Microstructure and Mechanical Properties of Hot-Extruded Mg–Zn–Ga–(Y) Biodegradable Alloys
Abstract
:1. Introduction
2. Materials and Methods
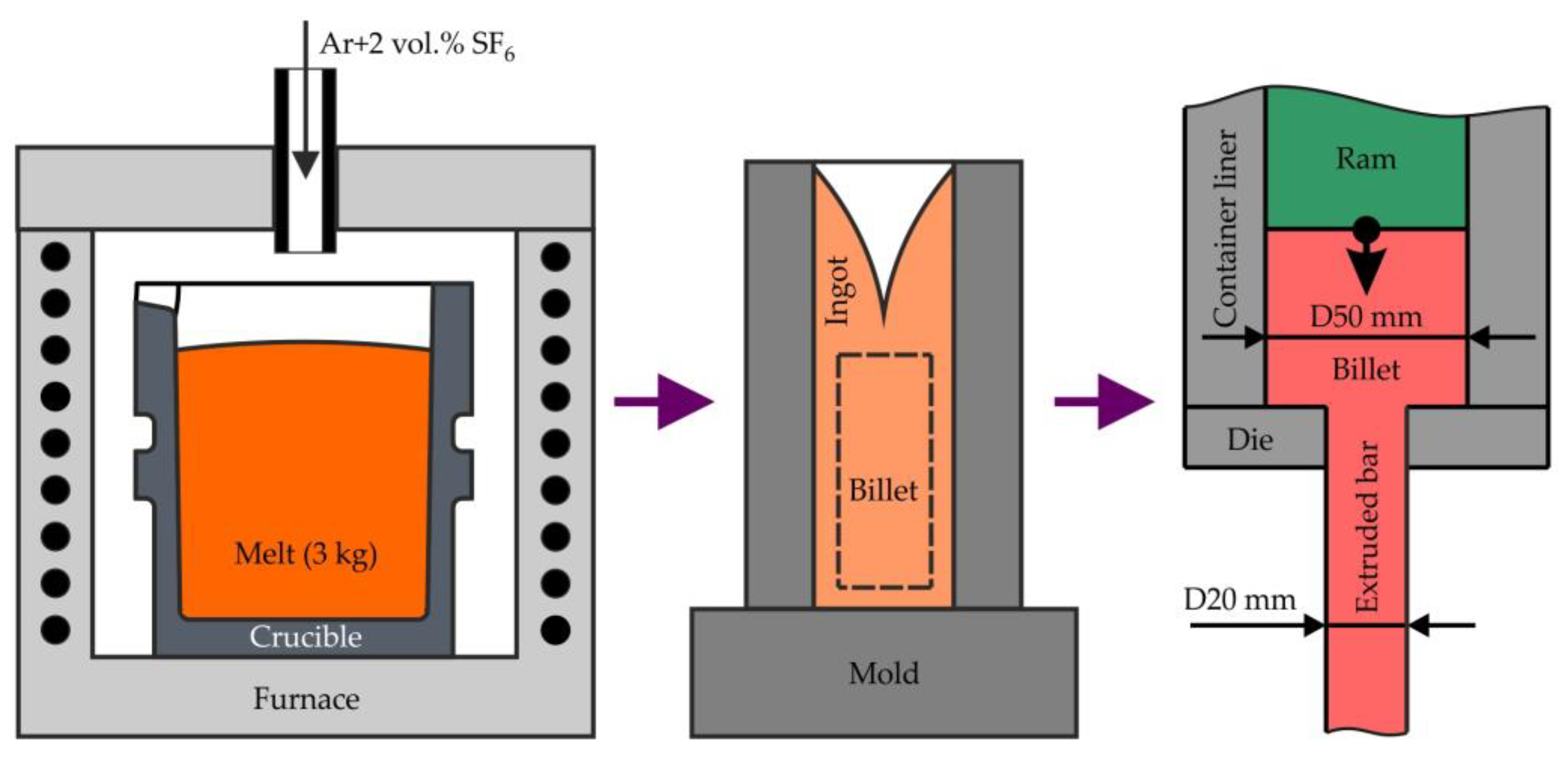
2.1. Alloy Preparation and Hot Extrusion
2.2. Microstructural Observations, Phase Composition, and Thermal Analysis
2.3. Mechanical Properties
3. Results and Discussion
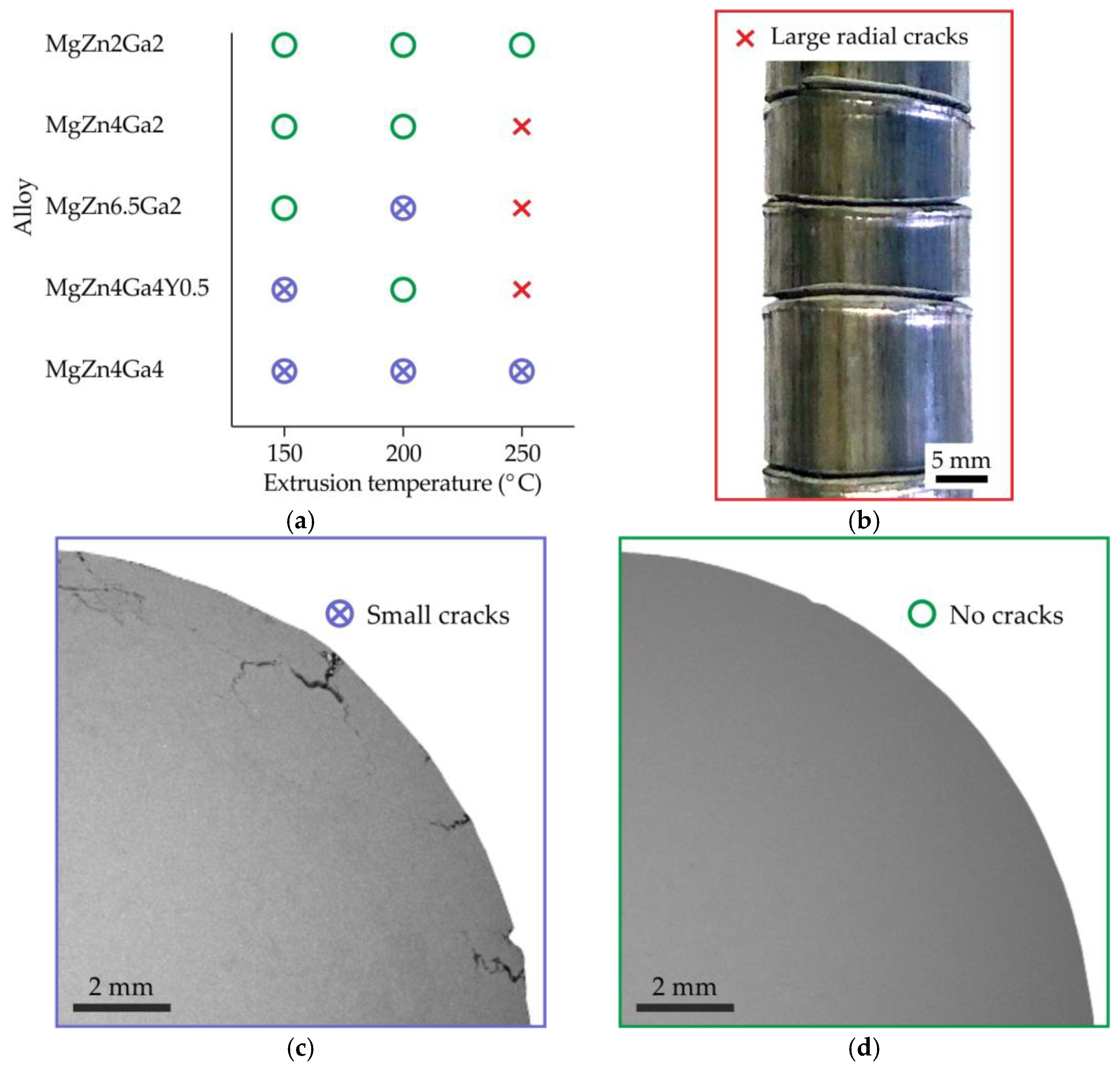
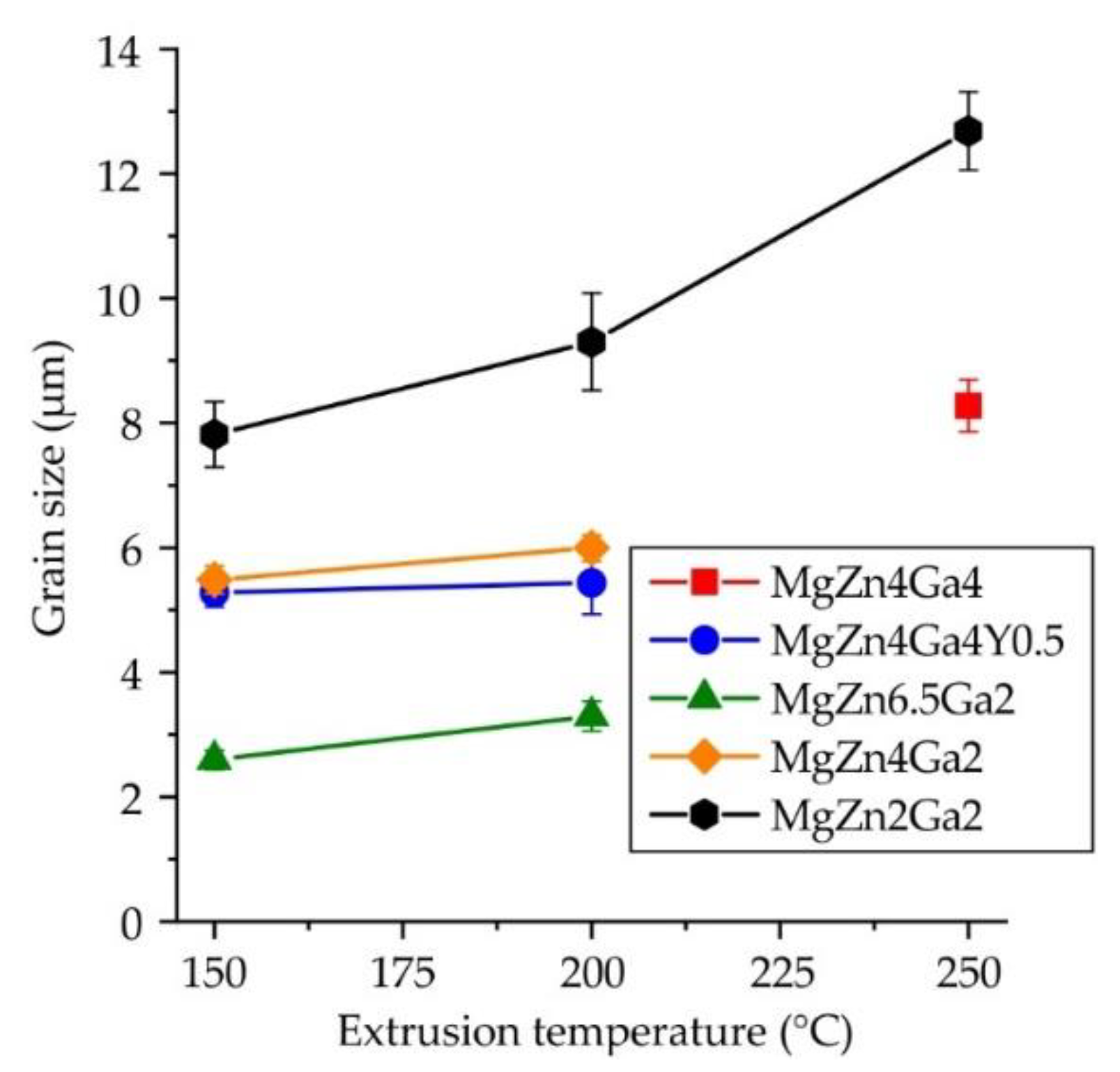
3.1. Quality of Extruded Bars
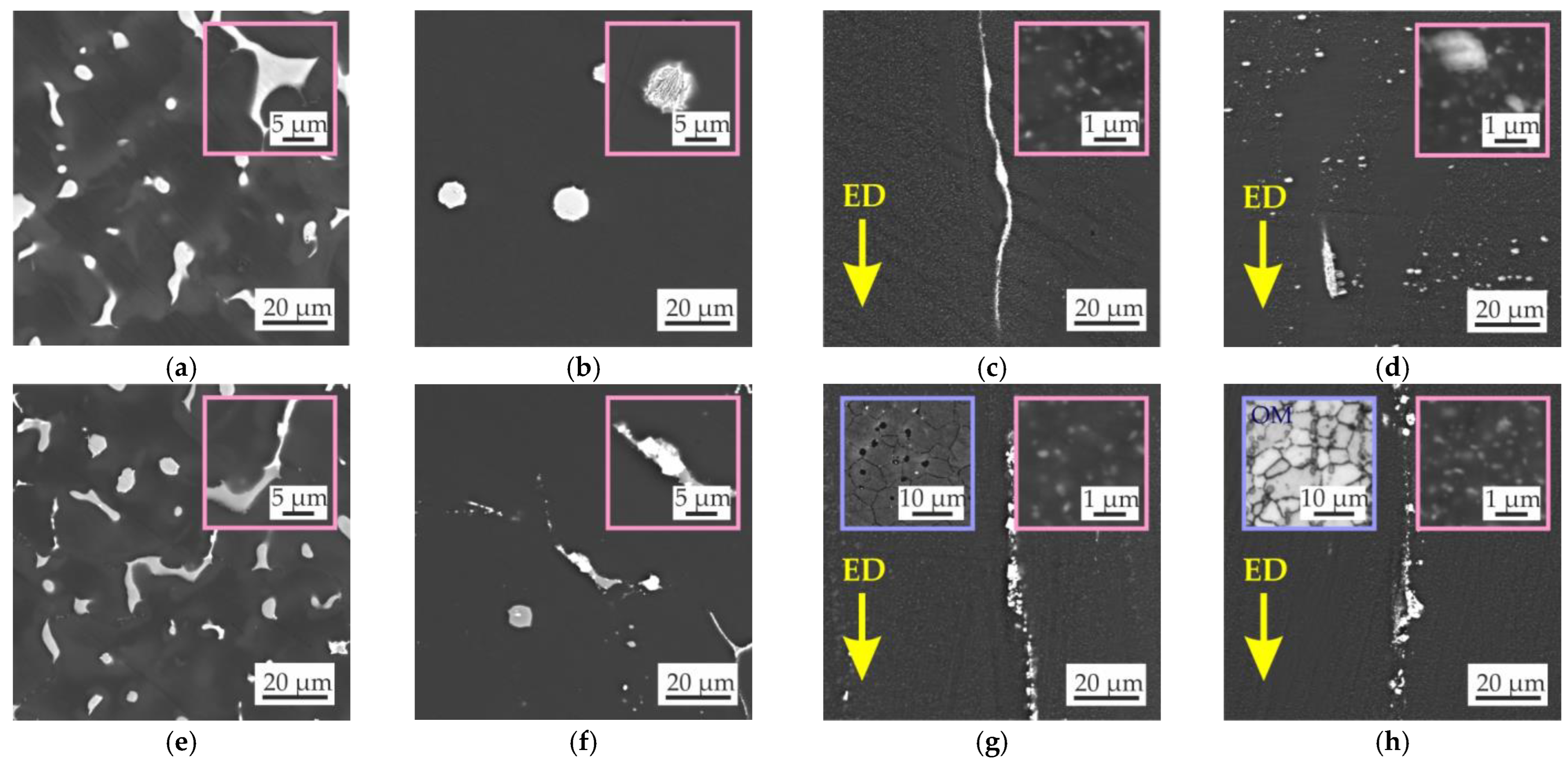
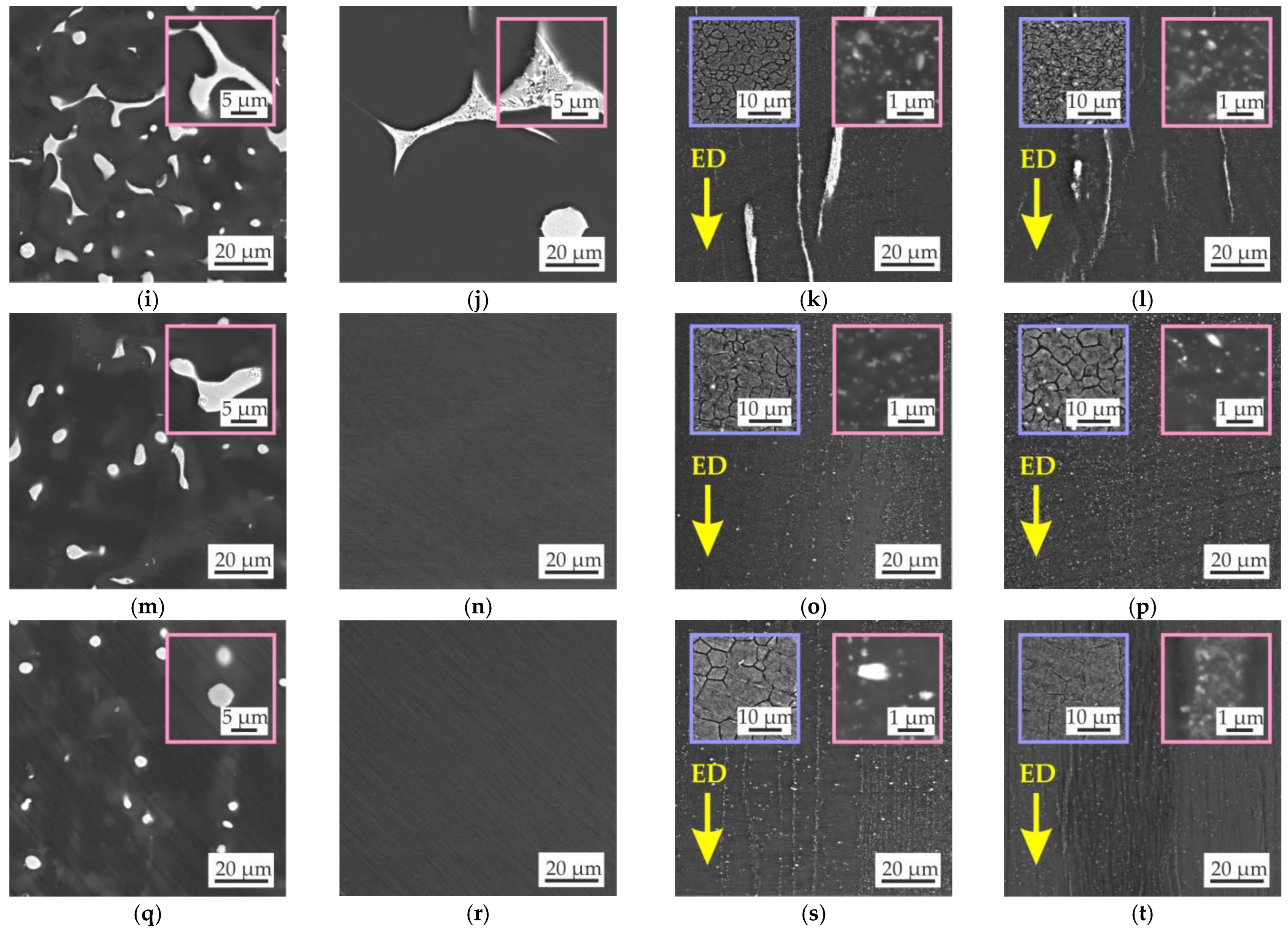
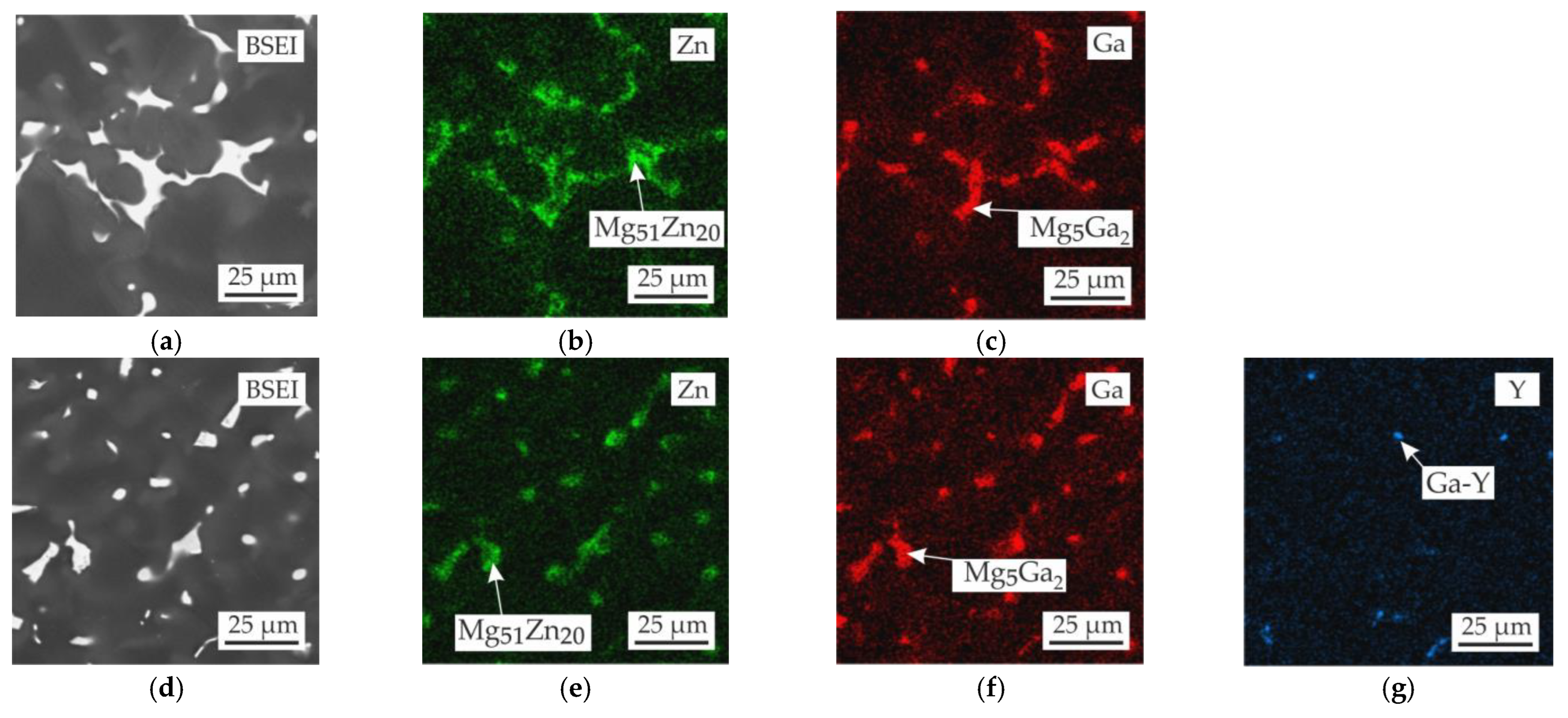
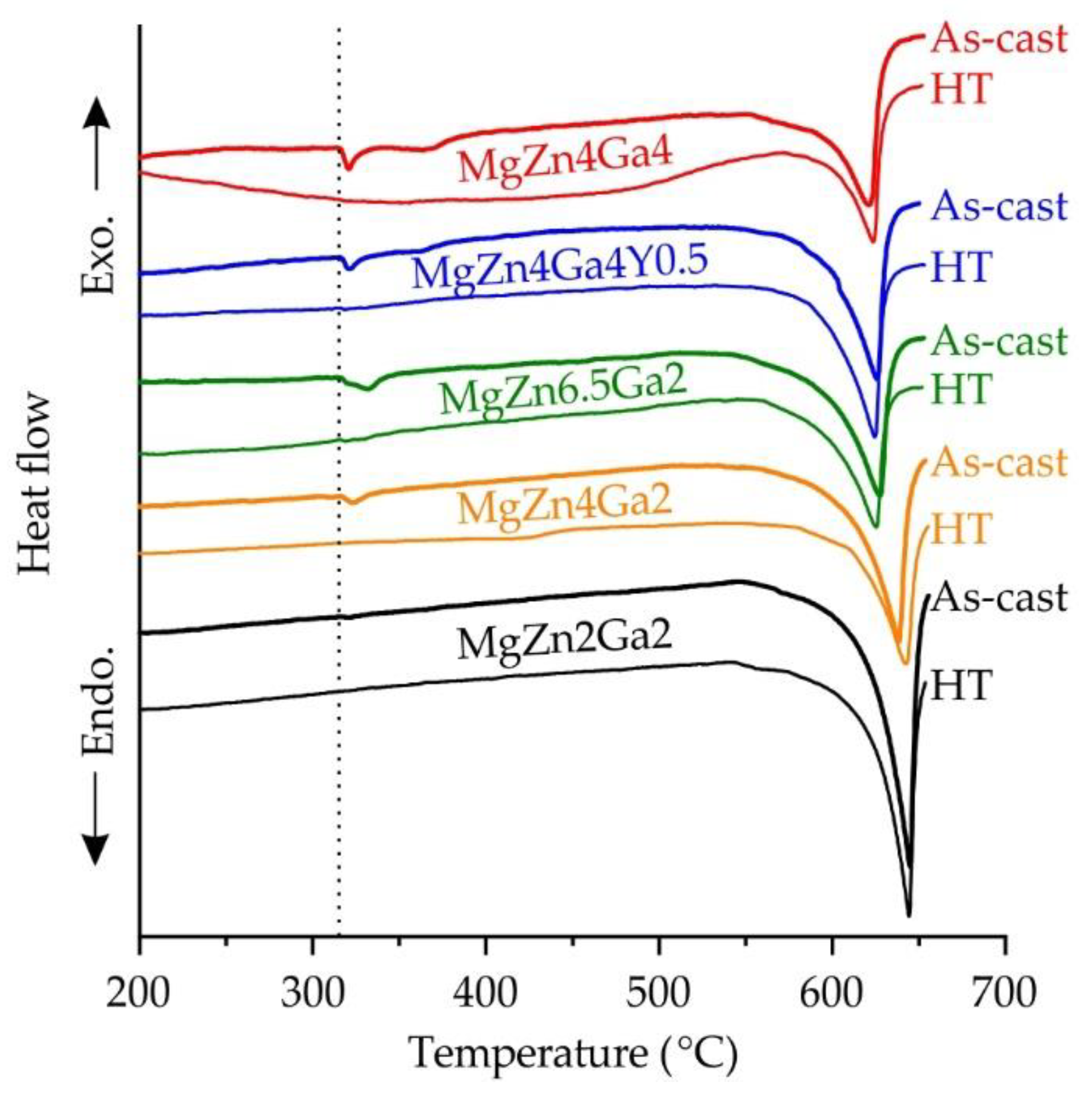
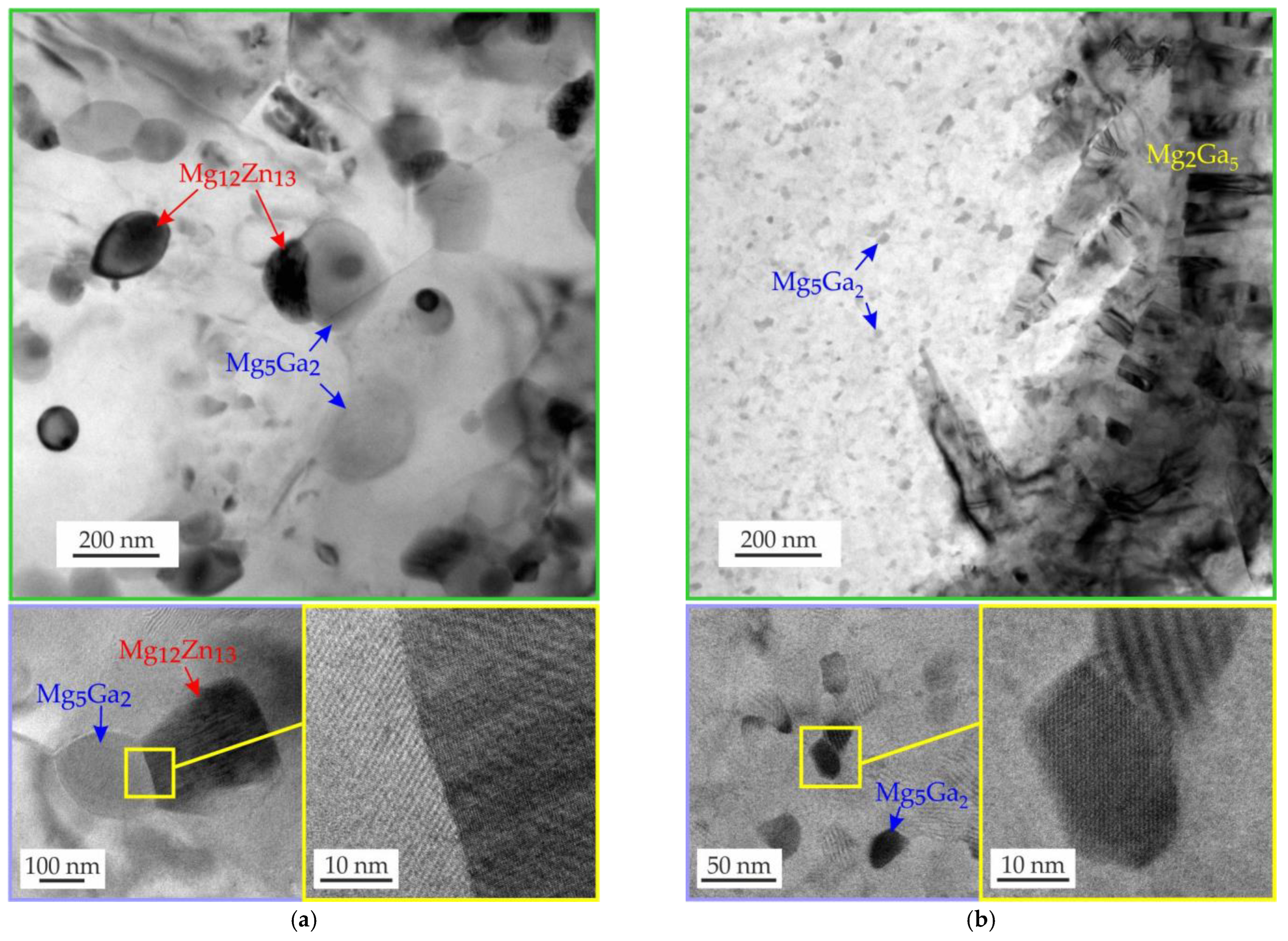
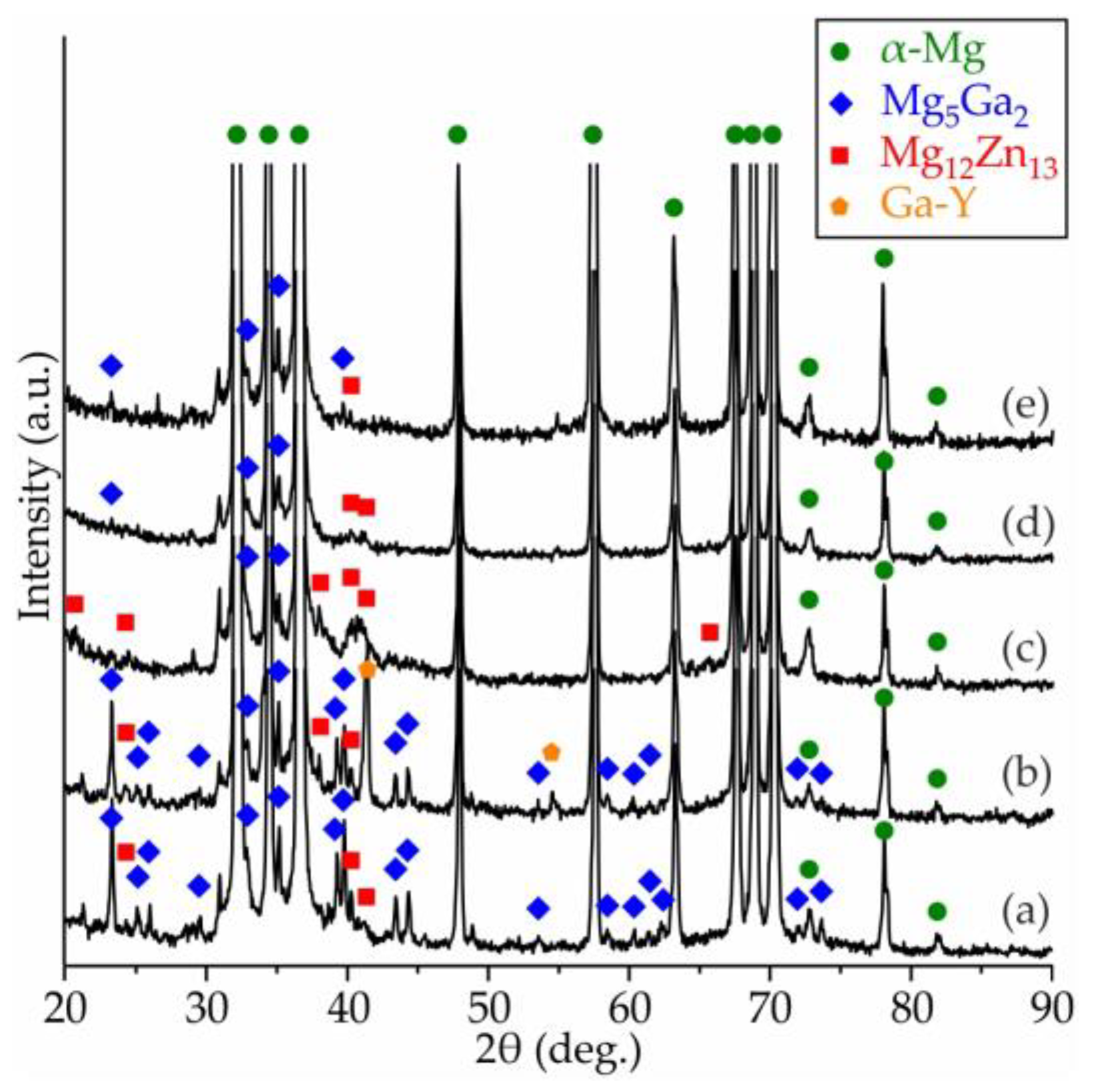
3.2. Microstructure and Phase Composition
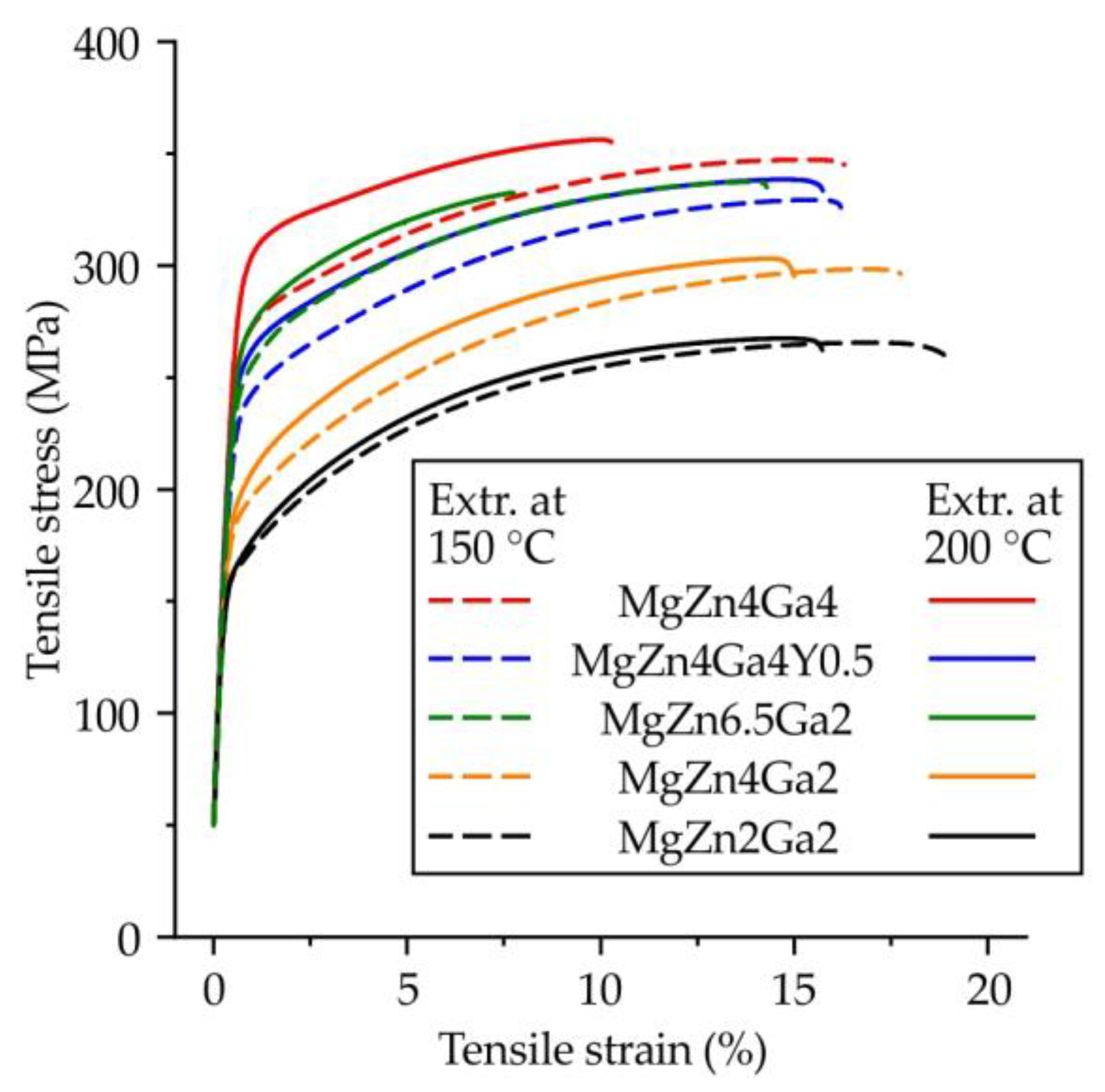
3.3. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gareb, B.; Van Bakelen, N.B.; Vissink, A.; Bos, R.R.M.; Van Minnen, B. Titanium or Biodegradable Osteosynthesis in Maxillofacial Surgery? In Vitro and In Vivo Performances. Polymers 2022, 14, 2782. [Google Scholar] [CrossRef] [PubMed]
- Vujović, S.; Desnica, J.; Stanišić, D.; Ognjanović, I.; Stevanovic, M.; Rosic, G. Applications of Biodegradable Magnesium-Based Materials in Reconstructive Oral and Maxillofacial Surgery: A Review. Molecules 2022, 27, 5529. [Google Scholar] [CrossRef] [PubMed]
- Kraus, T.; Fischerauer, S.F.; Hänzi, A.C.; Uggowitzer, P.J.; Löffler, J.F.; Weinberg, A.M. Magnesium alloys for temporary implants in osteosynthesis: In vivo studies of their degradation and interaction with bone. Acta Biomater. 2012, 8, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2010, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.-N.; Li, S.-S.; Li, X.-M.; Fan, Y.-B. Magnesium based degradable biomaterials: A review. Front. Mater. Sci. 2014, 8, 200–218. [Google Scholar] [CrossRef]
- Tran, N.T.; Kim, Y.-K.; Kim, S.-Y.; Lee, M.-H.; Lee, K.-B. Comparative Osteogenesis and Degradation Behavior of Magnesium Implant in Epiphysis and Diaphysis of the Long Bone in the Rat Model. Materials 2022, 15, 5630. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.-N.; Zheng, Y.-F. A review on magnesium alloys as biodegradable materials. Front. Mater. Sci. China 2010, 4, 111–115. [Google Scholar] [CrossRef]
- Nguyen, A.; Kunert, M.; Hort, N.; Schrader, C.; Weisser, J.; Schmidt, J. Cytotoxicity of the Ga-containing coatings on biodegradable magnesium alloys. Surf. Innov. 2015, 3, 10–19. [Google Scholar] [CrossRef]
- Rider, P.; Kačarević, Ž.P.; Elad, A.; Rothamel, D.; Sauer, G.; Bornert, F.; Windisch, P.; Hangyási, D.; Molnar, B.; Hesse, B.; et al. Biodegradation of a Magnesium Alloy Fixation Screw Used in a Guided Bone Regeneration Model in Beagle Dogs. Materials 2022, 15, 4111. [Google Scholar] [CrossRef]
- Delsmann, M.M.; Stürznickel, J.; Kertai, M.; Stücker, R.; Rolvien, T.; Rupprecht, M. Radiolucent Zones of Biodegradable Magnesium-Based Screws in Children and Adolescents—A Radiographic Analysis. Arch. Orthop. Trauma Surg. 2022, 2022, 1–9. [Google Scholar] [CrossRef]
- Kasinath, R.; Ernsberg, C.; Vass, S.; Ginn, S.N.; Qu, H.; Tong, W. Orthopedic Implant Having a Crystalline Gallium-Containing Hydroxyapatite Coating and Methods for Making the Same. US Patent No. 11,141,505, 21 January 2021. pp. 42–44. [Google Scholar]
- Melnikov, P.; Teixeira, A.R.; Malzac, A.; Coelho, M.d.B. Gallium-containing hydroxyapatite for potential use in orthopedics. Mater. Chem. Phys. 2009, 117, 86–90. [Google Scholar] [CrossRef]
- Ma, Z.; Fu, Q. Therapeutic Effect of Organic Gallium on Ovariectomized Osteopenic Rats by Decreased Serum Minerals and Increased Bone Mineral Content. Biol. Trace Elem. Res. 2010, 133, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, L.R. Mechanisms of Therapeutic Activity for Gallium. Pharmacol. Rev. 1998, 50, 665–682. [Google Scholar] [PubMed]
- Warrell, R.P., Jr.; Bockman, R.S.; Coonley, C.J.; Isaacs, M.; Staszewski, H. Gallium nitrate inhibits calcium resorption from bone and is effective treatment for cancer-related hypercalcemia. J. Clin. Investig. 1984, 73, 1487–1490. [Google Scholar] [CrossRef] [Green Version]
- Warrell, R.P., Jr.; Skelos, A.; Alcock, N.W.; Bockman, R.S. Gallium Nitrate for Acute Treatment of Cancer-related Hypercalcemia: Clinicopharmacological and Dose Response Analysis. Cancer Res. 1986, 46, 4208–4212. [Google Scholar]
- Warrell, R.P., Jr.; Israel, R.; Frisone, M.; Snyder, T.; Gaynor, J.J.; Bockman, R.S. Gallium Nitrate for Acute Treatment of Cancer-Related Hypercalcemia: A Randomized, Double-Blind Comparison to Calcitonin. Ann. Intern. Med. 1988, 108, 669–674. [Google Scholar] [CrossRef]
- Warrell, R.P., Jr.; Bosco, B.; Weinerman, S.; Levine, B.; Lane, J.; Bockman, R.S. Gallium Nitrate for Advanced Paget Disease of Bone: Effectiveness and Dose-Response Analysis. Ann. Intern. Med. 1990, 113, 847–851. [Google Scholar] [CrossRef] [Green Version]
- Matkovic, V.; Apseloff, G.; Shepard, D.R.; Gerber, N. Use of gallium to treat Paget’s disease of bone: A pilot study. Lancet 1990, 335, 72–75. [Google Scholar] [CrossRef]
- Niesvizky, R. Gallium nitrate in multiple myeloma: Prolonged survival in a cohort of patients with advanced-stage disease. Semin. Oncol. 2003, 30, 20–24. [Google Scholar] [CrossRef]
- Verron, E.; Masson, M.; Khoshniat, S.; Duplomb, L.; Wittrant, Y.; Baud’huin, M.; Badran, Z.; Bujoli, B.; Janvier, P.; Scimeca, J.-C.; et al. Gallium modulates osteoclastic bone resorption in vitro without affecting osteoblasts. Br. J. Pharmacol. 2010, 159, 1681–1692. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, R.; Bockman, R.S.; Doty, S.B.; Boskey, A.L. Bone particles from gallium-treated rats are resistant to resorption in vivo. Bone Miner. 1991, 12, 167–179. [Google Scholar] [CrossRef]
- Blair, H.C.; Teitelbaum, S.L.; Tan, H.-L.; Schlesinger, P.H. Reversible inhibition of osteoclastic activity by bone-bound gallium (III). J. Cell. Biochem. 1992, 48, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.F.; Islam, M.T.; Saheb, N.; Baig, M.M.A. Magnesium for Implants: A Review on the Effect of Alloying Elements on Biocompatibility and Properties. Materials 2022, 15, 5669. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Saxena, K.K.; Malik, V.; Mohammed, K.A.; Prakash, C.; Buddhi, D.; Dixit, S. Significance of Alloying Elements on the Mechanical Characteristics of Mg-Based Materials for Biomedical Applications. Crystals 2022, 12, 1138. [Google Scholar] [CrossRef]
- Amukarimi, S.; Mozafari, M. Biodegradable Magnesium Biomaterials—Road to the Clinic. Bioengineering 2022, 9, 107. [Google Scholar] [CrossRef]
- Liu, H.; Qi, G.; Ma, Y.; Hao, H.; Jia, F.; Ji, S.; Zhang, H.; Zhang, X. Microstructure and mechanical property of Mg–2.0Ga alloys. Mater. Sci. Eng. A 2009, 526, 7–10. [Google Scholar] [CrossRef]
- Huang, W.; Chen, J.; Yan, H.; Xia, W.; Su, B.; Zhu, W. Effects of Ga Content on Dynamic Recrystallization and Mechanical Properties of High Strain Rate Rolled Mg–Ga Alloys. Met. Mater. Int. 2020, 26, 747–759. [Google Scholar] [CrossRef]
- Huang, W.; Chen, J.; Yan, H.; Xia, W.; Su, B.; Yin, H.; Yan, X. Microstructure, texture modification and mechanical anisotropy of high strain rate rolled Mg–Ga alloy sheets. J. Mater. Sci. 2020, 55, 10242–10257. [Google Scholar] [CrossRef]
- Hernández-Cortés, A.A.; Escobedo-Bocardo, J.C.; Cortés-Hernández, D.A.; Almanza-Robles, J.M. Effect of Gallium Content and Heat Treatment on the Microstructure and Corrosion Rate of Magnesium Binary Alloys. Metals 2019, 9, 990. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Chen, J.; Yan, H.; Xia, W. Ga alloying for fabricating magnesium alloy sheet with uniform microstructure and excellent performance. Mater. Lett. 2021, 304, 130607. [Google Scholar] [CrossRef]
- Yin, H.; Chen, J.; Yan, H.; Xia, W.; Su, B.; Huang, W.; Yan, X. Effects of Zn addition on precipitation behavior and mechanical properties of Mg-5Ga alloy. Mater. Lett. 2021, 291, 129495. [Google Scholar] [CrossRef]
- Bazhenov, V.; Koltygin, A.; Komissarov, A.; Li, A.; Bautin, V.; Khasenova, R.; Anishchenko, A.; Seferyan, A.; Komissarova, J.; Estrin, Y. Gallium-containing magnesium alloy for potential use as temporary implants in osteosynthesis. J. Magnes. Alloy. 2020, 8, 352–363. [Google Scholar] [CrossRef]
- Tan, Q.; Atrens, A.; Mo, N.; Zhang, M.X. Oxidation of magnesium alloys at elevated temperatures in air: A review. Corros. Sci. 2016, 112, 734–759. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Z.; Stanford, N.; Davies, C.H.J.; Nie, J.-F.; Birbilis, N. Magnesium Extrusion Alloys: A Review of Developments and Prospects. Int. Mater. Rev. 2019, 64, 27–62. [Google Scholar] [CrossRef]
- Bazhenov, V.; Lyskovich, A.; Li, A.; Bautin, V.; Komissarov, A.; Koltygin, A.; Bazlov, A.; Tokar, A.; Ten, D.; Mukhametshina, A. Effect of Heat Treatment on the Mechanical and Corrosion Properties of Mg–Zn–Ga Biodegradable Mg Alloys. Materials 2021, 14, 7847. [Google Scholar] [CrossRef] [PubMed]
- Scheil, E. Bemerkungen zur Schichtkristallbildung. Z. Für Met. 1942, 34, 70–72. [Google Scholar] [CrossRef]
- Bazhenov, V.E.; Li, A.V.; Komissarov, A.A.; Koltygin, A.V.; Tavolzhanskii, S.A.; Bautin, V.A.; Voropaeva, O.O.; Mukhametshina, A.M.; Tokar, A.A. Microstructure and mechanical and corrosion properties of hot-extruded Mg–Zn–Ca–(Mn) biodegradable alloys. J. Magnes. Alloy. 2021, 9, 1428–1442. [Google Scholar] [CrossRef]
- Predel, B. Mg-Zn (Magnesium-Zinc). In Li-Mg–Nd-Zr. Landolt-Börnstein-Group IV Physical Chemistry (Numerical Data and Functional Relationships in Science and Technology; Madelung, O., Ed.; Springer: Berlin, Germany, 1997; Volume 5H. [Google Scholar] [CrossRef]
- Okamoto, H. Ga-Mg (Gallium-Magnesium). J. Phase Equilibria Diffus. 2013, 34, 148. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.B.; Jeong, J.; Oh, S.H. Critical evaluation and thermodynamic optimization of Mg–Ga system and effect of low pressure on phase equilibria. Calphad 2014, 46, 168–175. [Google Scholar] [CrossRef]
- Okamoto, H. Supplemental Literature Review of Binary Phase Diagrams: Al-Pt, As-U, C-Li, C-Mg, Cd-Nd, Co-Ta, Fe-Re, Ga-Y, La-Ni, O-V, P-Si, and Re-Zr. J. Phase Equilibria Diffus. 2020, 41, 722–733. [Google Scholar] [CrossRef]
- Robson, J.D.; Henry, D.T.; Davis, B. Particle effects on recrystallization in magnesium–manganese alloys: Particle-stimulated nucleation. Acta Mater. 2009, 57, 2739–2747. [Google Scholar] [CrossRef]
- Nes, E.; Ryum, N.; Hunderi, O. On the Zener drag. Acta Metall. 1985, 33, 11–22. [Google Scholar] [CrossRef]
- Jiang, M.G.; Xu, C.; Nakata, T.; Yan, H.; Chen, R.S.; Kamado, S. High-speed extrusion of dilute Mg-Zn-Ca-Mn alloys and its effect on microstructure, texture and mechanical properties. Mater. Sci. Eng. A 2016, 678, 329–338. [Google Scholar] [CrossRef] [Green Version]
- Yin, S.M.; Wang, C.H.; Diao, Y.D.; Wu, S.D.; Li, S.X. Influence of Grain Size and Texture on the Yield Asymmetry of Mg-3Al-1Zn Alloy. J. Mater. Sci. Technol. 2011, 27, 29–34. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Y.; Kolawole, S.K.; Wang, J.; Su, X.; Tan, L.; Yang, K. Systems, Properties, Surface Modification and Applications of Biodegradable Magnesium-Based Alloys: A Review. Materials 2022, 15, 5031. [Google Scholar] [CrossRef]
- Kubásek, J.; Vojtěch, D.; Dvorskỳ, D. Structural and mechanical study on Mg–xLM (x = 0–5 wt.%, LM = Sn, Ga) alloys. Int. J. Mater. Res. 2016, 107, 459–471. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef]



| Alloy | Element Content (wt.%) | |||
|---|---|---|---|---|
| Mg | Zn | Ga | Y | |
| MgZn4Ga4 | Bal. | 4.2 | 4.1 | - |
| MgZn4Ga4Y0.5 | Bal. | 4.2 | 4.1 | 0.4 |
| MgZn6.5Ga2 | Bal. | 6.5 | 2.0 | - |
| MgZn4Ga2 | Bal. | 4.2 | 2.2 | - |
| MgZn2Ga2 | Bal. | 2.3 | 2.3 | - |
| Alloy | Tliq.Sch (°C) | Tsol.eq (°C) | Meut.Sch (wt.%) | Eq. Phase Amount at RT (wt.%) | Eq. Precipit. Start. Temp. (°C) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mg5Ga2 | Mg12Zn13 | I | Mg5Ga2 | Mg12Zn13 | I | ||||
| MgZn4Ga4 | 624 | 370.5 | 9.82 | 7.5 | 5.41 | - | 266 | 258 | - |
| MgZn4Ga4Y0.5 | 623 | 412 | 6.58 | 7.5 | 3.05 | 2.49 | 264 | 189 | 434 |
| MgZn6.5Ga2 | 623 | 334 | 8.02 | 3.57 | 8.5 | - | 182 | 321 | - |
| MgZn4Ga2 | 630 | 421 | 8.70 | 3.94 | 5.41 | - | 188 | 250 | - |
| MgZn2Ga2 | 636 | 497 | 5.05 | 4.12 | 2.85 | - | 189.5 | 174 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazhenov, V.; Li, A.; Tavolzhanskii, S.; Bazlov, A.; Tabachkova, N.; Koltygin, A.; Komissarov, A.; Shin, K.S. Microstructure and Mechanical Properties of Hot-Extruded Mg–Zn–Ga–(Y) Biodegradable Alloys. Materials 2022, 15, 6849. https://doi.org/10.3390/ma15196849
Bazhenov V, Li A, Tavolzhanskii S, Bazlov A, Tabachkova N, Koltygin A, Komissarov A, Shin KS. Microstructure and Mechanical Properties of Hot-Extruded Mg–Zn–Ga–(Y) Biodegradable Alloys. Materials. 2022; 15(19):6849. https://doi.org/10.3390/ma15196849
Chicago/Turabian StyleBazhenov, Viacheslav, Anna Li, Stanislav Tavolzhanskii, Andrey Bazlov, Natalia Tabachkova, Andrey Koltygin, Alexander Komissarov, and Kwang Seon Shin. 2022. "Microstructure and Mechanical Properties of Hot-Extruded Mg–Zn–Ga–(Y) Biodegradable Alloys" Materials 15, no. 19: 6849. https://doi.org/10.3390/ma15196849
APA StyleBazhenov, V., Li, A., Tavolzhanskii, S., Bazlov, A., Tabachkova, N., Koltygin, A., Komissarov, A., & Shin, K. S. (2022). Microstructure and Mechanical Properties of Hot-Extruded Mg–Zn–Ga–(Y) Biodegradable Alloys. Materials, 15(19), 6849. https://doi.org/10.3390/ma15196849










