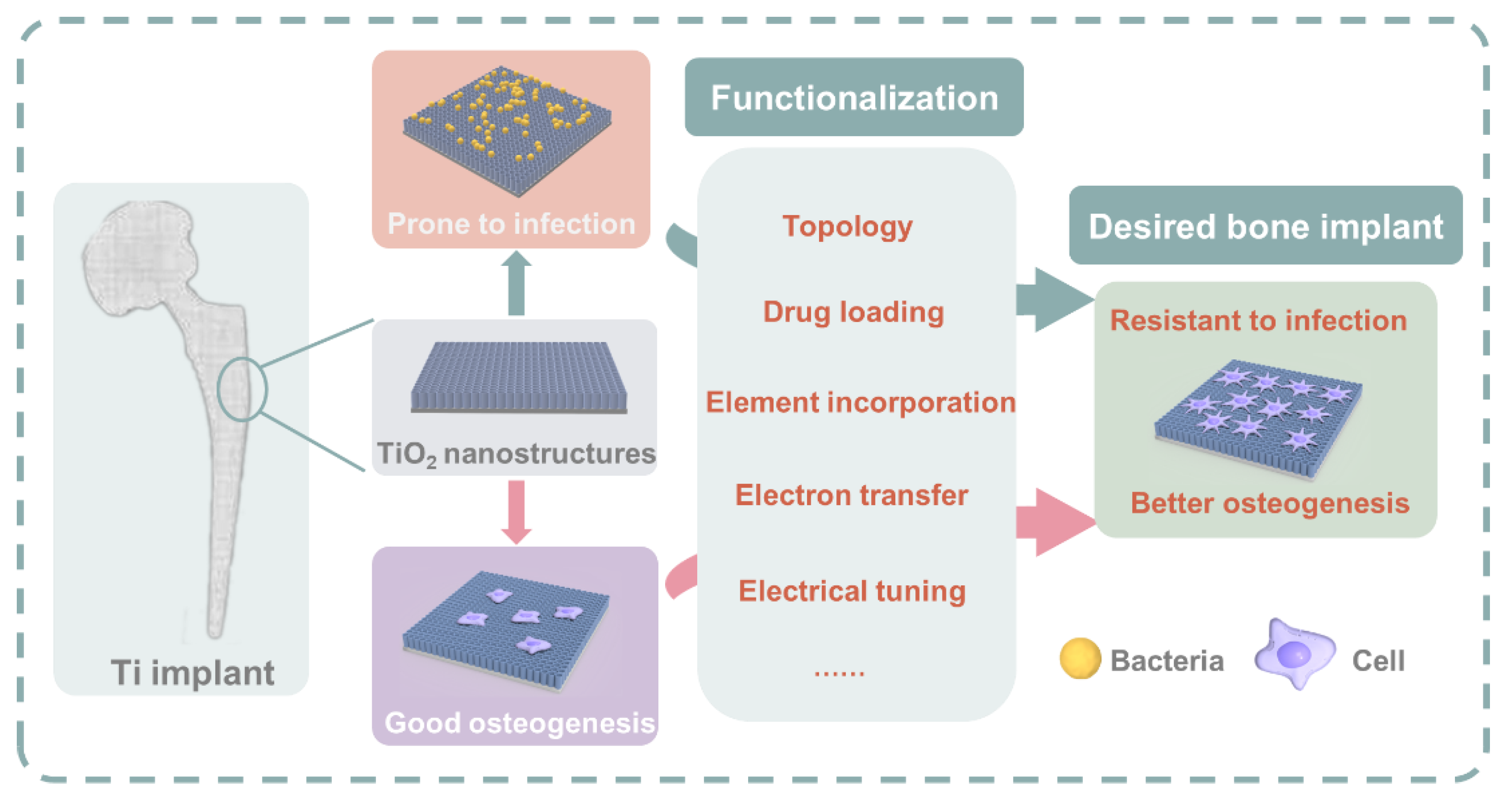
3. Functionalization Approaches of TiO2 for Better Antibacterial and Osteogenesis Property
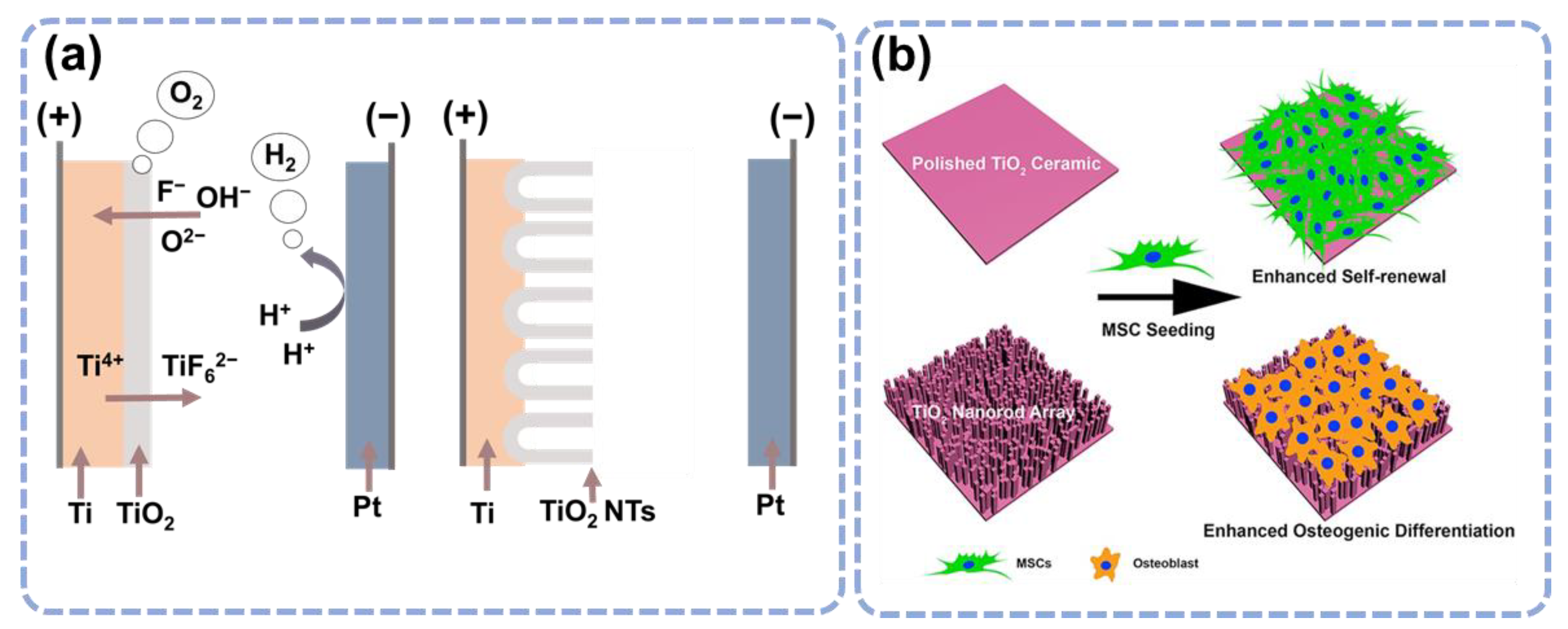
3.1. Topological Influence of the TiO2 Nanostructures
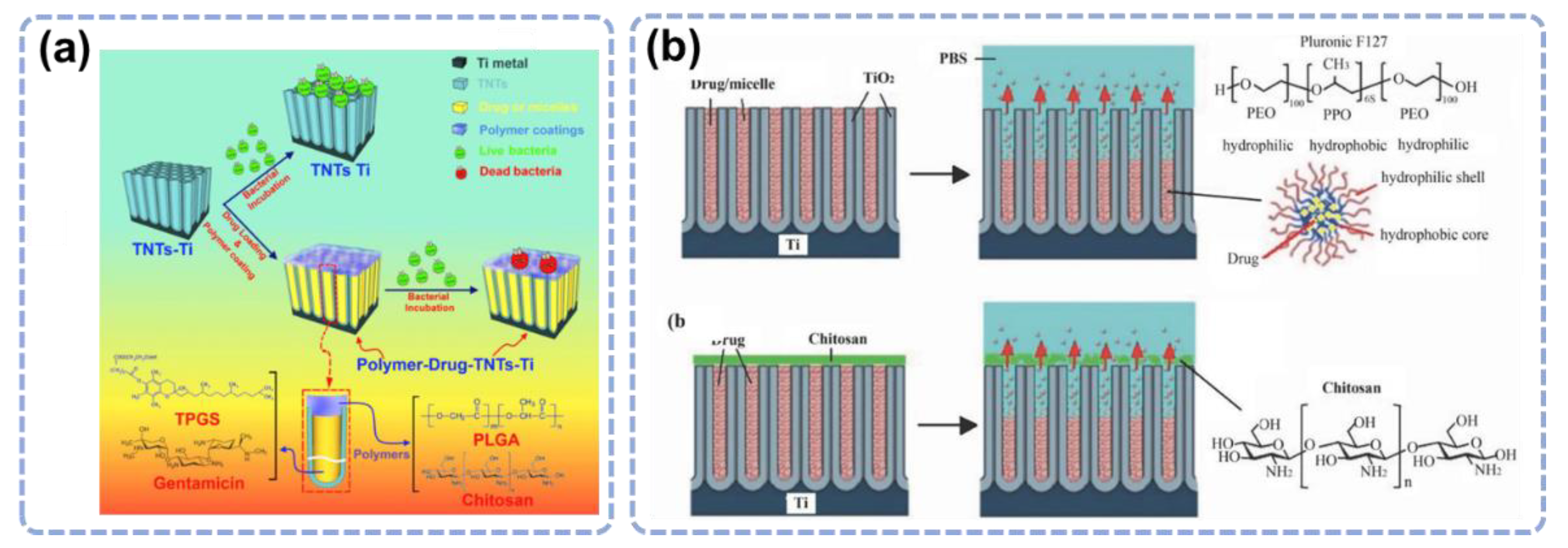
3.2. Drug Loading and Release Based on the TiO2 Nanostructures
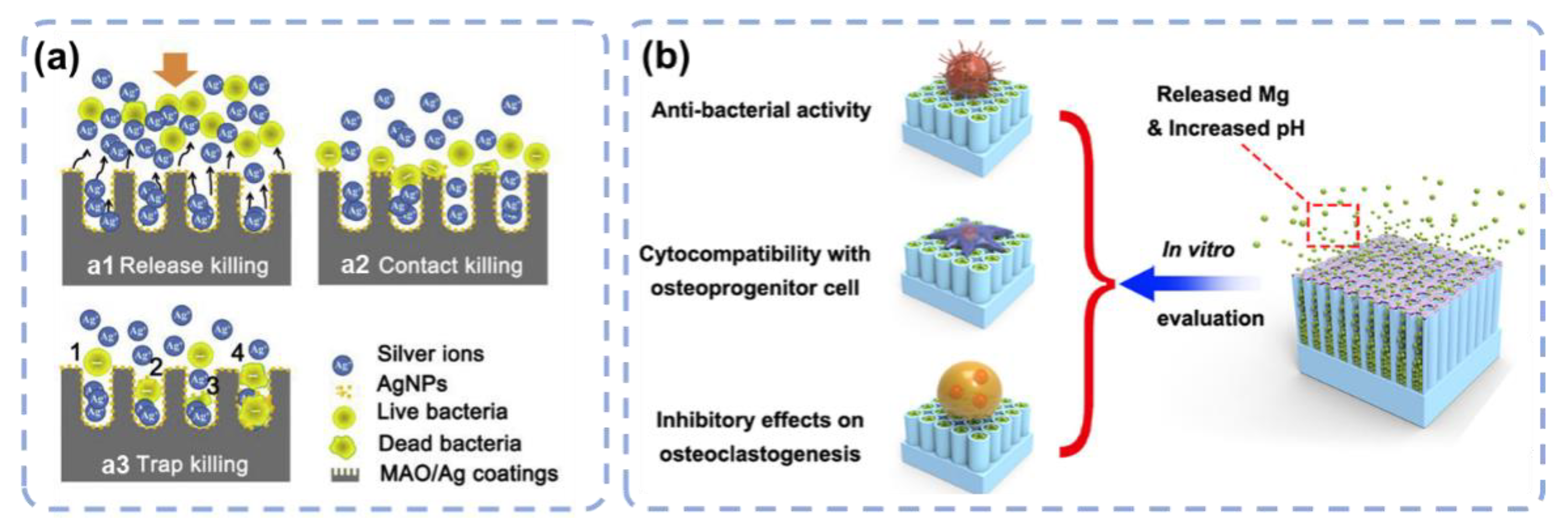
3.3. Element Incorporation
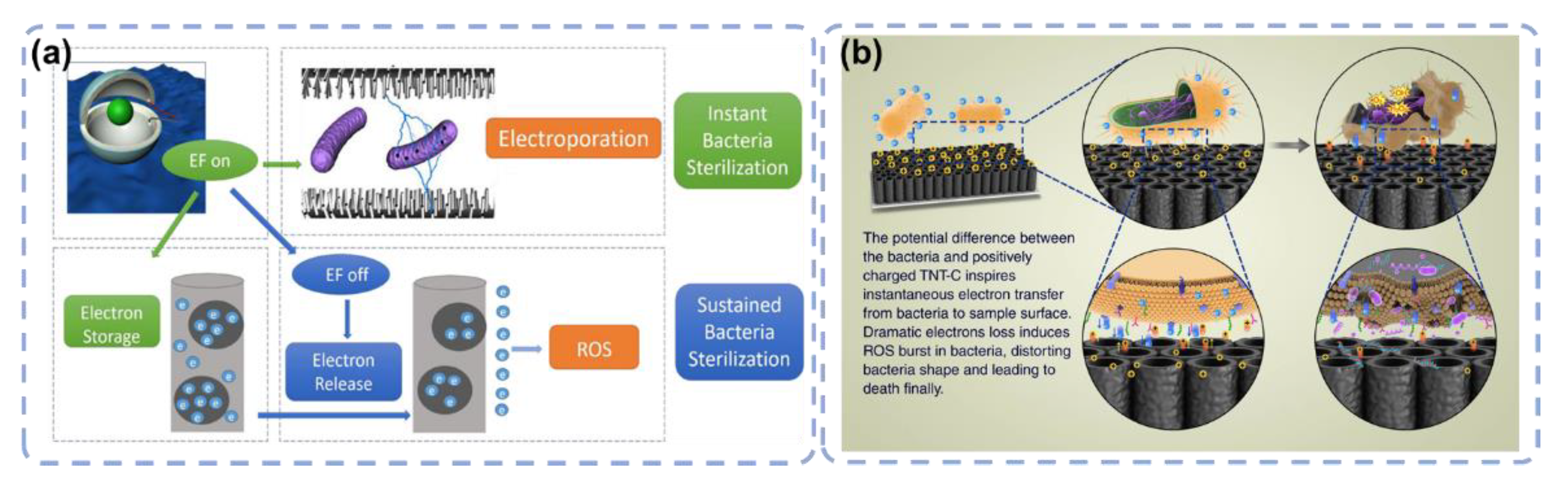
3.4. Electron Transfer
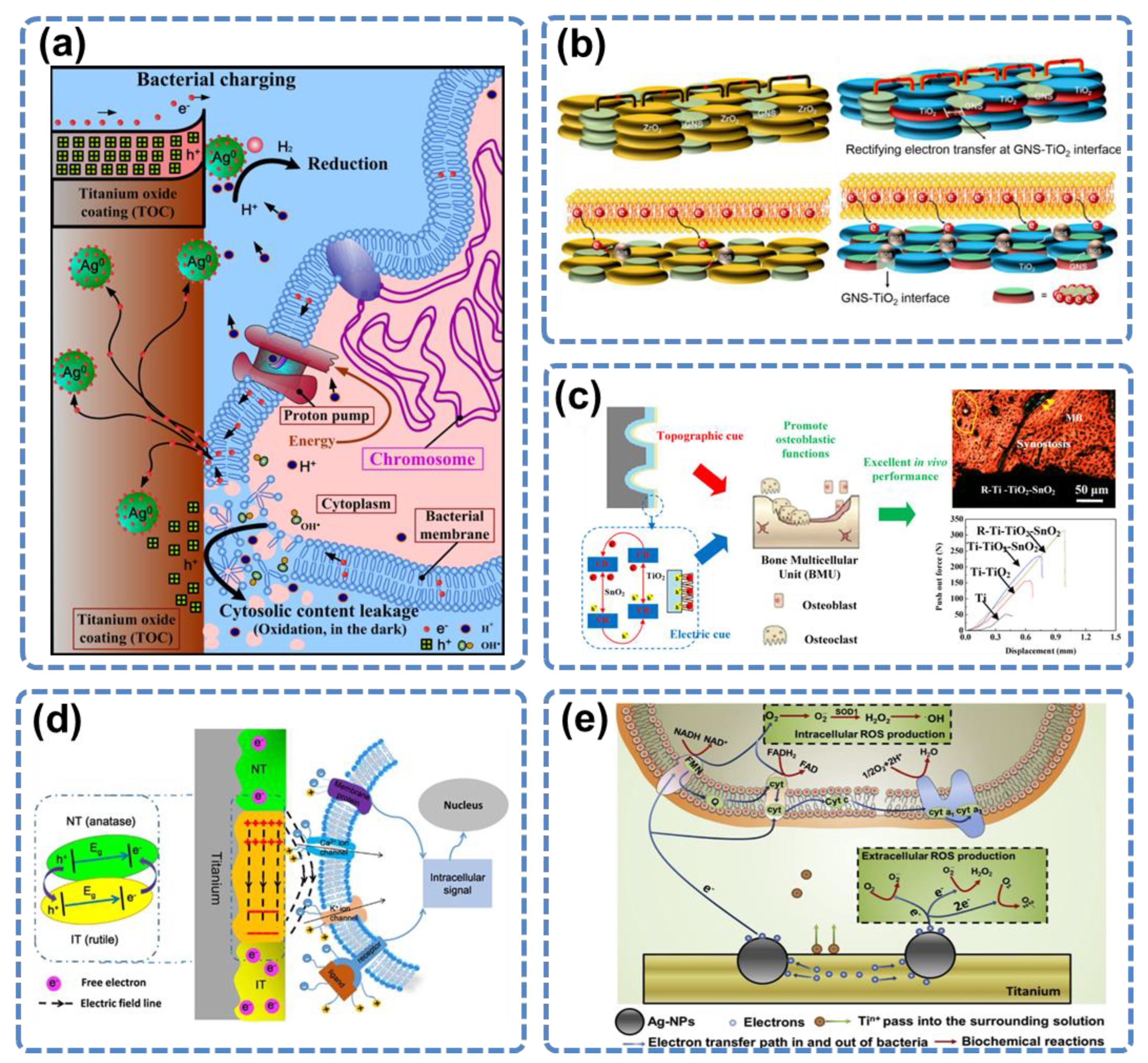
3.5. Electrical Functionalization
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baltatu, M.S.; Vizureanu, P.; Sandu, A.V.; Florido-Suarez, N.; Saceleanu, M.V.; Mirza-Rosca, J.C. New titanium alloys, promising materials for medical devices. Materials 2021, 14, 5934. [Google Scholar] [CrossRef] [PubMed]
- Filipović, U.; Dahmane, R.G.; Ghannouchi, S.; Zore, A.; Bohinc, K. Bacterial adhesion on orthopedic implants. Adv. Colloid Interface Sci. 2020, 283, 102228. [Google Scholar] [CrossRef]
- Losic, D.; Aw, M.S.; Santos, A.; Gulati, K.; Bariana, M. Titania nanotube arrays for local drug delivery: Recent advances and perspectives. Expert Opin. Drug Deliv. 2015, 12, 103–127. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M. Mechanical properties of biomedical titanium alloys. Mater. Sci. Eng. A 1998, 243, 231–236. [Google Scholar] [CrossRef]
- Elias, C.N.; Lima, J.H.C.; Valiev, R.; Meyers, M.A. Biomedical applications of titanium and its alloys. Jom 2008, 60, 46–49. [Google Scholar] [CrossRef]
- Sidambe, A.T. Biocompatibility of advanced manufactured titanium implants—A review. Materials 2014, 7, 8168–8188. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Song, Y.H.; An, J.H.; Song, H.J.; Anusavice, K.J. Cytocompatibility of pure metals and experimental binary titanium alloys for implant materials. J. Dent. 2013, 41, 1251–1258. [Google Scholar] [CrossRef]
- von Wilmowsky, C.; Bauer, S.; Lutz, R.; Meisel, M.; Neukam, F.W.; Toyoshima, T.; Schmuki, P.; Nkenke, E.; Schlegel, K.A. In vivo evaluation of anodic TiO2 nanotubes: An experimental study in the pig. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2009, 89, 165–171. [Google Scholar] [CrossRef]
- Zhao, G.; Schwartz, Z.; Wieland, M.; Rupp, F.; Geis-Gerstorfer, J.; Cochran, D.L.; Boyan, B.D. High surface energy enhances cell response to titanium substrate microstructure. J. Biomed. Mater. Res. Part A An Off. J. Soc. Biomater. Japanese Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2005, 74, 49–58. [Google Scholar] [CrossRef]
- Chevalier, J.; Gremillard, L. Ceramics for medical applications: A picture for the next 20 years. J. Eur. Ceram. Soc. 2009, 29, 1245–1255. [Google Scholar] [CrossRef]
- Moriarty, T.F.; Schlegel, U.; Perren, S.; Richards, R.G. Infection in fracture fixation: Can we influence infection rates through implant design? J. Mater. Sci. Mater. Med. 2010, 21, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef] [PubMed]
- Lindhe, J.; Meyle, J.; Group D of the European Workshop on Periodontology. Peri-implant diseases: Consensus report of the sixth European workshop on periodontology. J. Clin. Periodontol. 2008, 35, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Darby, I. Dental implants: Maintenance, care and treatment of peri-implant infection. Aust. Dent. J. 2003, 48, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Periodontology, A.A. of Parameter on placement and management of the dental implant. J. Periodontol. 2000, 71, 870–872. [Google Scholar]
- Tonetti, M.S. Risk factors for osseodisintegration. Periodontol. 2000 1998, 17, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, T.; Niimi, A.; Sawai, T.; Ueda, M. Effects of steroid-induced osteoporosis on osseointegration of titanium implants. Int. J. Oral Maxillofac. Implant. 1998, 13, 183–189. [Google Scholar]
- Farzad, P.; Andersson, L.; Nyberg, J. Dental implant treatment in diabetic patients. Implant Dent. 2002, 11, 262–267. [Google Scholar] [CrossRef]
- Halpern, M.; Kurtz, S.; Lau, E.; Mowat, F.; Ong, K. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Surg. 2007, 89, 780–785. [Google Scholar]
- Lentino, J.R. Prosthetic joint infections: Bane of orthopedists, challenge for infectious disease specialists. Clin. Infect. Dis. 2003, 36, 1157–1161. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials 2006, 27, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Bosco, R.; Van Den Beucken, J.; Leeuwenburgh, S.; Jansen, J. Surface engineering for bone implants: A trend from passive to active surfaces. Coatings 2012, 2, 95–119. [Google Scholar] [CrossRef]
- Brammer, K.S.; Frandsen, C.J.; Jin, S. TiO2 nanotubes for bone regeneration. Trends Biotechnol. 2012, 30, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; Gadegaard, N.; Tare, R.; Andar, A.; Riehle, M.O.; Herzyk, P.; Wilkinson, C.D.W.; Oreffo, R.O.C. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat. Mater. 2007, 6, 997–1003. [Google Scholar] [CrossRef]
- Ratner, B.D.; Bryant, S.J. Biomaterials: Where we have been and where we are going. Annu. Rev. Biomed. Eng. 2004, 6, 41–75. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Gilbert, P.M.; Blau, H.M. Designing materials to direct stem-cell fate. Nature 2009, 462, 433–441. [Google Scholar] [CrossRef]
- Chrzanowski, W.; Lee, J.H.; Kondyurin, A.; Lord, M.S.; Jang, J.; Kim, H.; Bilek, M.M.M. Nano-Bio-Chemical Braille for Cells: The Regulation of Stem Cell Responses using Bi-Functional Surfaces. Adv. Funct. Mater. 2015, 25, 193–205. [Google Scholar] [CrossRef]
- Huang, H.; He, L.; Zhou, W.; Qu, G.; Wang, J.; Yang, N.; Gao, J.; Chen, T.; Chu, P.K.; Yu, X.-F. Stable black phosphorus/Bi2O3 heterostructures for synergistic cancer radiotherapy. Biomaterials 2018, 171, 12–22. [Google Scholar] [CrossRef]
- Lu, H.; Hao, Q.; Chen, T.; Zhang, L.; Chen, D.; Ma, C.; Yao, W.; Zhu, Y. A high-performance Bi2O3/Bi2SiO5 pn heterojunction photocatalyst induced by phase transition of Bi2O3. Appl. Catal. B Environ. 2018, 237, 59–67. [Google Scholar] [CrossRef]
- Huang, X.; Xing, J.; Wang, Z.; Han, J.; Wang, R.; Li, C.; Xiao, C.; Lu, F.; Zhai, J.; Zhou, Z. 0D/1D Heterojunction Implant with Electro-Mechanobiological Coupling Cues Promotes Osteogenesis. Adv. Funct. Mater. 2021, 31, 2106249. [Google Scholar] [CrossRef]
- Fan, J.; Abedi-Dorcheh, K.; Vaziri, A.S.; Kazemi-Aghdam, F.; Rafieyan, S.; Sohrabinejad, M.; Ghorbani, M.; Adib, F.R.; Ghasemi, Z.; Klavins, K.; et al. A Review of Recent Advances in Natural Polymer-Based Scaffolds for Musculoskeletal Tissue Engineering. Polymers 2022, 14, 2097. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shen, X.; Luo, Z.; Hu, Y.; Li, M.; Ma, P.; Ran, Q.; Dai, L.; He, Y.; Cai, K. Osteogenesis potential of different titania nanotubes in oxidative stress microenvironment. Biomaterials 2018, 167, 44–57. [Google Scholar] [CrossRef]
- Calabrese, G.; Franco, D.; Petralia, S.; Monforte, F.; Condorelli, G.G.; Squarzoni, S.; Traina, F.; Conoci, S. Dual-Functional Nano-Functionalized Titanium Scaffolds to Inhibit Bacterial Growth and Enhance Osteointegration. Nanomaterials 2021, 11, 2634. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Fu, J. Integrated micro/nanoengineered functional biomaterials for cell mechanics and mechanobiology: A materials perspective. Adv. Mater. 2014, 26, 1494–1533. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Provenzano, P.P.; Smith, C.L.; Levchenko, A. Matrix nanotopography as a regulator of cell function. J. Cell Biol. 2012, 197, 351–360. [Google Scholar] [CrossRef]
- Dvir, T.; Timko, B.P.; Kohane, D.S.; Langer, R. Nanotechnological strategies for engineering complex tissues. Nat. Nanotechnology 2011, 6, 13–22. [Google Scholar] [CrossRef]
- Dalby, M.J.; Gadegaard, N.; Oreffo, R.O.C. Harnessing nanotopography and integrin–matrix interactions to influence stem cell fate. Nat. Mater. 2014, 13, 558–569. [Google Scholar] [CrossRef]
- Yao, X.; Peng, R.; Ding, J. Cell–material interactions revealed via material techniques of surface patterning. Adv. Mater. 2013, 25, 5257–5286. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Li, R.; Tang, X.; Guo, D.; Qing, Y.; Qin, Y. Enhanced antibacterial properties of orthopedic implants by titanium nanotube surface modification: A review of current techniques. Int. J. Nanomedicine 2019, 14, 7217–7236. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, L.; Wu, Z.; Zhang, Y.; Chu, P.K. Effects of micropitted/nanotubular titania topographies on bone mesenchymal stem cell osteogenic differentiation. Biomaterials 2012, 33, 2629–2641. [Google Scholar] [CrossRef]
- Descamps, S.; Awitor, K.O.; Raspal, V.; Johnson, M.B.; Bokalawela, R.S.P.; Larson, P.R.; Doiron, C.F. Mechanical properties of nanotextured titanium orthopedic screws for clinical applications. J. Med. Devices 2013, 7, 021005. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Brammer, K.S.; Li, Y.S.J.; Teng, D.; Engler, A.J.; Chien, S.; Jin, S. Stem cell fate dictated solely by altered nanotube dimension. Proc. Natl. Acad. Sci. USA 2009, 106, 2130–2135. [Google Scholar] [CrossRef]
- Lv, L.; Liu, Y.; Zhang, P.; Zhang, X.; Liu, J.; Chen, T.; Su, P.; Li, H.; Zhou, Y. The nanoscale geometry of TiO2 nanotubes influences the osteogenic differentiation of human adipose-derived stem cells by modulating H3K4 trimethylation. Biomaterials 2015, 39, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cai, T.; Huang, Y.; Xia, X.; Cole, S.P.C.; Cai, Y. A review of the structure, preparation, and application of NLCs, PNPs, and PLNs. Nanomaterials 2017, 7, 122. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Ni, J.; Zheng, K.; Shen, Y.; Wang, X.; He, G.; Jin, S.; Tang, T. Dual effects and mechanism of TiO2 nanotube arrays in reducing bacterial colonization and enhancing C3H10T1/2 cell adhesion. Int. J. Nanomedicine 2013, 8, 3093. [Google Scholar] [PubMed]
- Ercan, B.; Taylor, E.; Alpaslan, E.; Webster, T.J. Diameter of titanium nanotubes influences anti-bacterial efficacy. Nanotechnology 2011, 22, 295102. [Google Scholar] [CrossRef] [PubMed]
- Puckett, S.D.; Taylor, E.; Raimondo, T.; Webster, T.J. The relationship between the nanostructure of titanium surfaces and bacterial attachment. Biomaterials 2010, 31, 706–713. [Google Scholar] [CrossRef]
- Qiu, J.; Li, J.; Wang, S.; Ma, B.; Zhang, S.; Guo, W.; Zhang, X.; Tang, W.; Sang, Y.; Liu, H. TiO2 nanorod array constructed nanotopography for regulation of mesenchymal stem cells fate and the realization of location-committed stem cell differentiation. Small 2016, 12, 1770–1778. [Google Scholar] [CrossRef]
- Buensuceso, C.S.; Woodside, D.; Huff, J.L.; Plopper, G.E.; O’Toole, T.E. The WD protein Rack1 mediates protein kinase C and integrin-dependent cell migration. J. Cell Sci. 2001, 114, 1691–1698. [Google Scholar] [CrossRef]
- Pegueroles, M.; Aparicio, C.; Bosio, M.; Engel, E.; Gil, F.J.; Planell, J.A.; Altankov, G. Spatial organization of osteoblast fibronectin matrix on titanium surfaces: Effects of roughness, chemical heterogeneity and surface energy. Acta Biomater. 2010, 6, 291–301. [Google Scholar] [CrossRef]
- Geesink, R.G.T.; de Groot, K.; KLEIN, C.P.A.T. Chemical implant fixation using hydroxyl-apatite coatings: The development of a human total hip prosthesis for chemical fixation to bone using hydroxyl-apatite coatings on titanium substrates. Clin. Orthop. Relat. Res. 1987, 225, 147–170. [Google Scholar] [CrossRef]
- Lee, J.J.; Rouhfar, L.; Beirne, O.R. Survival of hydroxyapatite-coated implants: A meta-analytic review. J. Oral Maxillofac. Surg. 2000, 58, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; Hart, A.; Yarwood, S.J. The effect of the RACK1 signalling protein on the regulation of cell adhesion and cell contact guidance on nanometric grooves. Biomaterials 2008, 29, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.B.; Yao, Z.; Keeney, M.; Yang, F. The future of biologic coatings for orthopaedic implants. Biomaterials 2013, 34, 3174–3183. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Yang, L.; Guo, M.; Pan, C.; Cai, Q.; Yao, S. Biocompatibility and in vitro antineoplastic drug-loaded trial of titania nanotubes prepared by anodic oxidation of a pure titanium. Sci. China Ser. B Chem. 2009, 52, 2161–2165. [Google Scholar] [CrossRef]
- Yang, D.-J.; Kim, H.-G.; Cho, S.-J.; Choi, W.-Y. Thickness-conversion ratio from titanium to TiO2 nanotube fabricated by anodization method. Mater. Lett. 2008, 62, 775–779. [Google Scholar] [CrossRef]
- Kulkarni, M.; Mazare, A.; Gongadze, E.; Perutkova, Š.; Kralj-Iglič, V.; Milošev, I.; Schmuki, P.; Iglič, A.; Mozetič, M. Titanium nanostructures for biomedical applications. Nanotechnology 2015, 26, 62002. [Google Scholar] [CrossRef]
- Andersson, R.E.; Lukas, G.; Skullman, S.; Hugander, A. Local administration of antibiotics by gentamicin–collagen sponge does not improve wound healing or reduce recurrence rate after pilonidal excision with primary suture: A prospective randomized controlled trial. World J. Surg. 2010, 34, 3042–3046. [Google Scholar] [CrossRef] [PubMed]
- Kumeria, T.; Mon, H.; Aw, M.S.; Gulati, K.; Santos, A.; Griesser, H.J.; Losic, D. Advanced biopolymer-coated drug-releasing titania nanotubes (TNTs) implants with simultaneously enhanced osteoblast adhesion and antibacterial properties. Colloids Surf. B Biointerfaces 2015, 130, 255–263. [Google Scholar] [CrossRef]
- Aw, M.S.; Gulati, K.; Losic, D. Controlling drug release from titania nanotube arrays using polymer nanocarriers and biopolymer coating. J. Biomater. Nanobiotechnol. 2011, 2, 477. [Google Scholar] [CrossRef]
- Gulati, K.; Ramakrishnan, S.; Aw, M.S.; Atkins, G.J.; Findlay, D.M.; Losic, D. Biocompatible polymer coating of titania nanotube arrays for improved drug elution and osteoblast adhesion. Acta Biomater. 2012, 8, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Xiu, P.; Li, M.; Xu, X.; Shi, Y.; Cheng, Y.; Wei, S.; Zheng, Y.; Xi, T.; Cai, H. Bioinspired anchoring AgNPs onto micro-nanoporous TiO2 orthopedic coatings: Trap-killing of bacteria, surface-regulated osteoblast functions and host responses. Biomaterials 2016, 75, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Song, J.; Jung, Y.; Kweon, O.Y.; Song, H.; Jang, J. ElectrospunZnO/TiO2 composite nanofibers as a bactericidal agent. Chem. Commun. 2011, 47, 9164–9166. [Google Scholar] [CrossRef] [PubMed]
- Paladini, F.; Pollini, M.; Sannino, A.; Ambrosio, L. Metal-based antibacterial substrates for biomedical applications. Biomacromolecules 2015, 16, 1873–1885. [Google Scholar] [CrossRef]
- Chernousova, S.; Epple, M. Silver as antibacterial agent: Ion, nanoparticle, and metal. Angew. Chemie Int. Ed. 2013, 52, 1636–1653. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, C.; Yang, S.; Chiu, T.-W.; Wu, J.; Xiao, K.; Huang, Y.; Li, X. Enhanced silver loaded antibacterial titanium implant coating with novel hierarchical effect. J. Biomater. Appl. 2018, 32, 1289–1299. [Google Scholar] [CrossRef]
- Hou, X.; Mao, D.; Ma, H.; Ai, Y.; Zhao, X.; Deng, J.; Li, D.; Liao, B. Antibacterial ability of Ag–TiO2 nanotubes prepared by ion implantation and anodic oxidation. Mater. Lett. 2015, 161, 309–312. [Google Scholar] [CrossRef]
- Shanmuganathan, R.; MubarakAli, D.; Prabakar, D.; Muthukumar, H.; Thajuddin, N.; Kumar, S.S.; Pugazhendhi, A. An enhancement of antimicrobial efficacy of biogenic and ceftriaxone-conjugated silver nanoparticles: Green approach. Environ. Sci. Pollut. Res. 2018, 25, 10362–10370. [Google Scholar] [CrossRef]
- Tang, Y.; Chappell, H.F.; Dove, M.T.; Reeder, R.J.; Lee, Y.J. Zinc incorporation into hydroxylapatite. Biomaterials 2009, 30, 2864–2872. [Google Scholar] [CrossRef]
- Tas, A.C.; Bhaduri, S.B.; Jalota, S. Preparation of Zn-doped β-tricalcium phosphate (β-Ca3(PO4)2) bioceramics. Mater. Sci. Eng. C 2007, 27, 394–401. [Google Scholar]
- Lowe, N.M.; Fraser, W.D.; Jackson, M.J. Is there a potential therapeutic value of copper and zinc for osteoporosis? Proc. Nutr. Soc. 2002, 61, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Storrie, H.; Stupp, S.I. Cellular response to zinc-containing organoapatite: An in vitro study of proliferation, alkaline phosphatase activity and biomineralization. Biomaterials 2005, 26, 5492–5499. [Google Scholar] [CrossRef] [PubMed]
- Applerot, G.; Lipovsky, A.; Dror, R.; Perkas, N.; Nitzan, Y.; Lubart, R.; Gedanken, A. Enhanced antibacterial activity of nanocrystalline ZnO due to increased ROS-mediated cell injury. Adv. Funct. Mater. 2009, 19, 842–852. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, X.; Li, B.; Cao, C.; Dong, Y.; Ding, C.; Chu, P.K. UV-irradiation-induced bioactivity on TiO2 coatings with nanostructural surface. Acta Biomater. 2008, 4, 544–552. [Google Scholar] [CrossRef] [PubMed]
- de Assis, S.L.; Wolynec, S.; Costa, I. Corrosion characterization of titanium alloys by electrochemical techniques. Electrochim. Acta 2006, 51, 1815–1819. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Diez-Vicente, A.L. Development of nanocomposites reinforced with carboxylated poly (ether ether ketone) grafted to zinc oxide with superior antibacterial properties. ACS Appl. Mater. Interfaces 2014, 6, 3729–3741. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, L.; Luo, H.; Zhang, D.; Lei, S.; Zhou, K. Dual-purpose magnesium-incorporated titanium nanotubes for combating bacterial infection and ameliorating osteolysis to realize better osseointegration. ACS Biomater. Sci. Eng. 2019, 5, 5368–5383. [Google Scholar] [CrossRef]
- Steinmetz, O.; Hoch, S.; Avniel-Polak, S.; Gavish, K.; Eli-Berchoer, L.; Wilensky, A.; Nussbaum, G. CX3CR1hi Monocyte/Macrophages Support Bacterial Survival and Experimental Infection–Driven Bone Resorption. J. Infect. Dis. 2016, 213, 1505–1515. [Google Scholar] [CrossRef]
- Wang, J.; Wu, X.; Duan, Y. Magnesium lithospermate B protects against lipopolysaccharide-induced bone loss by inhibiting RANKL/RANK pathway. Front. Pharmacol. 2018, 9, 64. [Google Scholar] [CrossRef]
- Chopra, D.; Gulati, K.; Ivanovski, S. Understanding and optimizing the antibacterial functions of anodized nano-engineered titanium implants. Acta Biomater. 2021, 127, 80–101. [Google Scholar] [CrossRef] [PubMed]
- Choe, H.J.; Kwon, S.-H.; Lee, J.-J. Tribological properties and thermal stability of TiAlCN coatings deposited by ICP-assisted sputtering. Surf. Coatings Technol. 2013, 228, 282–285. [Google Scholar] [CrossRef]
- Harris, H.W.; El-Naggar, M.Y.; Bretschger, O.; Ward, M.J.; Romine, M.F.; Obraztsova, A.Y.; Nealson, K.H. Electrokinesis is a microbial behavior that requires extracellular electron transport. Proc. Natl. Acad. Sci. USA 2010, 107, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Dong, H.; Reguera, G.; Beyenal, H.; Lu, A.; Liu, J.; Yu, H.-Q.; Fredrickson, J.K. Extracellular electron transfer mechanisms between microorganisms and minerals. Nat. Rev. Microbiol. 2016, 14, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tan, J.; Zhu, H.; Mei, Y.; Liu, X. Biomedical Implants with Charge-Transfer Monitoring and Regulating Abilities. Adv. Sci. 2021, 8, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Vecitis, C.D.; Zodrow, K.R.; Kang, S.; Elimelech, M. Electronic-structure-dependent bacterial cytotoxicity of single-walled carbon nanotubes. ACS Nano 2010, 4, 5471–5479. [Google Scholar] [CrossRef] [PubMed]
- de Faria, A.F.; Martinez, D.S.T.; Meira, S.M.M.; de Moraes, A.C.M.; Brandelli, A.; Souza Filho, A.G.; Alves, O.L. Anti-adhesion and antibacterial activity of silver nanoparticles supported on graphene oxide sheets. Colloids Surf. B Biointerfaces 2014, 113, 115–124. [Google Scholar] [CrossRef]
- Strahl, H.; Hamoen, L.W. Membrane potential is important for bacterial cell division. Proc. Natl. Acad. Sci. USA 2010, 107, 12281–12286. [Google Scholar] [CrossRef]
- Cao, H.; Qiao, Y.; Liu, X.; Lu, T.; Cui, T.; Meng, F.; Chu, P.K. Electron storage mediated dark antibacterial action of bound silver nanoparticles: Smaller is not always better. Acta Biomater. 2013, 9, 5100–5110. [Google Scholar] [CrossRef]
- Li, J.; Zhou, H.; Qian, S.; Liu, Z.; Feng, J.; Jin, P.; Liu, X. Plasmonic gold nanoparticles modified titania nanotubes for antibacterial application. Appl. Phys. Lett. 2014, 104, 261110. [Google Scholar] [CrossRef]
- Wang, G.; Feng, H.; Gao, A.; Hao, Q.; Jin, W.; Peng, X.; Li, W.; Wu, G.; Chu, P.K. Extracellular electron transfer from aerobic bacteria to Au-loaded TiO2 semiconductor without light: A new bacteria-killing mechanism other than localized surface plasmon resonance or microbial fuel cells. ACS Appl. Mater. Interfaces 2016, 8, 24509–24516. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, G.; Zhu, H.; Zhang, M.; Zheng, X.; Di, Z.; Liu, X.; Wang, X. Antibacterial activity of large-area monolayer graphene film manipulated by charge transfer. Sci. Rep. 2014, 4, 1–8. [Google Scholar] [CrossRef]
- Wang, G.; Jin, W.; Qasim, A.M.; Gao, A.; Peng, X.; Li, W.; Feng, H.; Chu, P.K. Antibacterial effects of titanium embedded with silver nanoparticles based on electron-transfer-induced reactive oxygen species. Biomaterials 2017, 124, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, H.; Qiu, C.; Iatsunskyi, I.; Coy, E.; Moya, S.; Wang, Z.; Wu, W.; Zhao, X.; Wang, G. Electron transfer correlated antibacterial activity of biocompatible graphene Nanosheets-TiO2 coatings. Carbon N. Y. 2020, 166, 350–360. [Google Scholar] [CrossRef]
- Zhou, R.; Han, Y.; Cao, J.; Li, M.; Jin, G.; Du, Y.; Luo, H.; Yang, Y.; Zhang, L.; Su, B. Enhanced osseointegration of hierarchically structured Ti implant with electrically bioactive SnO2–TiO2 bilayered surface. ACS Appl. Mater. Interfaces 2018, 10, 30191–30200. [Google Scholar] [CrossRef]
- Wang, D.; Li, Q.; Qiu, J.; Zhang, X.; Ge, N.; Liu, X. Corrosion motivated ROS generation helps endow titanium with broad-spectrum antibacterial abilities. Adv. Mater. Interfaces 2019, 6, 1900514. [Google Scholar] [CrossRef]
- Ning, C.; Yu, P.; Zhu, Y.; Yao, M.; Zhu, X.; Wang, X.; Lin, Z.; Li, W.; Wang, S.; Tan, G. Built-in microscale electrostatic fields induced by anatase–rutile-phase transition in selective areas promote osteogenesis. NPG Asia Mater. 2016, 8, e243. [Google Scholar] [CrossRef]
- Tian, J.; Feng, H.; Yan, L.; Yu, M.; Ouyang, H.; Li, H.; Jiang, W.; Jin, Y.; Zhu, G.; Li, Z. A self-powered sterilization system with both instant and sustainable anti-bacterial ability. Nano Energy 2017, 36, 241–249. [Google Scholar] [CrossRef]
- Wang, G.; Feng, H.; Hu, L.; Jin, W.; Hao, Q.; Gao, A.; Peng, X.; Li, W.; Wong, K.-Y.; Wang, H. An antibacterial platform based on capacitive carbon-doped TiO2 nanotubes after direct or alternating current charging. Nat. Commun. 2018, 9, 1–12. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noreen, S.; Wang, E.; Feng, H.; Li, Z. Functionalization of TiO2 for Better Performance as Orthopedic Implants. Materials 2022, 15, 6868. https://doi.org/10.3390/ma15196868
Noreen S, Wang E, Feng H, Li Z. Functionalization of TiO2 for Better Performance as Orthopedic Implants. Materials. 2022; 15(19):6868. https://doi.org/10.3390/ma15196868
Chicago/Turabian StyleNoreen, Sehrish, Engui Wang, Hongqing Feng, and Zhou Li. 2022. "Functionalization of TiO2 for Better Performance as Orthopedic Implants" Materials 15, no. 19: 6868. https://doi.org/10.3390/ma15196868
APA StyleNoreen, S., Wang, E., Feng, H., & Li, Z. (2022). Functionalization of TiO2 for Better Performance as Orthopedic Implants. Materials, 15(19), 6868. https://doi.org/10.3390/ma15196868





