Reaching Visible Light Photocatalysts with Pt Nanoparticles Supported in TiO2-CeO2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Support Preparation
2.2.1. Impregnation Synthesis
2.2.2. Sol-Gel Synthesis
2.3. Pt Catalysts Preparation
2.4. Characterization Techniques
2.5. Photocatalytic Reaction
3. Results and Discussion
3.1. Specific Area by the BET Method
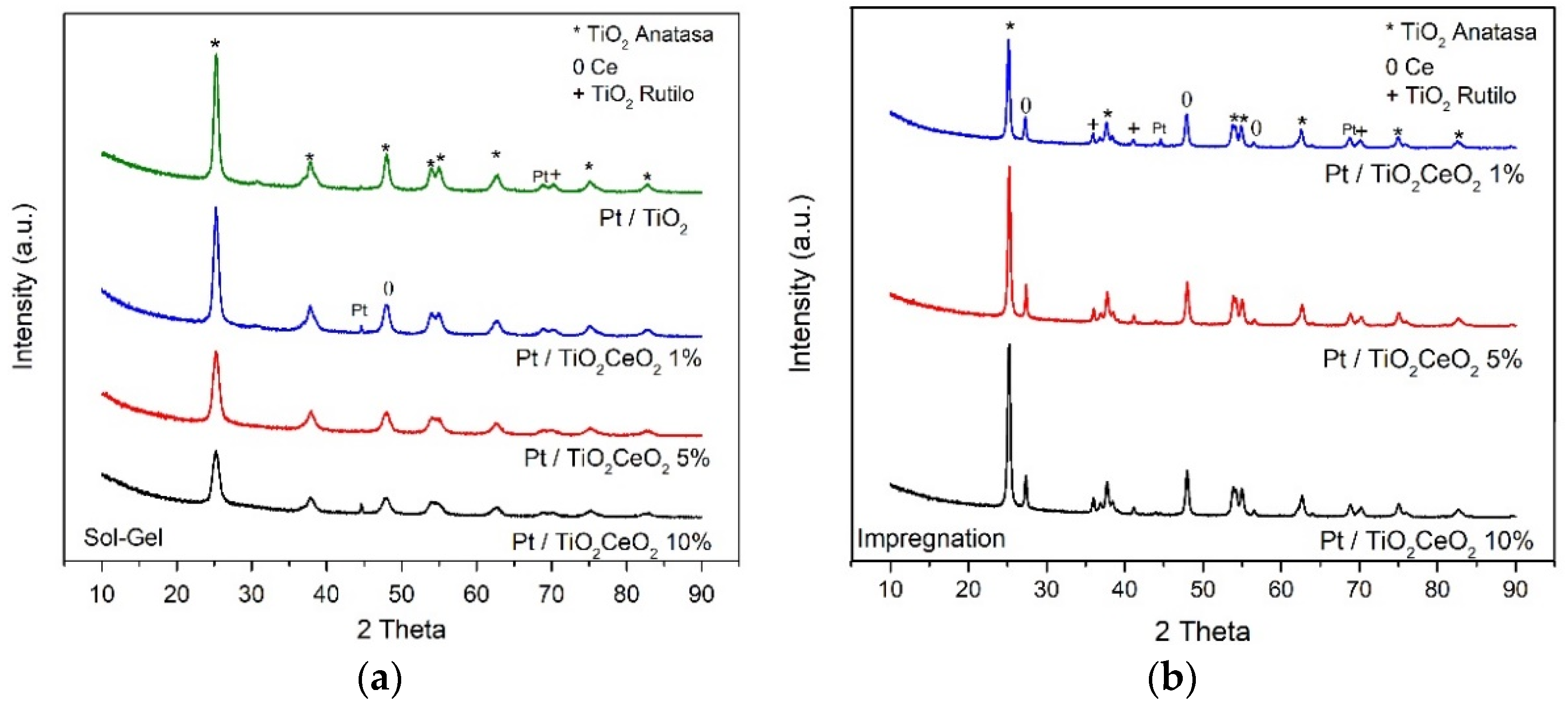
3.2. X-ray Diffraction (XRD)
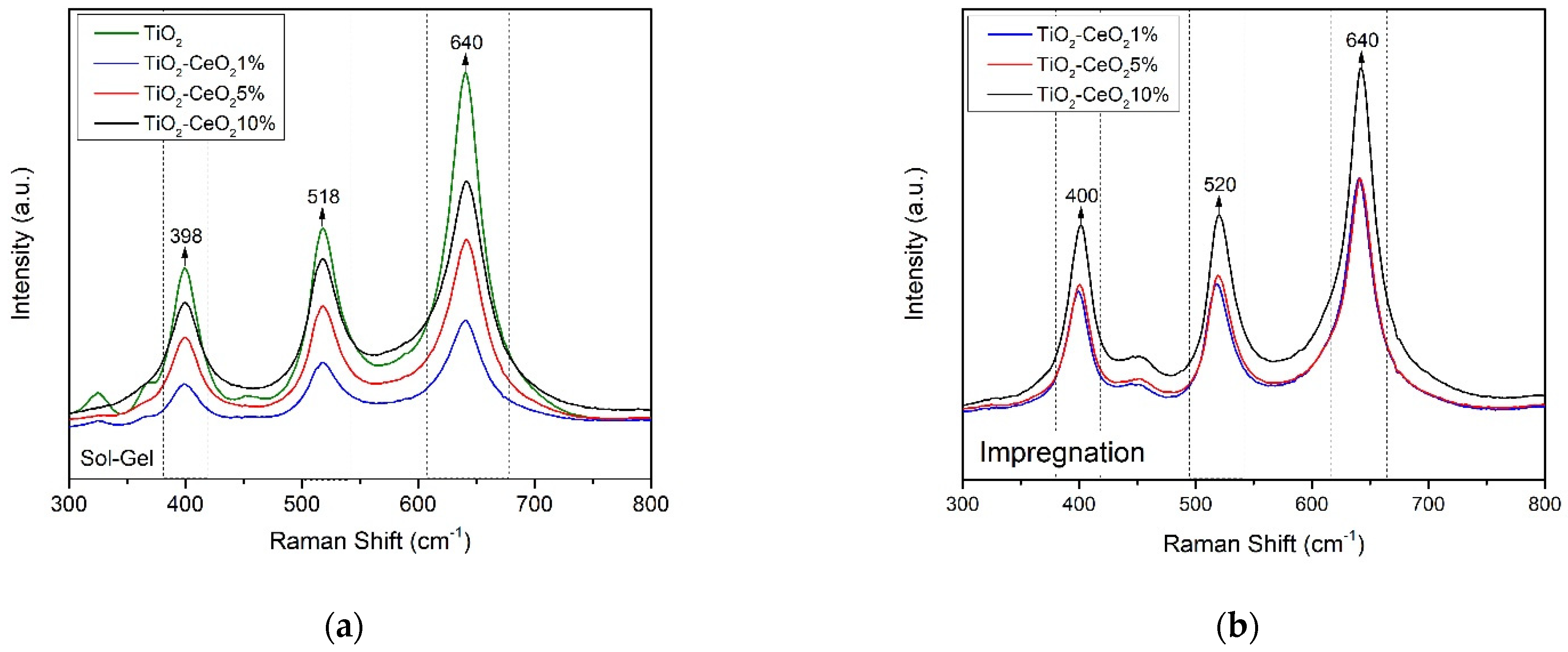
3.3. Raman Spectroscopy
3.4. Transmission Electron Microscope (TEM)
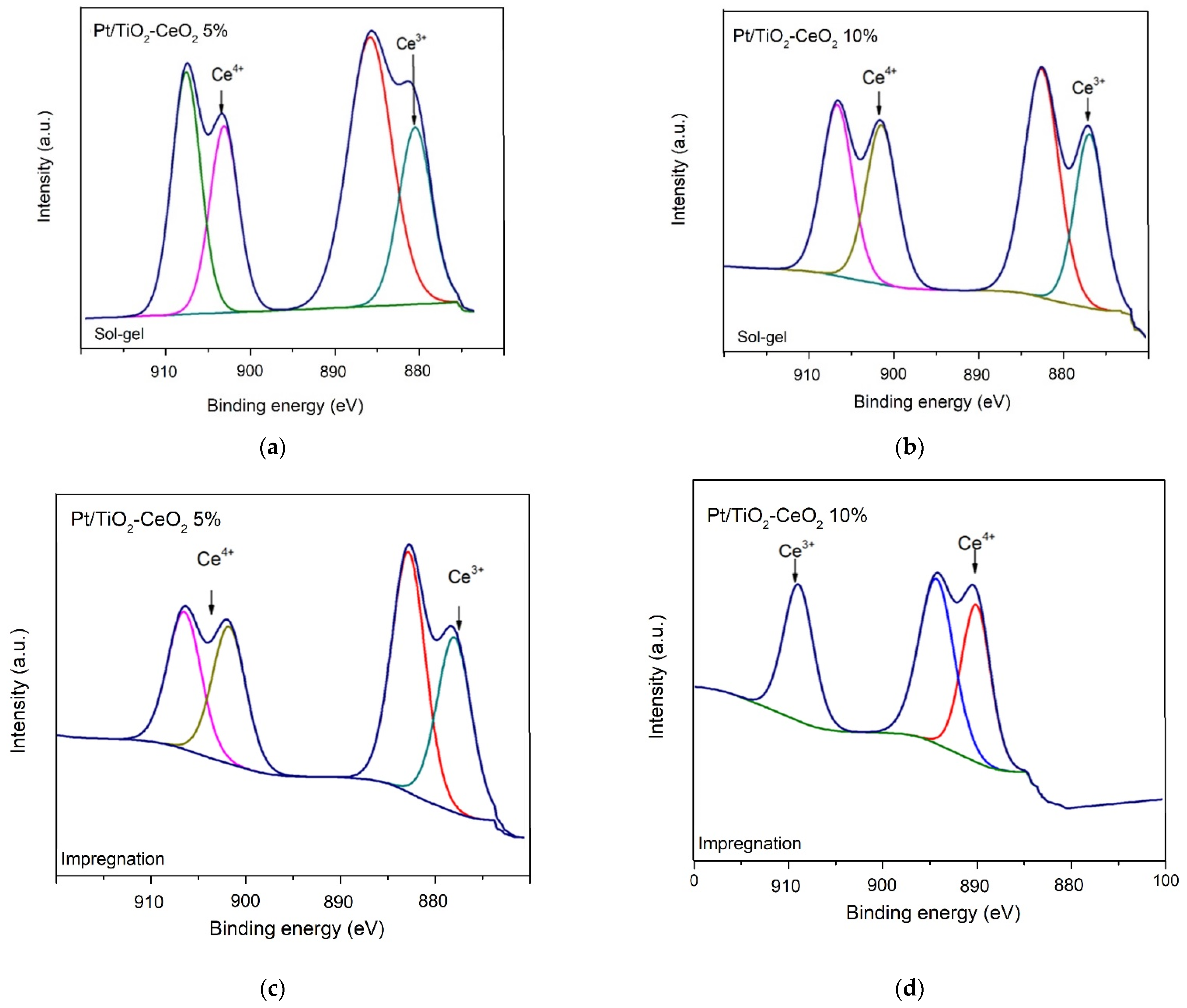
3.5. X-ray Photoelectron Spectrometry (XPS)
3.6. UV–Vis for Band Gap
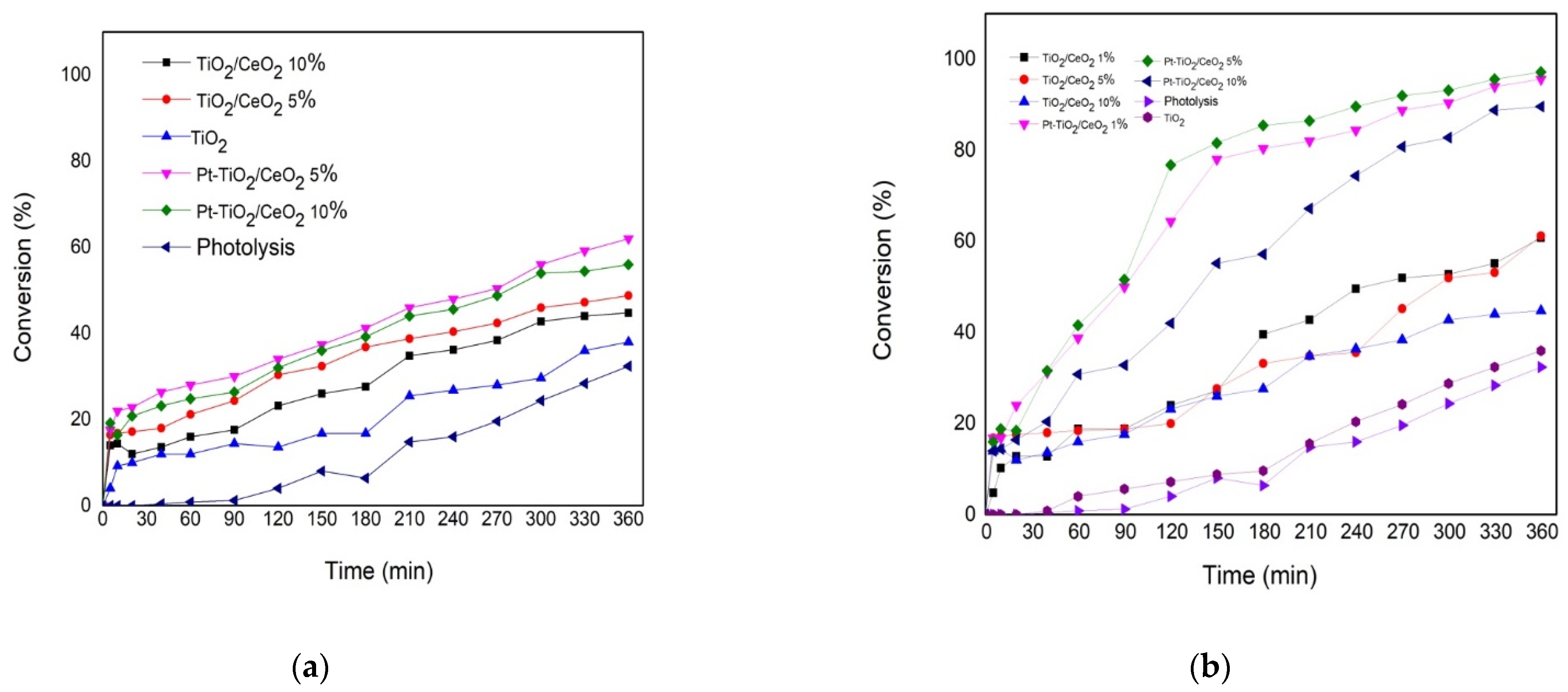
3.7. Photocatalysts Degradation of 2,4-Dichlorophenoxyacetic Acid
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gopinath, K.; Madhav, N.; Krishnan, A.; Malolan, R.; Rangarajan, G. Present applications of titanium dioxide for the photocatalytic removal of pollutants from water: A review. J. Environ. Manag. 2020, 270, 110906. [Google Scholar] [CrossRef] [PubMed]
- Borges, M.E.; Sierra, M.; Cuevas, E.; García, R.D.; Esparza, P. Photocatalysis with solar energy: Sunlight-responsive photocatalyst based on TiO2 loaded on a natural material for wastewater treatment. Sol. Energy 2016, 135, 527–535. [Google Scholar] [CrossRef]
- Van Deelen, T.W.; Hernández Mejía, C.; de Jong, K.P. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2019, 2, 955–970. [Google Scholar] [CrossRef]
- Yunarti, R.T.; Isa, I.D.; Dimonti, L.C.C.; Dwiatmoko, A.A.; Ridwan, M.; Ha, J.-M. Study of Ag2O/TiO2 nanowires synthesis and characterization for heterogeneous reduction reaction catalysis of 4-nitrophenol. Nano-Struct. Nano-Objects 2021, 26, 100719. [Google Scholar] [CrossRef]
- Ayesha, B.; Jabeen, U.; Naeem, A.; Kasi, P.; Malghani, M.N.K.; Khan, S.U.; Akhtar, J.; Aamir, M. Synthesis of zinc stannate nanoparticles by sol-gel method for photocatalysis of commercial dyes. Results Chem. 2020, 2, 100023. [Google Scholar] [CrossRef]
- Fonseca-Cervantes, O.; Pérez-Larios, A.; Romero Arellano, V.; Sulbaran-Rangel, B.; Guzmán González, C. Effects in Band Gap for Photocatalysis in TiO2 Support by Adding Gold and Ruthenium. Processes 2020, 8, 1032. [Google Scholar] [CrossRef]
- Pragathiswaran, C.; Smitha, C.; Mahin Abbubakkar, B.; Govindhan, P.; Anantha Krishnan, N. Synthesis and characterization of TiO2/ZnO–Ag nanocomposite for photocatalytic degradation of dyes and anti-microbial activity. Mater. Today Proc. 2021, 45, 3357–3364. [Google Scholar] [CrossRef]
- Tian, J.; Sang, Y.; Zhao, Z.; Zhou, W.; Wang, D.; Kang, X.; Liu, H.; Wang, J.; Chen, S.; Cai, H.; et al. Enhanced Photocatalytic Performances of CeO2/TiO2 Nanobelt Heterostructures. Small 2013, 9, 3864–3872. [Google Scholar] [CrossRef]
- Chatterjee, D.; Dasgupta, S. Visible light indu ced photocatalytic degradation of organic pollutants. J. Photochem. Photobiol. C Photochem. Rev. 2005, 6, 186–205. [Google Scholar] [CrossRef]
- Matte, L.P.; Kilian, A.S.; Luza, L.; Alves, M.C.M.; Morais, J.; Baptista, D.L.; Dupont, J.; Bernardi, F. Influence of the CeO2 Support on the Reduction Properties of Cu/CeO2 and Ni/CeO2 Nanoparticles. J. Phys. Chem. C 2015, 119, 26459–26470. [Google Scholar] [CrossRef]
- Bellardita, M.; Fiorenza, R.; Urso, L.; Spitaleri, L.; Gulino, A.; Compagnini, G.; Scirè, S.; Palmisano, L. Exploring the Photothermo-Catalytic Performance of Brookite TiO2-CeO2 Composites. Catalysts 2020, 10, 765. [Google Scholar] [CrossRef]
- Henych, J.; Šťastný, M.; Němečková, Z.; Mazanec, K.; Tolasz, J.; Kormunda, M.; Ederer, J.; Janoš, P. Bifunctional TiO2/CeO2 reactive adsorbent/photocatalyst for degradation of bis-p-nitrophenyl phosphate and CWAs. Chem. Eng. J. 2021, 414, 128822. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, X.; Zhang, N.; Zhao, Q.; He, X.; Feng, J. Photocatalytic mechanism of TiO2-CeO2 films prepared by magnetron sputtering under UV and visible light. Surf. Sci. 2005, 595, 203–211. [Google Scholar] [CrossRef]
- Gnanasekaran, L.; Rajendran, S.; Priya, A.K.; Durgalakshmi, D.; Vo, D.-V.N.; Cornejo-Ponce, L.; Gracia, F.; Soto-Moscoso, M. Photocatalytic degradation of 2,4-dichlorophenol using bio-green assisted TiO2-CeO2 nanocomposite system. Environ. Res. 2021, 195, 110852. [Google Scholar] [CrossRef]
- García-Domínguez, Á.E.; Torres-Torres, G.; Arévalo-Pérez, J.C.; Silahua-Pavón, A.; Sánchez-Trinidad, C.; Godavarthi, S.; Ojeda-López, R.; Sierra-Gómez, U.A.; Cervantes-Uribe, A. Urea assisted synthesis of TiO2-CeO2 composites for photocatalytic acetaminophen degradation via simplex-centroid mixture design. Results Eng. 2022, 14, 100443. [Google Scholar] [CrossRef]
- Petrović, S.; Stanković, M.; Pavlović, S.; Mojović, Z.; Radić, N.; Mojović, M.; Rožić, L. Nickel oxide on mechanochemically synthesized TiO2-CeO2: Photocatalytic and electrochemical activity. React. Kinet. Mech. Catal. 2021, 133, 1097–1110. [Google Scholar] [CrossRef]
- Khan, M.A.R.; Mamun, M.S.A.; Ara, M.H. Review on platinum nanoparticles: Synthesis, characterization, and applications. Microchem. J. 2021, 171, 106840. [Google Scholar] [CrossRef]
- Jeyaraj, M.; Gurunathan, S.; Qasim, M.; Kang, M.-H.; Kim, J.-H. A Comprehensive Review on the Synthesis, Characterization, and Biomedical Application of Platinum Nanoparticles. Nanomaterials 2019, 9, 1719. [Google Scholar] [CrossRef]
- Guzmán, C.; del Ángel, G.; Gómez, R.; Galindo-Hernández, F.; Ángeles-Chavez, C. Degradation of the herbicide 2,4-dichlorophenoxyacetic acid over Au/TiO2-CeO2 photocatalysts: Effect of the CeO2 content on the photoactivity. Catal. Today 2011, 166, 146–151. [Google Scholar] [CrossRef]
- Guzmán, C.; Del Angel, G.; Fierro, J.L.G.; Bertin, V. Role of Pt Oxidation State on the Activity and Selectivity for Crotonaldehyde Hydrogenation Over Pt–Sn/Al2O3–La and Pt–Pb/Al2O3–La Catalysts. Top. Catal. 2010, 53, 1142–1144. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. Understanding Polymorphic Phase Transformation Behavior during Growth of Nanocrystalline Aggregates: Insights from TiO2. J. Phys. Chem. B 2000, 104, 3481–3487. [Google Scholar] [CrossRef]
- He, Z.; Cai, Q.; Fang, H.; Situ, G.; Qiu, J.; Song, S.; Chen, J. Photocatalytic activity of TiO2 containing anatase nanoparticles and rutile nanoflower structure consisting of nanorods. J. Environ. Sci. 2013, 25, 2460–2468. [Google Scholar] [CrossRef]
- Rocha-Ortiz, G.; Tessensohn, M.E.; Salas-Reyes, M.; Flores-Moreno, R.; Webster, R.D.; Astudillo-Sánchez, P.D. Homogeneous electron-transfer reaction between anionic species of anthraquinone derivatives and molecular oxygen in acetonitrile solutions: Electrochemical properties of disperse red 60. Electrochim. Acta 2020, 354, 136601. [Google Scholar] [CrossRef]
- Yang, W.-D.; Hsu, Y.-C.; Lin, W.-C.; Huang, I.-L. Characterization and photocatalytic activity of N and Pt doped titania prepared by microemulsion technique. Adv. Mater. Sci. 2018, 3, 1–5. [Google Scholar] [CrossRef]
- Dauscher, A.; Hilaire, L.; Le Normand, F.; Müller, W.; Maire, G.; Vasquez, A. Characterization by XPS and XAS of supported Pt/TiO2—CeO2 catalysts. Surf. Interface Anal. 1990, 16, 341–346. [Google Scholar] [CrossRef]
- Rocha, M.A.L.; Del Ángel, G.; Torres-Torres, G.; Cervantes, A.; Vázquez, A.; Arrieta, A.; Beltramini, J.N. Effect of the Pt oxidation state and Ce3+/Ce4+ ratio on the Pt/TiO2-CeO2 catalysts in the phenol degradation by catalytic wet air oxidation (CWAO). Catal. Today 2015, 250, 145–154. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific. Titanium Transition Metal Primary XPS Region: Ti2p. Available online: https://xpssimplified.com/elements/titanium.php (accessed on 24 July 2022).
- Thermo Fisher Scientific. Platinum Transition Metal. Primary XPS Region: Pt4f. Available online: https://xpssimplified.com/elements/platinum.php#appnotes (accessed on 24 July 2022).
- Ramos-Ramírez, E.; Gutiérrez-Ortega, N.L.; Tzompantzi-Morales, F.; Barrera-Rodríguez, A.; Castillo-Rodríguez, J.C.; Tzompantzi-Flores, C.; Santolalla-Vargas, C.E.; Guevara-Hornedo, M.d.P. Photocatalytic Degradation of 2,4-Dichlorophenol on NiAl-Mixed Oxides Derivatives of Activated Layered Double Hydroxides. Top. Catal. 2020, 63, 546–563. [Google Scholar] [CrossRef]
- Ba-Abbad, M.M.; Kadhum, A.A.H.; Mohamad, A.B.; Takriff, M.S.; Sopian, K. Photocatalytic degradation of chlorophenols under direct solar radiation in the presence of ZnO catalyst. Res. Chem. Intermed. 2013, 39, 1981–1996. [Google Scholar] [CrossRef]



| Support | Method | Diameter Pore (Å) | Specific Surface Area (m2/g) |
|---|---|---|---|
| TiO2 | Sol-gel | 52.54 | 185.59 |
| TiO2-CeO2 1% | Sol-gel | 53.08 | 181.46 |
| TiO2-CeO2 10% | Sol-gel | 77.51 | 104.36 |
| TiO2-CeO2 1% | Impregnation | 295.06 | 43.56 |
| TiO2-CeO2 10% | Impregnation | 304.05 | 43.61 |
| Support | Method | Binding Energy (eV) | Relative Abundance (%) | ||||
|---|---|---|---|---|---|---|---|
| Pt (4f7/2) | Ti (2p3/2) | Ce (3d5/2) | Pt0− Pt2+ | Ti4+ | Ce3+ Ce4+ | ||
| Pt/TiO2 | Sol-gel | 73.0 75.9 | 458.1 | - | 80–20 | 100 | - |
| Pt/TiO2-CeO2 5% | Sol-gel | 75.4 77.09–78.09 | 458 | 880–900 | 53–47 | 100 | 56–44 |
| Pt/TiO2-CeO2 10% | Sol-gel | 75.9 77.09–78.09 | 458.1 | 881–900.1 | 47–53 | 100 | 52–47 |
| Pt/TiO2-CeO2 5% | Impregnation | - | 457.7 | 880–900 | - | 100 | 48.8–51.19 |
| Pt/TiO2-CeO2 10% | Impregnation | - | 465 | 880–900 | - | 100 | 38–62 |
| Catalyst ID Name | Band Gap (eV) | Wavelengths (nm) |
|---|---|---|
| TiO2 | 3.45 | 359 |
| Pt/TiO2 | 3.39 | 365 |
| Pt/TiO2-CeO2- 1% | 3.05 | 406 |
| Pt/TiO2-CeO2- 5% | 2.82 | 439 |
| Catalysts | Method | Pt (wt%) | X% | Cf (ppm) |
|---|---|---|---|---|
| TiO2 | Commercial | - | 38 | 160 |
| TiO2-CeO2 5% | Impregnation | - | 49 | 128 |
| TiO2-CeO2 10% | Impregnation | - | 45 | 138 |
| Pt-TiO2-CeO2 5% | Impregnation | 1 | 62 | 95 |
| Pt-TiO2-CeO2 10% | Impregnation | 1 | 56 | 110 |
| TiO2 | Sol-Gel | - | 38 | 155 |
| TiO2-CeO2 1% | Sol-Gel | - | 61 | 98 |
| TiO2-CeO2 5% | Sol-Gel | - | 61 | 66 |
| TiO2-CeO2 10% | Sol-Gel | - | 45 | 138 |
| Pt-TiO2-CeO2 1% | Sol-Gel | 1 | 95 | 11 |
| Pt-TiO2-CeO2 5% | Sol-Gel | 1 | 97 | 7 |
| Pt-TiO2-CeO2 10% | Sol-Gel | 1 | 89 | 27 |
| Photolysis | - | - | 32 | 169 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mejia-Estrella, I.A.; Pérez Larios, A.; Sulbarán-Rangel, B.; Guzmán González, C.A. Reaching Visible Light Photocatalysts with Pt Nanoparticles Supported in TiO2-CeO2. Materials 2022, 15, 6784. https://doi.org/10.3390/ma15196784
Mejia-Estrella IA, Pérez Larios A, Sulbarán-Rangel B, Guzmán González CA. Reaching Visible Light Photocatalysts with Pt Nanoparticles Supported in TiO2-CeO2. Materials. 2022; 15(19):6784. https://doi.org/10.3390/ma15196784
Chicago/Turabian StyleMejia-Estrella, Ixchel Alejandra, Alejandro Pérez Larios, Belkis Sulbarán-Rangel, and Carlos Alberto Guzmán González. 2022. "Reaching Visible Light Photocatalysts with Pt Nanoparticles Supported in TiO2-CeO2" Materials 15, no. 19: 6784. https://doi.org/10.3390/ma15196784
APA StyleMejia-Estrella, I. A., Pérez Larios, A., Sulbarán-Rangel, B., & Guzmán González, C. A. (2022). Reaching Visible Light Photocatalysts with Pt Nanoparticles Supported in TiO2-CeO2. Materials, 15(19), 6784. https://doi.org/10.3390/ma15196784








