Eutectic Fatty Acids Phase Change Materials Improved with Expanded Graphite
Abstract
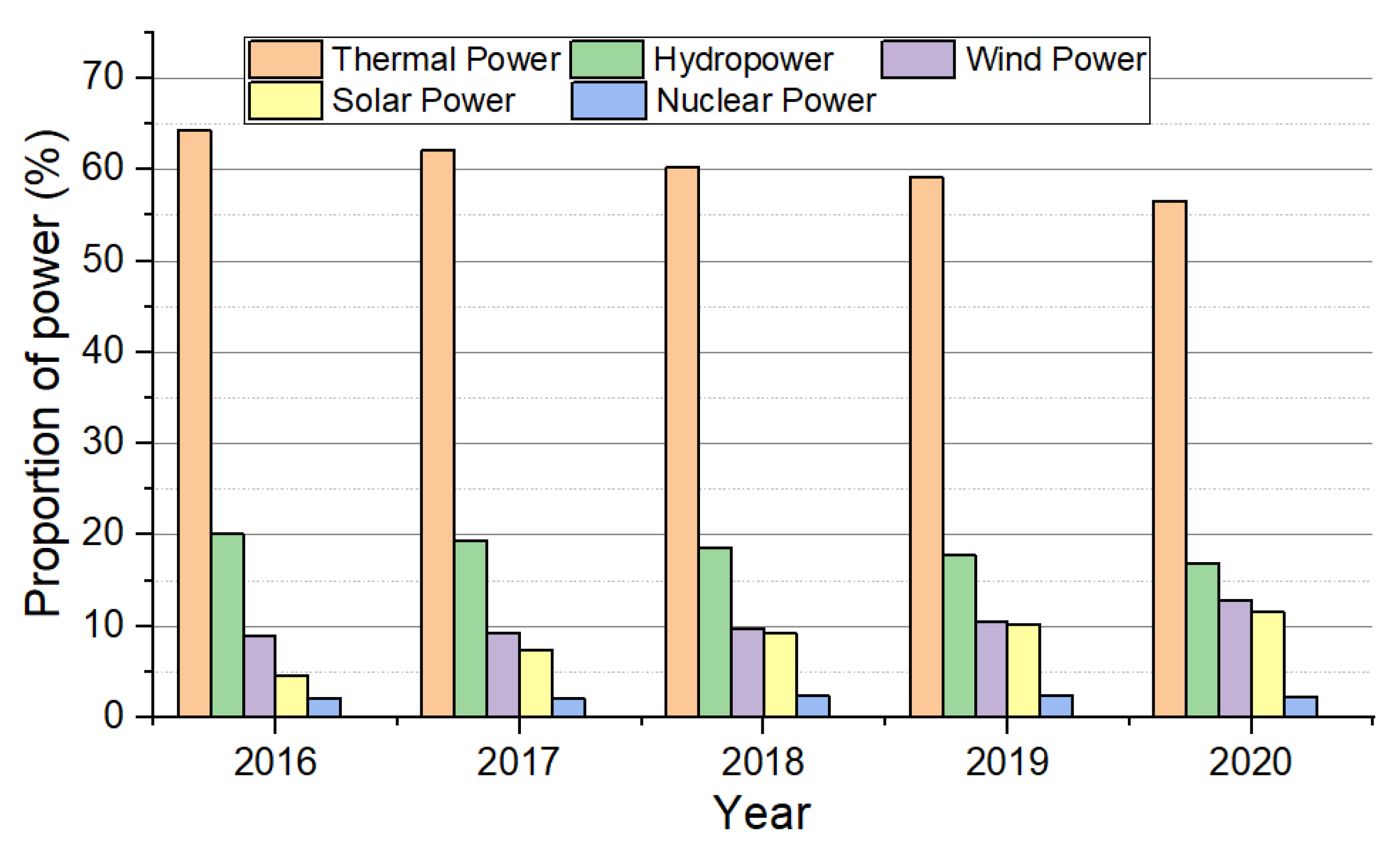
1. Introduction
2. Materials and Methods
2.1. Materials
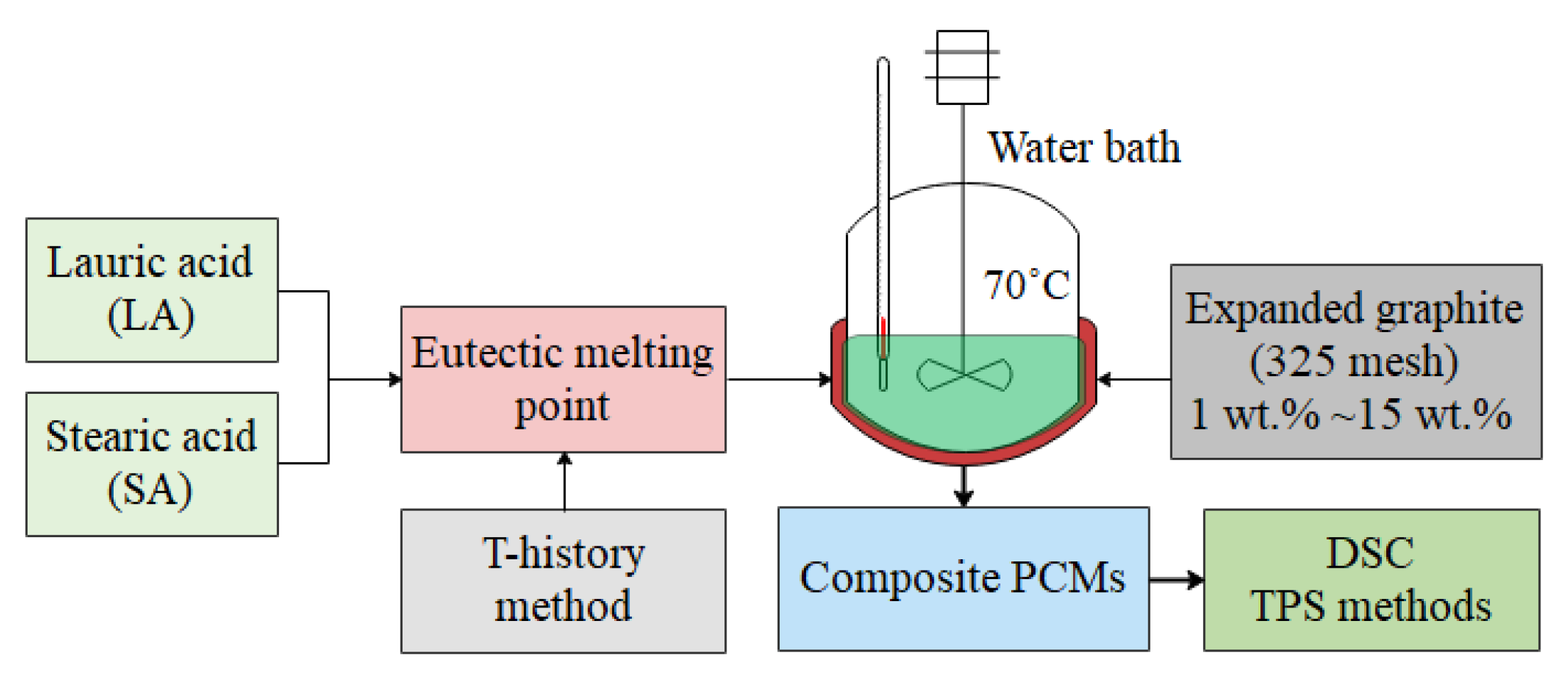
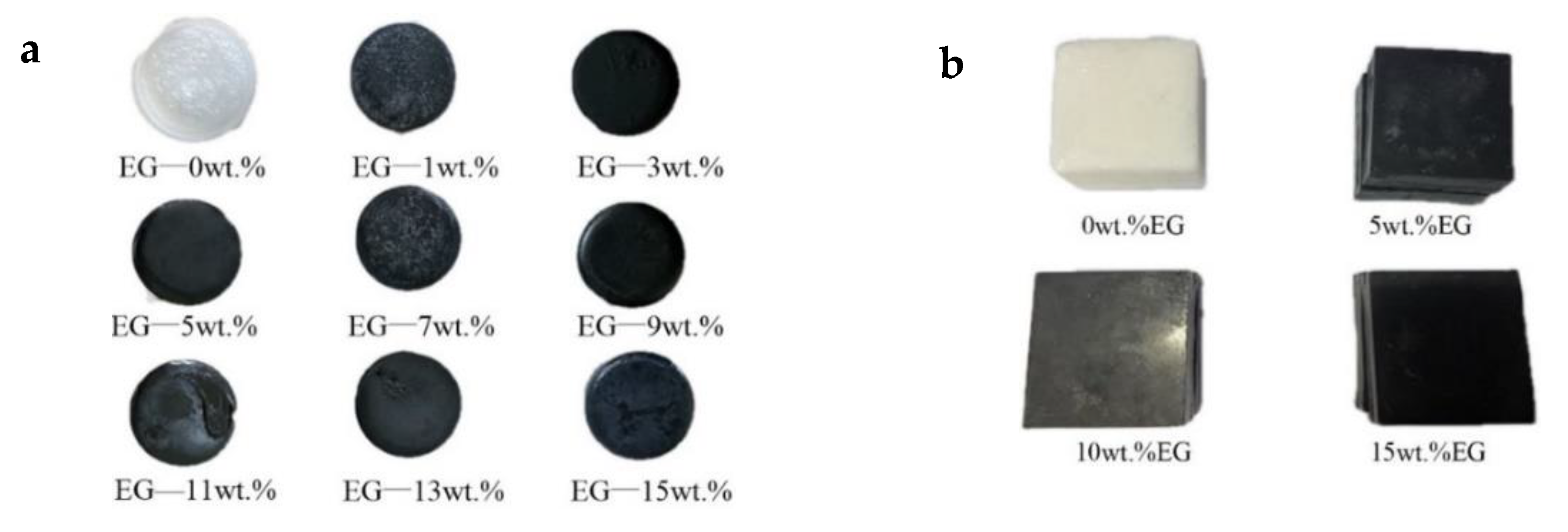
2.2. Preparation and Process Methods
2.3. Differential Scanning Calorimetry (DSC)
2.4. Transient Plane Source (TPS) Methods
2.5. Low-Temperature Thermal Energy Storage Experiment
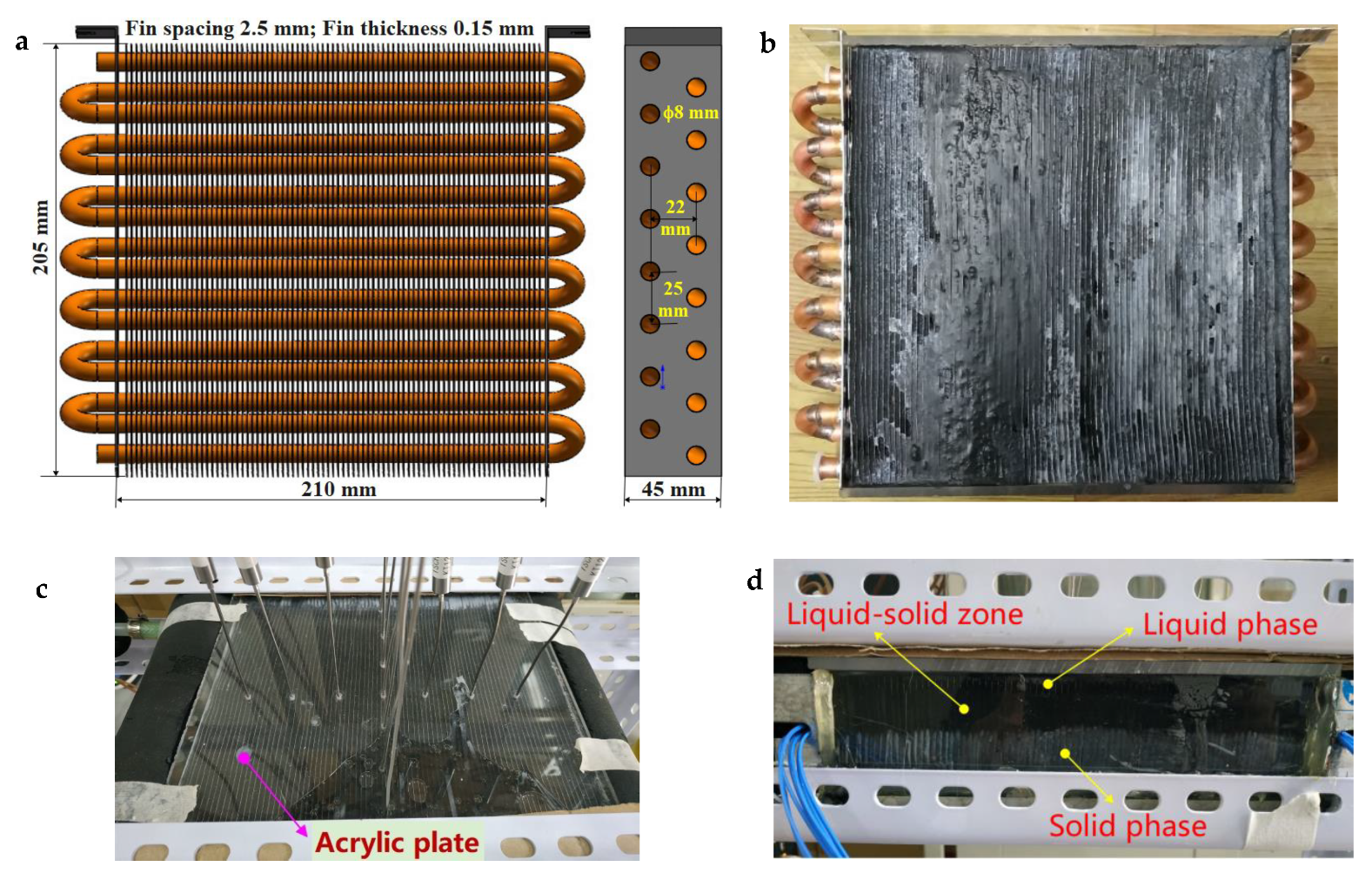
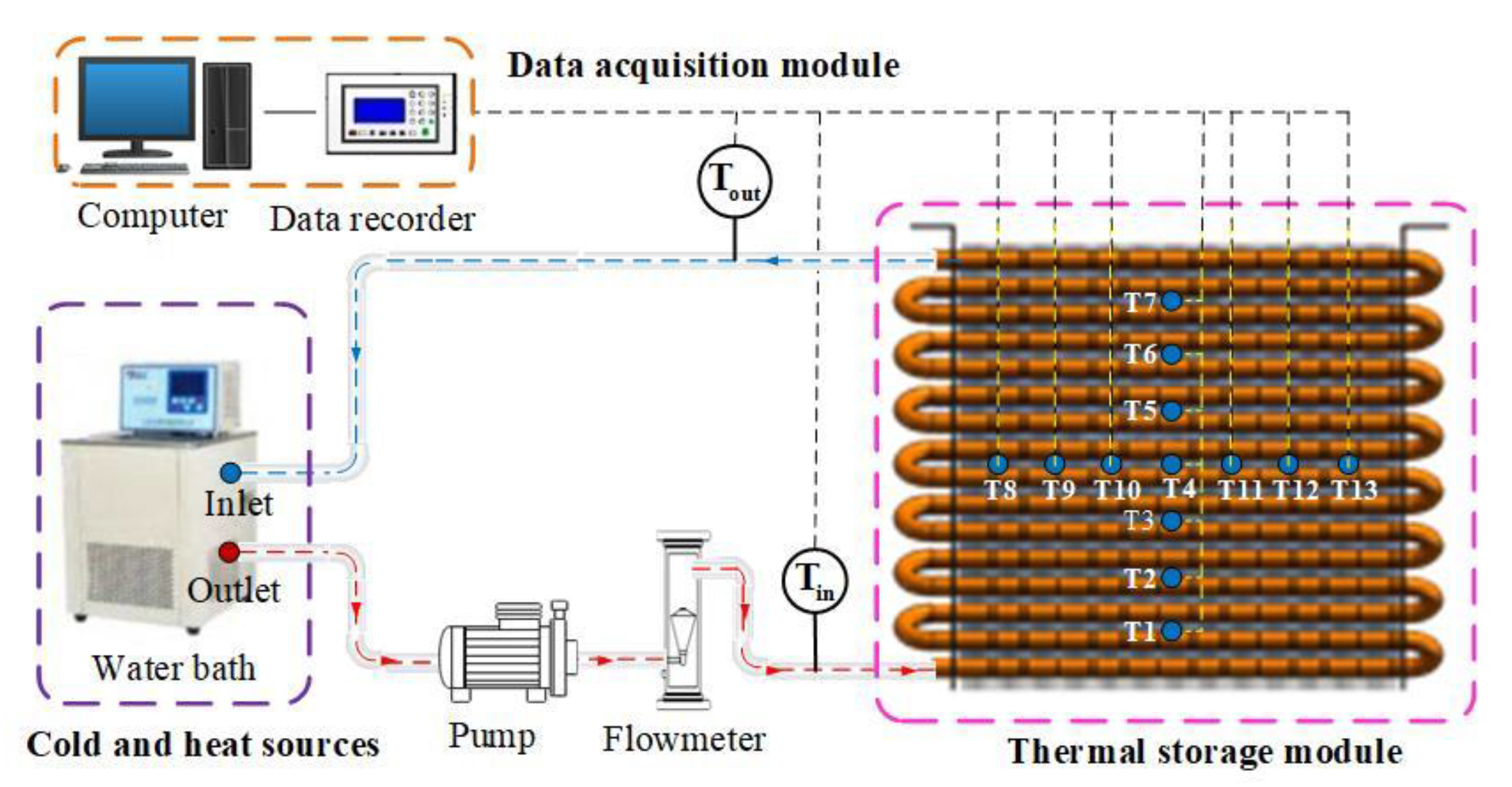
2.5.1. Finned-Coil-Type Heat Reservoir
2.5.2. Testing System
3. Results and Discussion
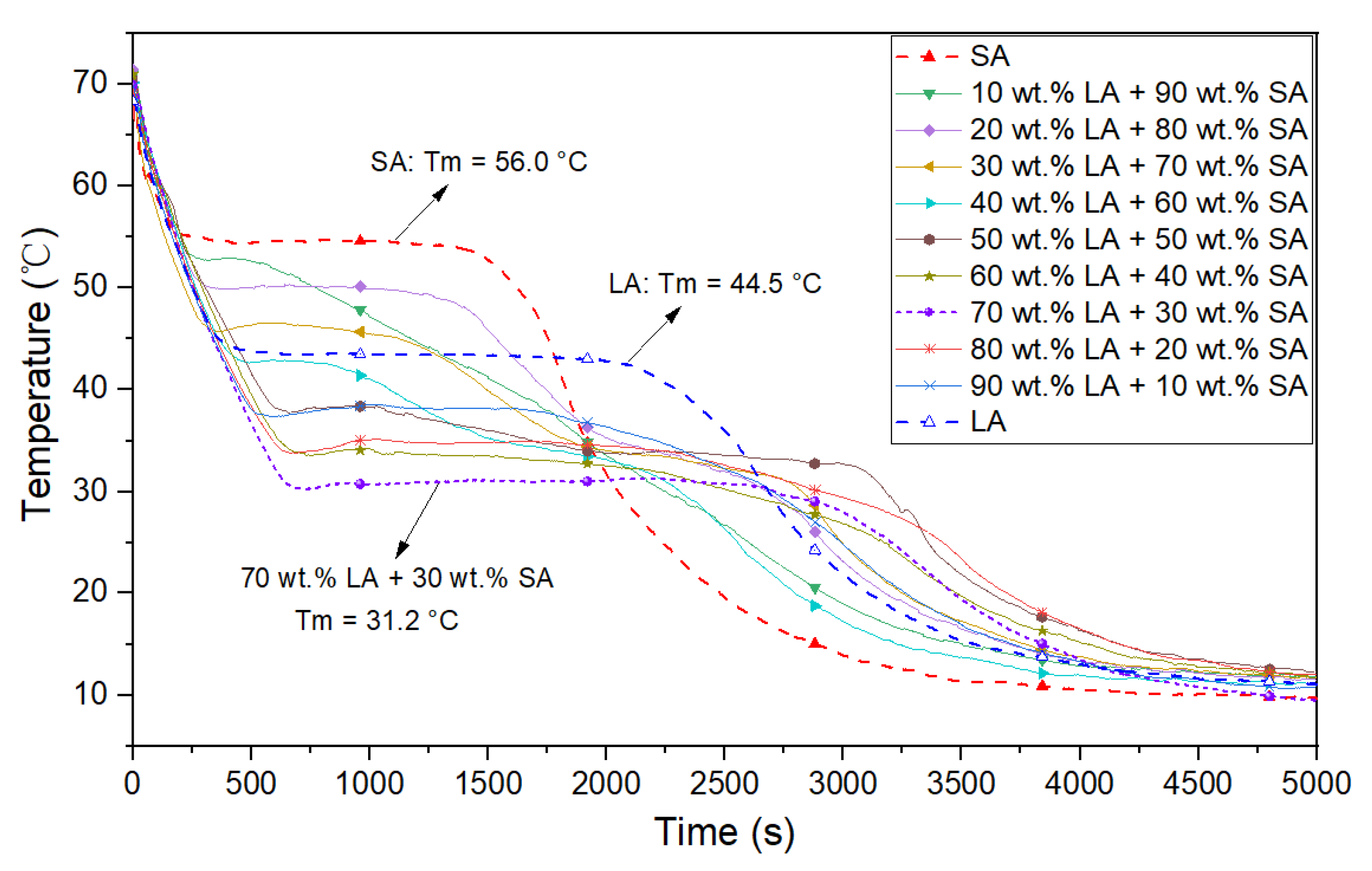
3.1. The Eutectic Point of LA and SA
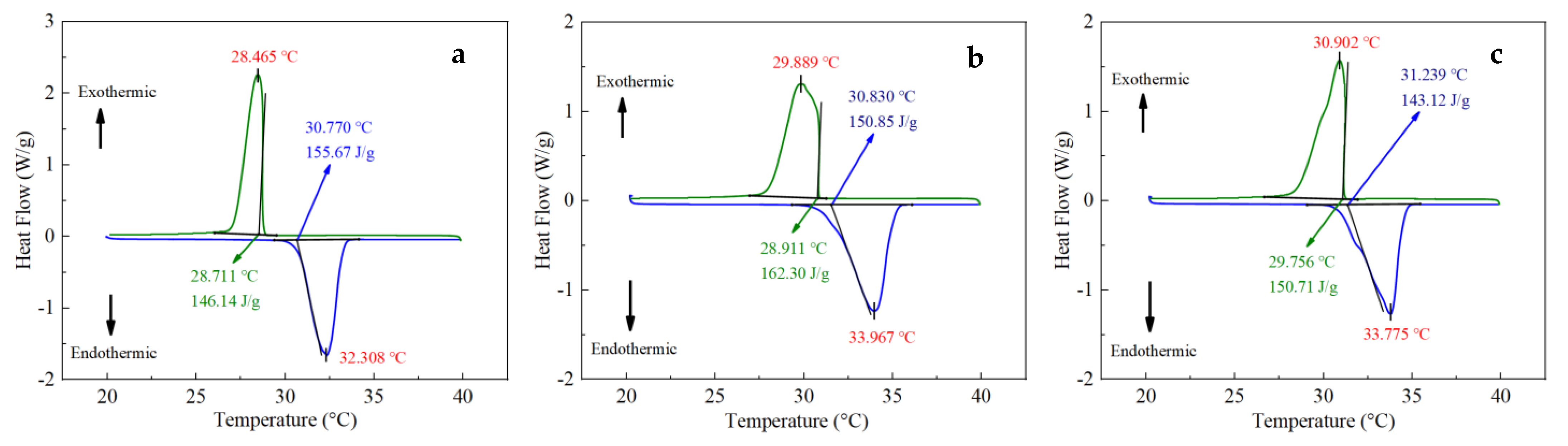
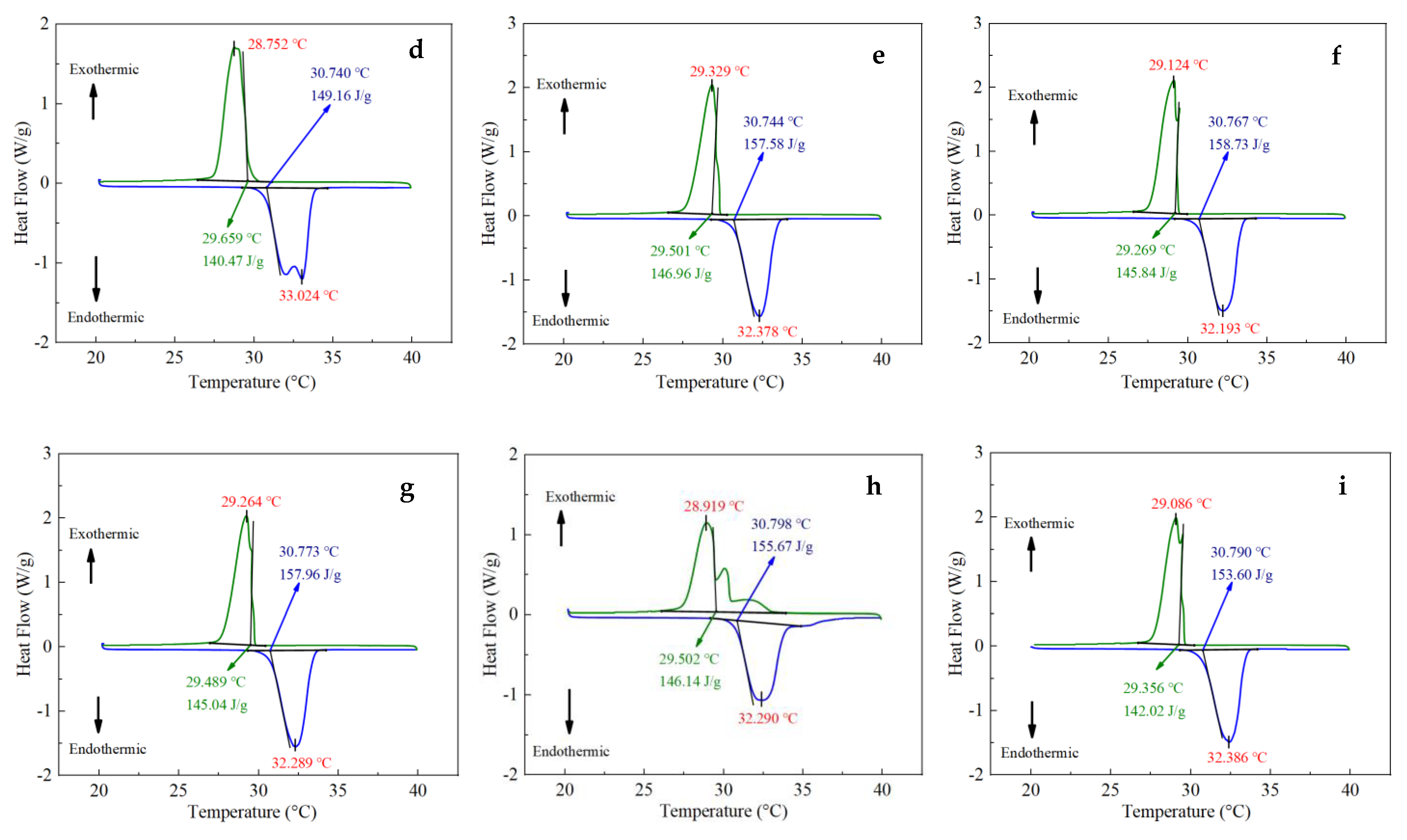
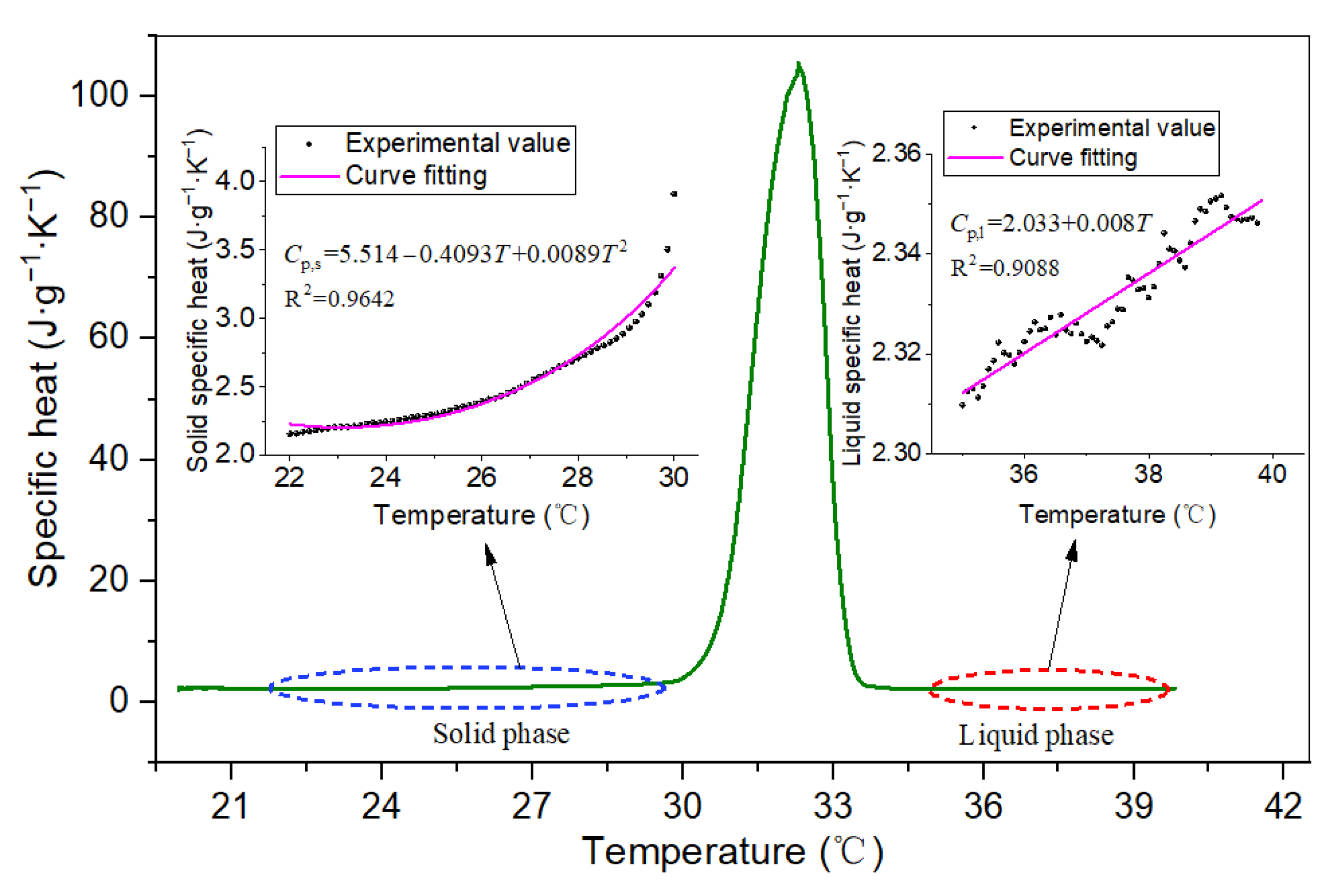
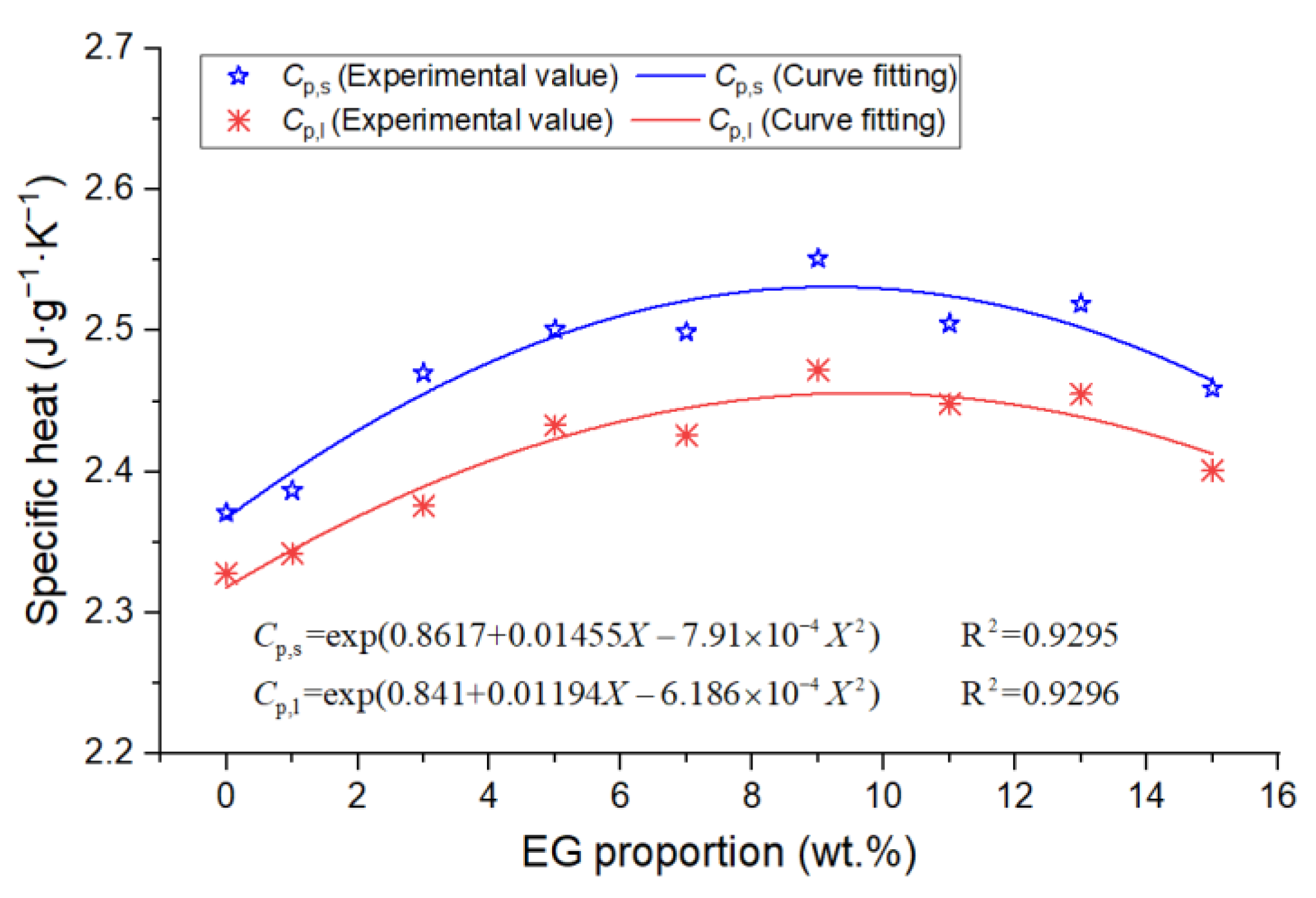
3.2. DSC Results
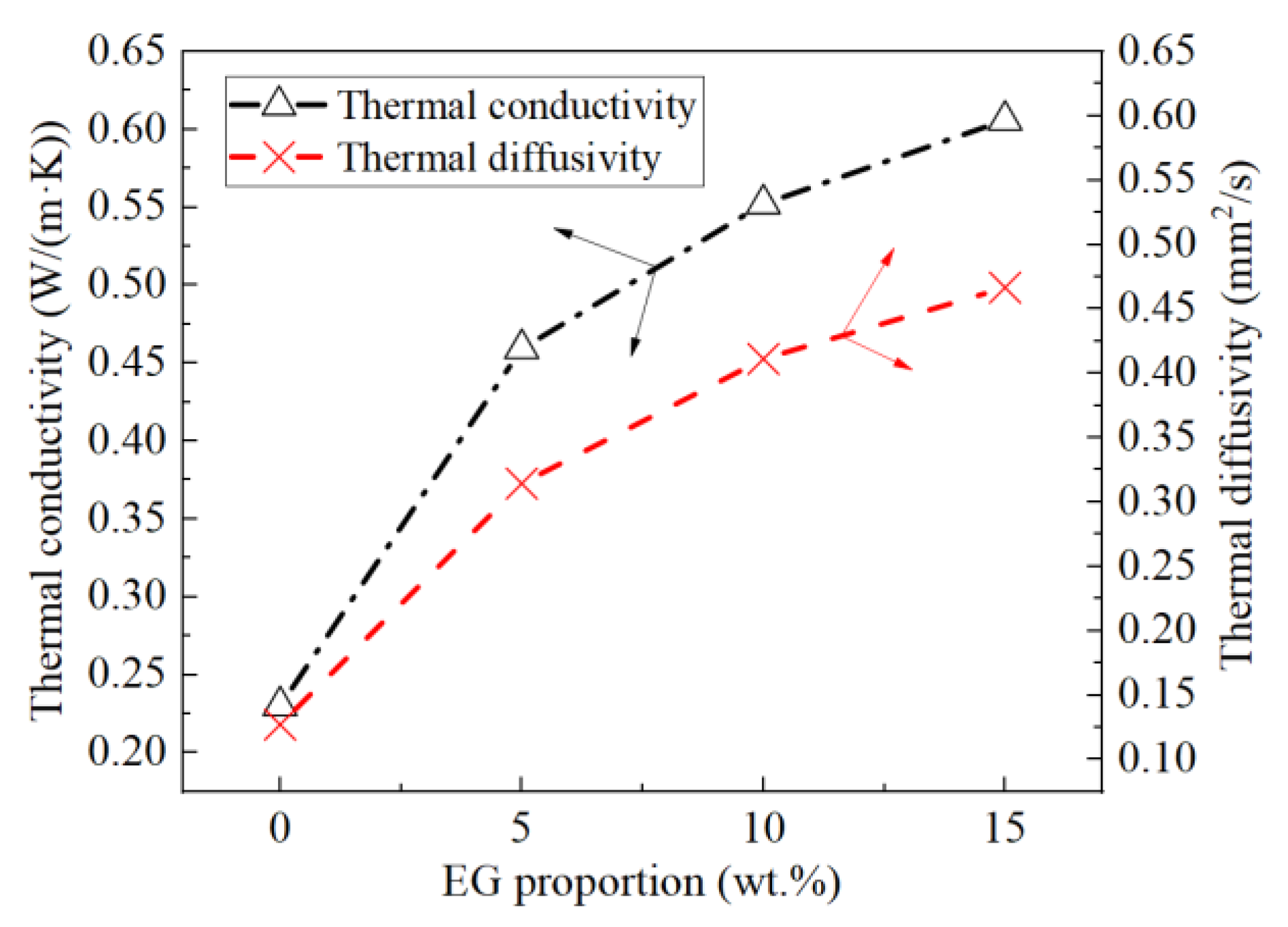
3.3. TPS Results
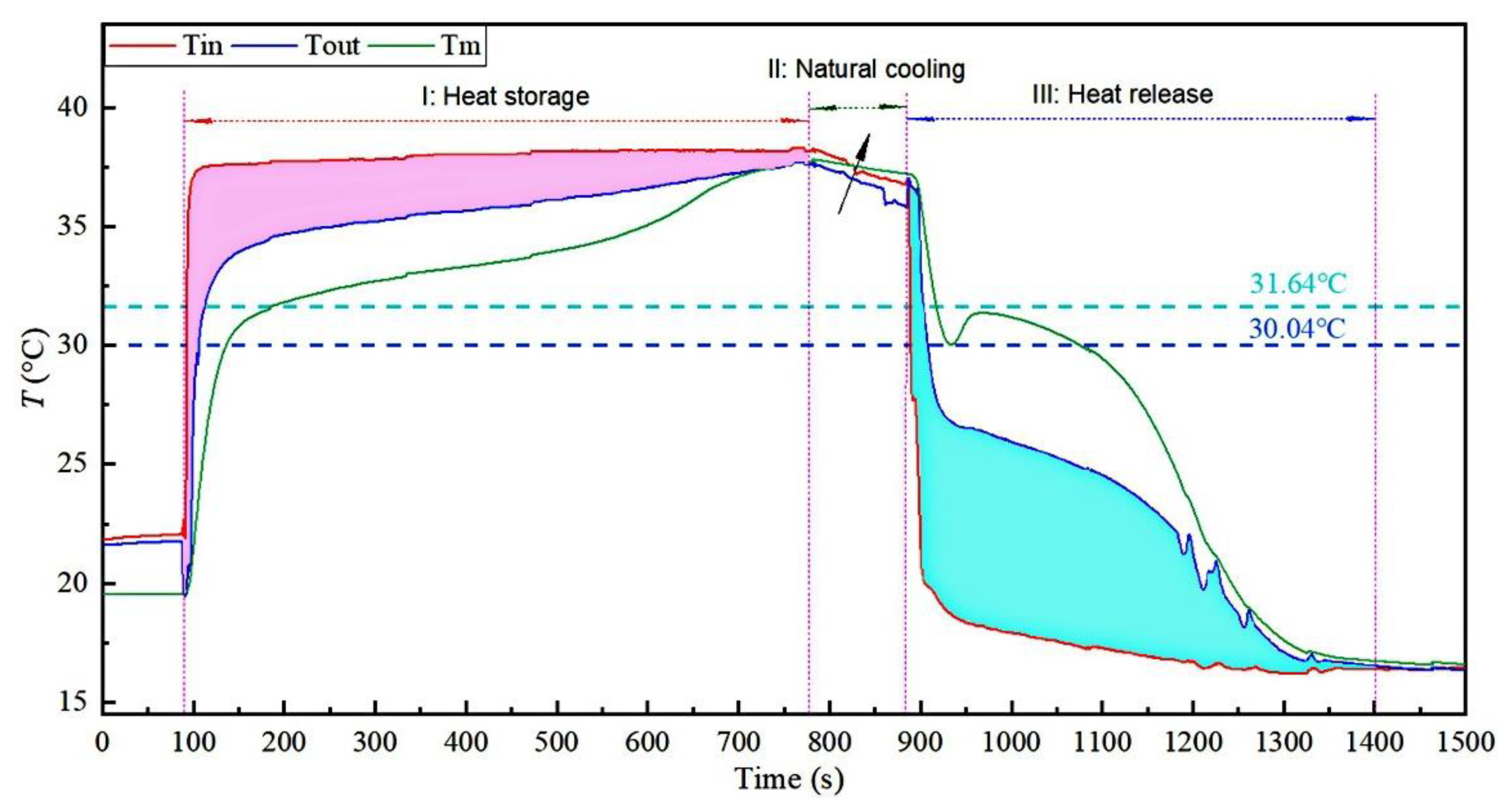
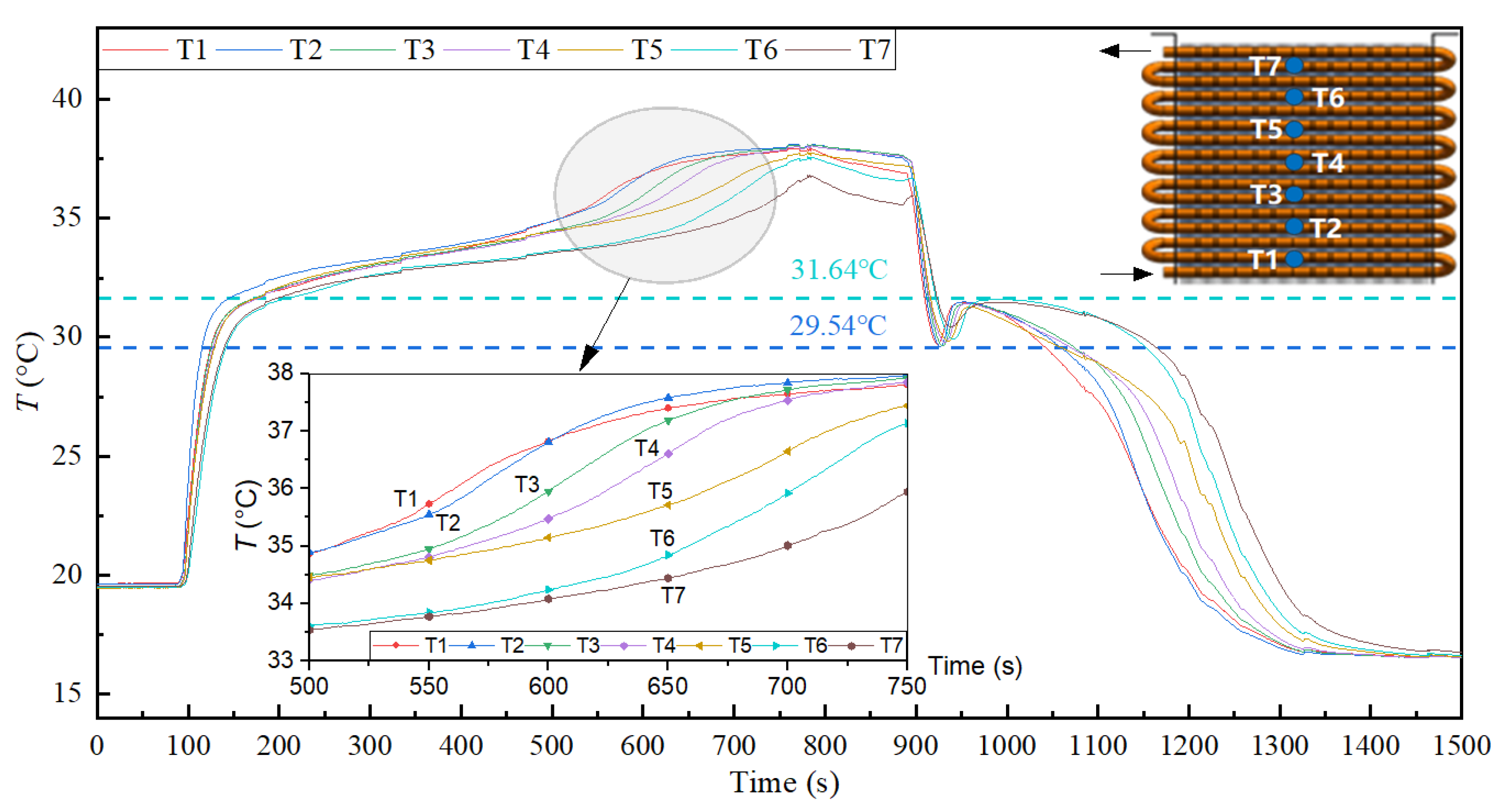
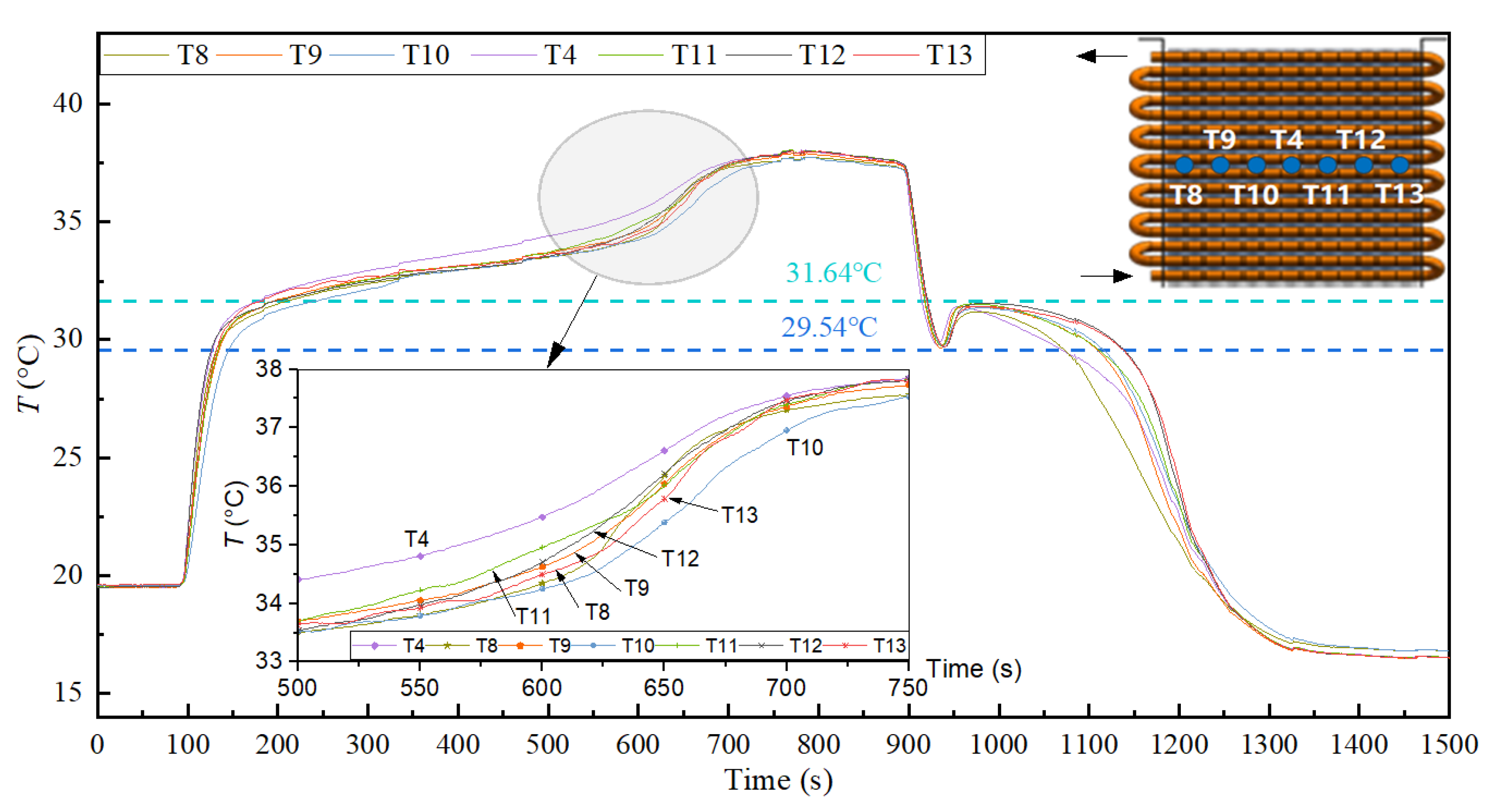
3.4. Low-Temperature Thermal Energy Storage Experiment Results
4. Conclusions
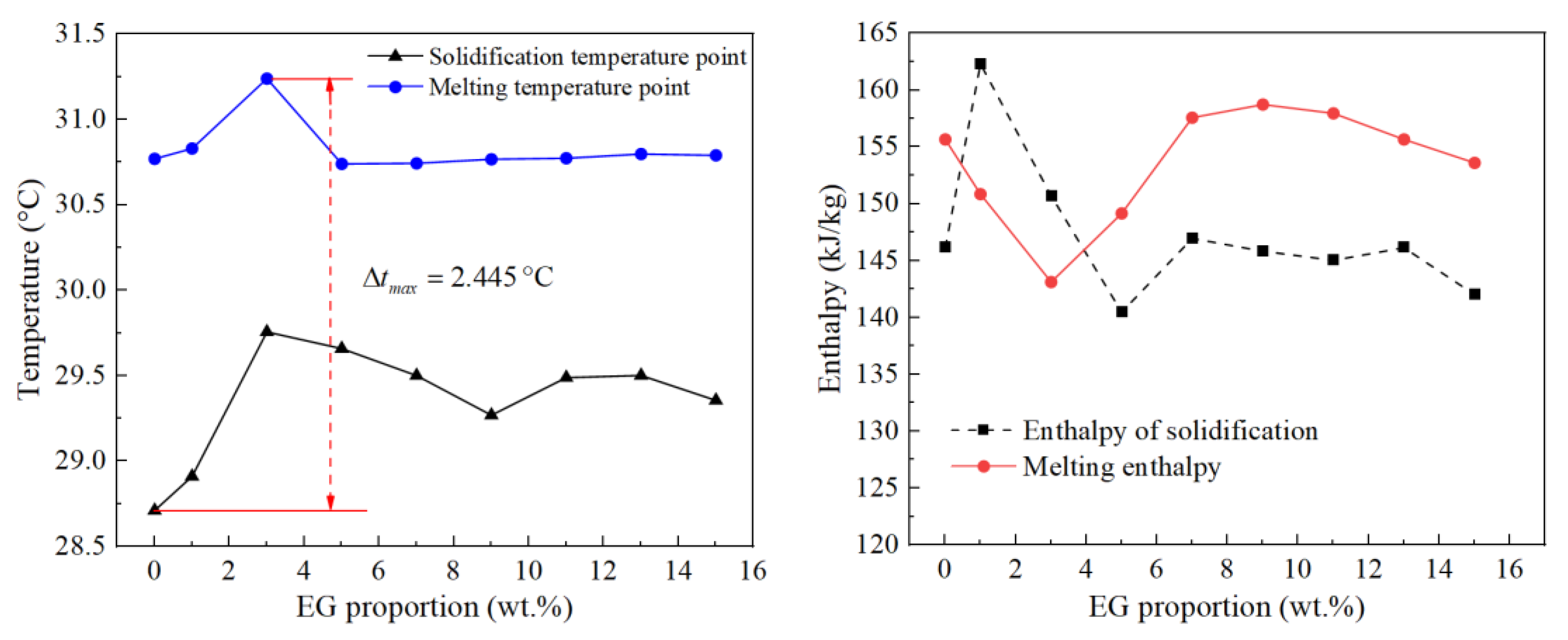
- According to the step cooling curve, the eutectic point of lauric acid and stearic acid was 31.2 °C when the mass proportion of LA was 70 wt.% and that of SA was 30 wt.%.
- Based on the DSC and TPS results, the properties of composite PCMs remained stable when the EG content was greater than 5 wt.%, and the specific heat capacity reached the maximum when the EG ratio was about 9%. The thermal conductivity and thermal diffusion coefficient of the composite PCMs (10−15 wt.%) increased by 2.4–2.6 times and 3.2–3.7 times compared with those of pure eutectic acid, respectively. This indicated EG could enhance heat conduction.
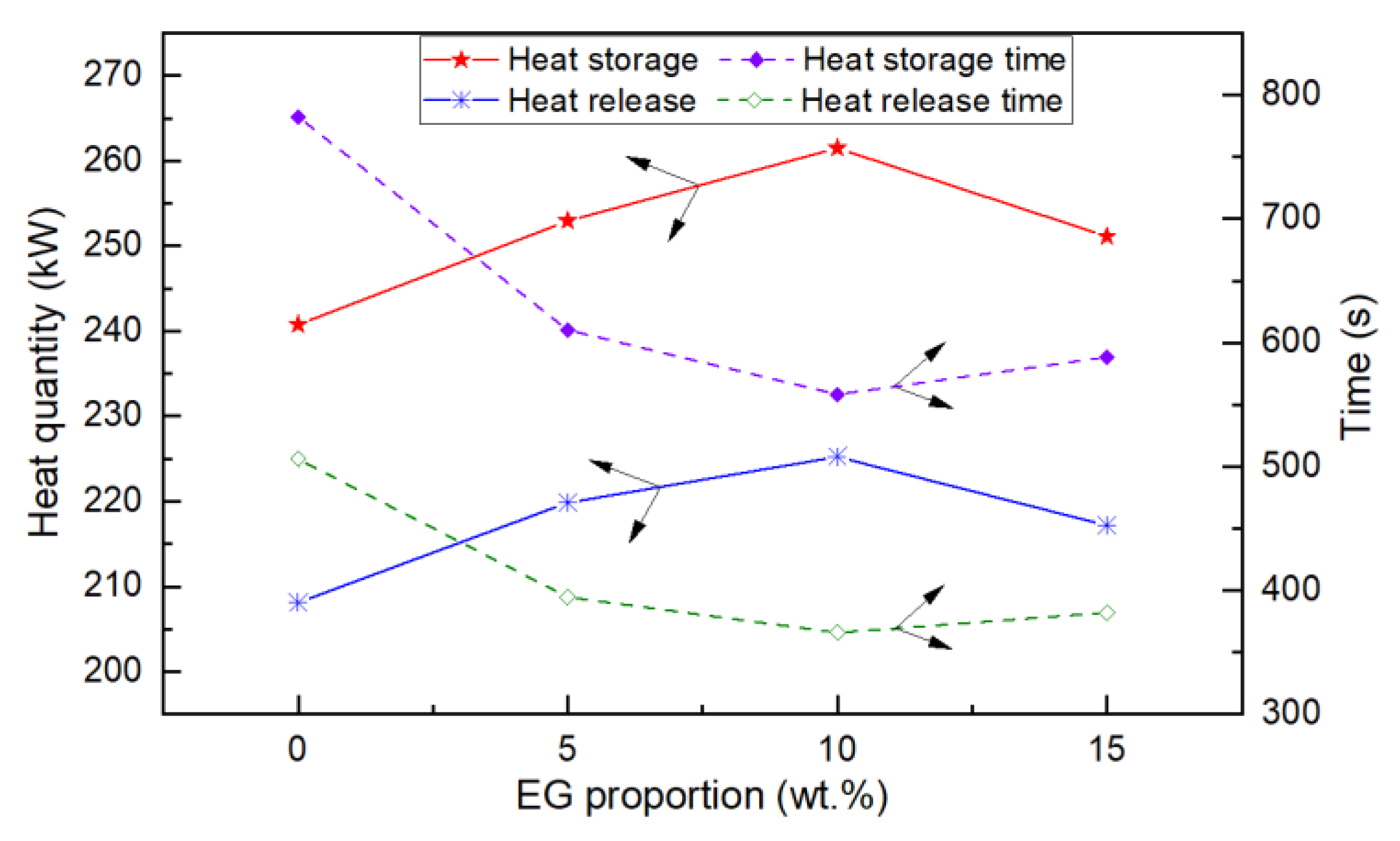
- The experimental results of the finned-coil-type heat reservoirs showed that the optimum ratio of EG was 10 wt.%. The heat storage time was reduced by 20.4%, 8.1%, and 6.2% compared with the other three EG ratios; meanwhile, the heat release time was decreased by 19.3%, 6.7%, and 5.3%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NBS (National Bureau of Statistics of China). Online Statistical Database: Installed Capacity of Power Generation. 2021. Available online: http://www.stats.gov.cn/ (accessed on 26 September 2022).
- Zalba, B.; Marin, J.M.; Cabeza, L.F.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Zhou, D.; Zhao, C.Y.; Tian, Y. Review on thermal energy storage with phase change materials (PCMs) in building applications. Appl. Energy 2012, 92, 593–605. [Google Scholar] [CrossRef]
- Yang, T.Y.; King, W.P.; Miljkovic, N. Phase change material-based thermal energy storage. Cell Rep. Phys. Sci. 2021, 2, 100540. [Google Scholar] [CrossRef]
- Pomianowski, M.; Heiselberg, P.; Zhang, Y.P. Review of thermal energy storage technologies based on PCM application in buildings. Energy Build. 2013, 67, 56–69. [Google Scholar] [CrossRef]
- Bao, X.H.; Yang, H.B.; Xu, X.X.; Xu, T.; Cui, H.Z.; Tang, W.C.; Sang, G.C.; Fung, W.H. Development of a stable inorganic phase change material for thermal energy storage in buildings. Sol. Energy Mater. Sol. Cells 2020, 208, 11. [Google Scholar] [CrossRef]
- Gorbacheva, S.N.; Makarova, V.V.; Ilyin, S.O. Hydrophobic nanosilica-stabilized graphite particles for improving thermal conductivity of paraffin wax-based phase-change materials. J. Energy Storage 2021, 36, 102417. [Google Scholar] [CrossRef]
- Bulk, A.; Odukomaiya, A.; Simmons, E.; Woods, J. Processing Compressed Expanded Natural Graphite for Phase Change Material Composites. J. Therm. Sci. 2022. [Google Scholar] [CrossRef]
- Li, M. A nano-graphite/paraffin phase change material with high thermal conductivity. Appl. Energy 2013, 106, 25–30. [Google Scholar] [CrossRef]
- Mills, A.; Farid, M.; Selman, J.R.; Al-Hallaj, S. Thermal conductivity enhancement of phase change materials using a graphite matrix. Appl. Therm. Eng. 2006, 26, 1652–1661. [Google Scholar] [CrossRef]
- Sedeh, M.M.; Khodadadi, J.M. Thermal conductivity improvement of phase change materials/graphite foam composites. Carbon 2013, 60, 117–128. [Google Scholar] [CrossRef]
- Fang, G.Y.; Li, H.I.; Liu, X. Preparation and properties of lauric acid/silicon dioxide composites as form-stable phase change materials for thermal energy storage. Mater. Chem. Phys. 2010, 122, 533–536. [Google Scholar] [CrossRef]
- Guo, Y.L.; Yang, W.B.; Jiang, Z.N.; He, F.F.; Zhang, K.; He, R.; Wu, J.Y.; Fan, J.H. Silicone rubber/paraffin@silicon dioxide form-stable phase change materials with thermal energy storage and enhanced mechanical property. Sol. Energy Mater. Sol. Cells 2019, 196, 16–24. [Google Scholar] [CrossRef]
- Li, H.; Fang, G.Y.; Liu, X. Synthesis of shape-stabilized paraffin/silicon dioxide composites as phase change material for thermal energy storage. J. Mater. Sci. 2010, 45, 1672–1676. [Google Scholar] [CrossRef]
- Wang, W.L.; Yang, X.X.; Fang, Y.T.; Ding, J. Preparation and performance of form-stable polyethylene glycol/silicon dioxide composites as solid-liquid phase change materials. Appl. Energy 2009, 86, 170–174. [Google Scholar] [CrossRef]
- Hua, J.S.; Yuan, C.; Zhao, X.; Zhang, J.; Du, J.X. Structure and thermal properties of expanded graphite/paraffin composite phase change material. Energy Sources Part A-Recovery Util. Environ. Eff. 2019, 41, 86–93. [Google Scholar] [CrossRef]
- Jeon, J.; Park, J.H.; Wi, S.; Kim, K.H.; Kim, S. Thermal performance enhancement of a phase change material with expanded graphite via ultrasonication. J. Ind. Eng. Chem. 2019, 79, 437–442. [Google Scholar] [CrossRef]
- Kao, H.T.; Li, M.; Lv, X.W.; Tan, J.M. Preparation and thermal properties of expanded graphite/paraffin/organic montmorillonite composite phase change material. J. Therm. Anal. Calorim. 2012, 107, 299–303. [Google Scholar] [CrossRef]
- Li, W.; Zhang, R.; Jiang, N.; Tang, X.F.; Shi, H.F.; Zhang, X.X.; Zhang, Y.K.; Dong, L.; Zhang, N.X. Composite macrocapsule of phase change materials/expanded graphite for thermal energy storage. Energy 2013, 57, 607–614. [Google Scholar] [CrossRef]
- Xia, L.; Zhang, P.; Wang, R.Z. Preparation and thermal characterization of expanded graphite/paraffin composite phase change material. Carbon 2010, 48, 2538–2548. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, P.; Meng, Z.N.; Li, M. Thermal Characterization of Lauric-Stearic Acid/Expanded Graphite Eutectic Mixture as Phase Change Materials. J. Nanosci. Nanotechnol. 2015, 15, 3288–3294. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- de Gracia, A.; Cabeza, L.F. Phase change materials and thermal energy storage for buildings. Energy Build. 2015, 103, 414–419. [Google Scholar] [CrossRef]
- Demirbas, M.F. Thermal energy storage and phase change materials: An overview. Energy Sources Part B 2006, 1, 85–95. [Google Scholar] [CrossRef]
- Ammar, Y.; Joyce, S.; Norman, R.; Wang, Y.D.; Roskilly, A.P. Low grade thermal energy sources and uses from the process industry in the UK. Appl. Energy 2012, 89, 3–20. [Google Scholar] [CrossRef]
- Soda, M.; Beyene, A. Multiphase ultra-low grade thermal energy storage for organic Rankine cycle. Int. J. Energy Res. 2016, 40, 51–60. [Google Scholar] [CrossRef]
- Kishore, R.A.; Priya, S. A Review on Low-Grade Thermal Energy Harvesting: Materials, Methods and Devices. Materials 2018, 11, 1433. [Google Scholar] [CrossRef] [PubMed]
- Marumo, K.; Kobayashi, N.; Nakagawa, T.; Fukai, J.; Itaya, Y. Lithium Bromide/Water Absorption Heat Pump for Simultaneous Production of Heated Air and Steam from Waste Heat. J. Chem. Eng. Jpn. 2016, 49, 268–273. [Google Scholar] [CrossRef]
- Xu, A.X.; Xu, M.J.; Xie, N.; Liang, J.W.; Zeng, K.M.; Kou, G.X.; Liu, Z.Q.; Yang, S. Performance analysis of a cascade lithium bromide absorption refrigeration/dehumidification process driven by low-grade waste heat for hot summer and cold winter climate area in China. Energy Convers. Manag. 2021, 228, 14. [Google Scholar] [CrossRef]
- Hung, T.C.; Shai, T.Y.; Wang, S.K. A review of organic Rankine cycles (ORCs) for the recovery of low-grade waste heat. Energy 1997, 22, 661–667. [Google Scholar] [CrossRef]
- Lecompte, S.; Huisseune, H.; van den Broek, M.; Vanslambrouck, B.; De Paepe, M. Review of organic Rankine cycle (ORC) architectures for waste heat recovery. Renew. Sustain. Energy Rev. 2015, 47, 448–461. [Google Scholar] [CrossRef]
- Liu, B.T.; Chien, K.H.; Wang, C.C. Effect of working fluids on organic Rankine cycle for waste heat recovery. Energy 2004, 29, 1207–1217. [Google Scholar] [CrossRef]
- Lee, C.E.; Yu, B.J.; Kim, D.H.; Jang, S.H. Analysis of the thermodynamic performance of a waste-heat-recovery boiler with additional water spray onto combustion air stream. Appl. Therm. Eng. 2018, 135, 197–205. [Google Scholar] [CrossRef]
- Crane, D.T.; Jackson, G.S. Optimization of cross flow heat exchangers for thermoelectric waste heat recovery. Energy Convers. Manag. 2004, 45, 1565–1582. [Google Scholar] [CrossRef]
- Cao, X.Q.; Yang, W.W.; Zhou, F.; He, Y.L. Performance analysis of different high-temperature heat pump systems for low-grade waste heat recovery. Appl. Therm. Eng. 2014, 71, 291–300. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Wang, R.Z.; Yang, C. Perspectives for low-temperature waste heat recovery. Energy 2019, 176, 1037–1043. [Google Scholar] [CrossRef]
- Cuce, P.M.; Riffat, S. A comprehensive review of heat recovery systems for building applications. Renew. Sustain. Energy Rev. 2015, 47, 665–682. [Google Scholar] [CrossRef]
- Culha, O.; Gunerhan, H.; Biyik, E.; Ekren, O.; Hepbasli, A. Heat exchanger applications in wastewater source heat pumps for buildings: A key review. Energy Build. 2015, 104, 215–232. [Google Scholar] [CrossRef]
- Hepbasli, A. Low exergy (LowEx) heating and cooling systems for sustainable buildings and societies. Renew. Sustain. Energy Rev. 2012, 16, 73–104. [Google Scholar] [CrossRef]
- Pu, W.H.; Yue, C.; Han, D.; He, W.F.; Liu, X.; Zhang, Q.; Chen, Y.T. Experimental study on Organic Rankine cycle for low grade thermal energy recovery. Appl. Therm. Eng. 2016, 94, 221–227. [Google Scholar] [CrossRef]
- Ziviani, D.; Beyene, A.; Venturini, M. Advances and challenges in ORC systems modeling for low grade thermal energy recovery. Appl. Energy 2014, 121, 79–95. [Google Scholar] [CrossRef]
- Bruckner, S.; Liu, S.L.; Miro, L.; Radspieler, M.; Cabeza, L.F.; Lavemanna, E. Industrial waste heat recovery technologies: An economic analysis of heat transformation technologies. Appl. Energy 2015, 151, 157–167. [Google Scholar] [CrossRef]
- Sari, A. Thermal reliability test of some fatty acids as PCMs used for solar thermal latent heat storage applications. Energy Convers. Manag. 2003, 44, 2277–2287. [Google Scholar] [CrossRef]
- Kenisarin, M.M. Thermophysical properties of some organic phase change materials for latent heat storage. A review. Sol. Energy 2014, 107, 553–575. [Google Scholar] [CrossRef]
- San, A.; Kaygusuz, K. Some fatty acids used for latent heat storage: Thermal stability and corrosion of metals with respect to thermal cycling. Renew. Energy 2003, 28, 939–948. [Google Scholar] [CrossRef]
- Sari, A. Eutectic mixtures of some fatty acids for latent heat storage: Thermal properties and thermal reliability with respect to thermal cycling. Energy Convers. Manag. 2006, 47, 1207–1221. [Google Scholar] [CrossRef]
- Keles, S.; Kaygusuz, K.; Sari, A. Lauric and myristic acids eutectic mixture as phase change material for low-temperature heating applications. Int. J. Energy Res. 2005, 29, 857–870. [Google Scholar] [CrossRef]
- Sari, A. Eutectic mixtures of some fatty acids for low temperature solar heating applications: Thermal properties and thermal reliability. Appl. Therm. Eng. 2005, 25, 2100–2107. [Google Scholar] [CrossRef]
- Liu, C.; Yuan, Y.P.; Zhang, N.; Cao, X.L.; Yang, X.J. A novel PCM of lauric-myristic-stearic acid/expanded graphite composite for thermal energy storage. Mater. Lett. 2014, 120, 43–46. [Google Scholar] [CrossRef]
| Material | Melting Temperature (°C) | Specific Heat (J·g−1·K−1) | Thermal Conductivity (W·m−1·K−1) | Latent Heat (J·g−1) | Density (g·m−3) |
|---|---|---|---|---|---|
| LA | 44.0–46.0 | 1.60 | 0.147 | 184.4 | 870.0 |
| SA | 55.0–69.0 | 2.35 | 0.172 | 259.0 | 941.0 |
| PCMs | 0 wt.% EG | 5 wt.% EG | 10 wt.% EG | 15 wt.% EG |
|---|---|---|---|---|
| Net weight (g) | 1428.2 | 1437.3 | 1444.8 | 1431.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Huang, G.; Jia, Z.; Gao, Q.; Li, Y.; Gu, Z. Eutectic Fatty Acids Phase Change Materials Improved with Expanded Graphite. Materials 2022, 15, 6856. https://doi.org/10.3390/ma15196856
Wang Z, Huang G, Jia Z, Gao Q, Li Y, Gu Z. Eutectic Fatty Acids Phase Change Materials Improved with Expanded Graphite. Materials. 2022; 15(19):6856. https://doi.org/10.3390/ma15196856
Chicago/Turabian StyleWang, Zanshe, Guoqiang Huang, Zhaoying Jia, Qi Gao, Yanping Li, and Zhaolin Gu. 2022. "Eutectic Fatty Acids Phase Change Materials Improved with Expanded Graphite" Materials 15, no. 19: 6856. https://doi.org/10.3390/ma15196856
APA StyleWang, Z., Huang, G., Jia, Z., Gao, Q., Li, Y., & Gu, Z. (2022). Eutectic Fatty Acids Phase Change Materials Improved with Expanded Graphite. Materials, 15(19), 6856. https://doi.org/10.3390/ma15196856







