Effect of Microstructure Refinement on the Corrosion Behavior of the Bioresorbable Mg-1Zn-0.2Ca and Mg-1Ca Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Study of the Corrosion Behavior Based on Mass Loss
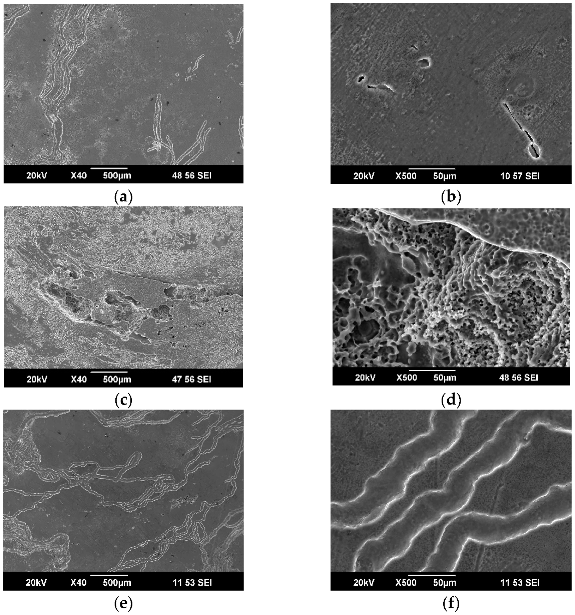
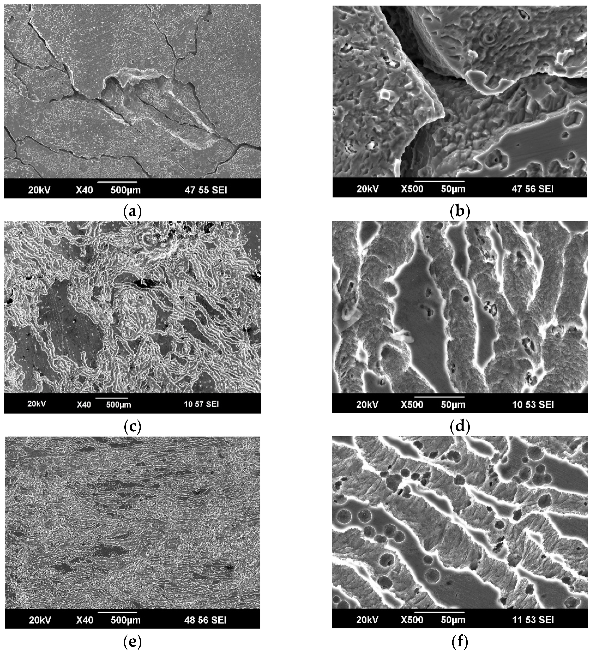
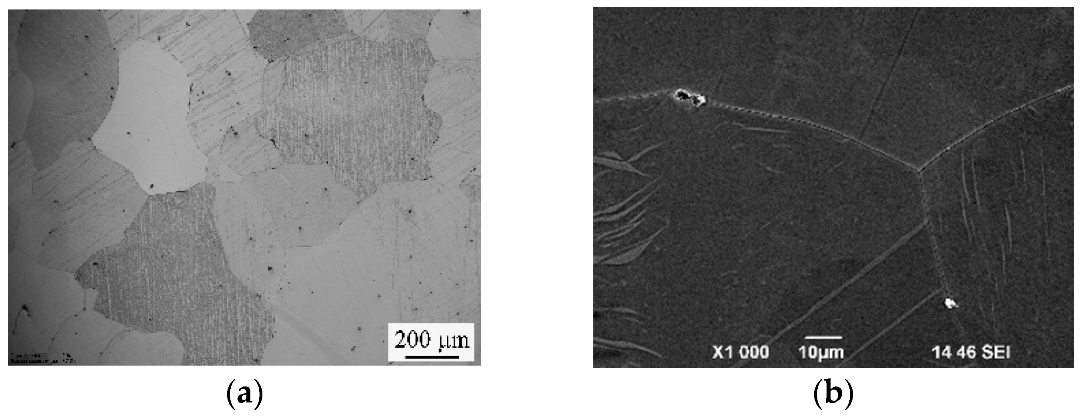
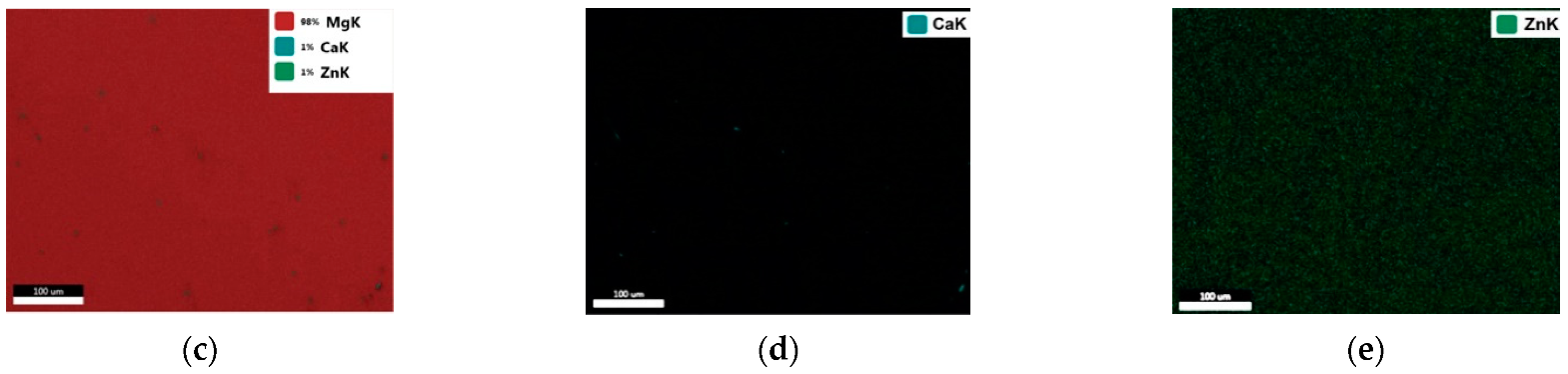
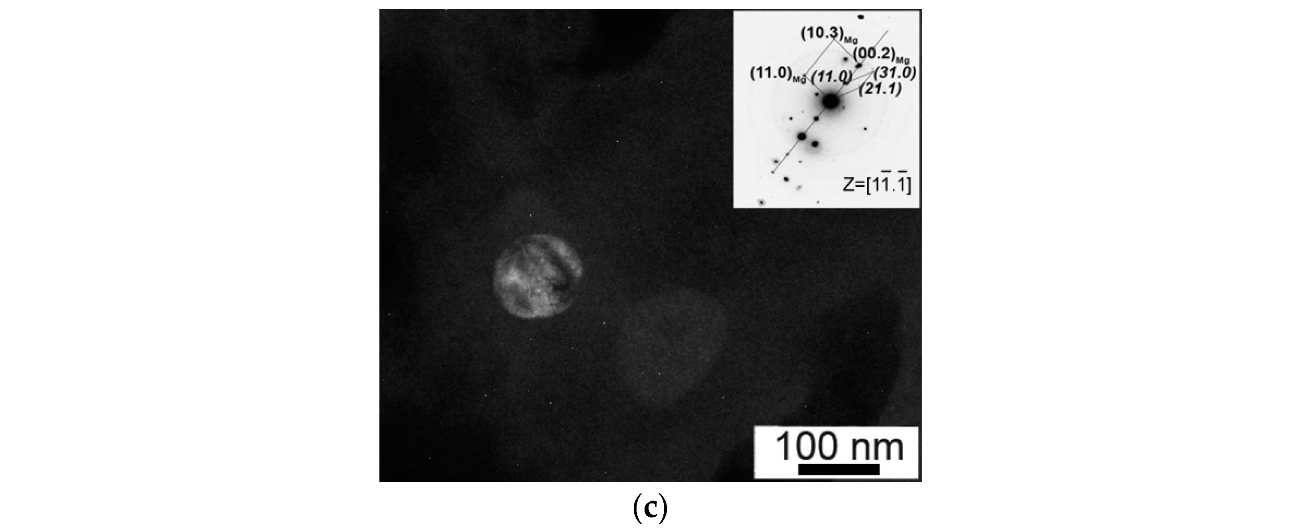
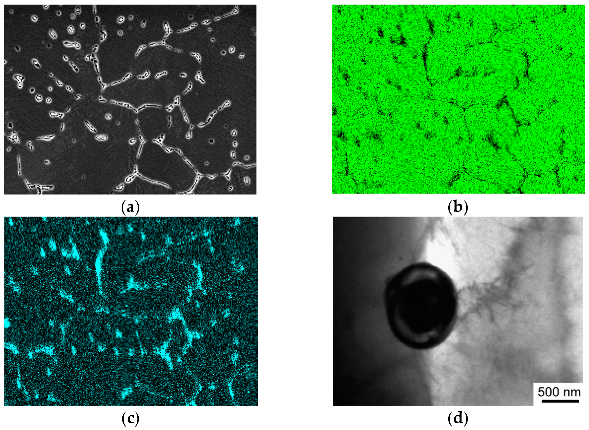
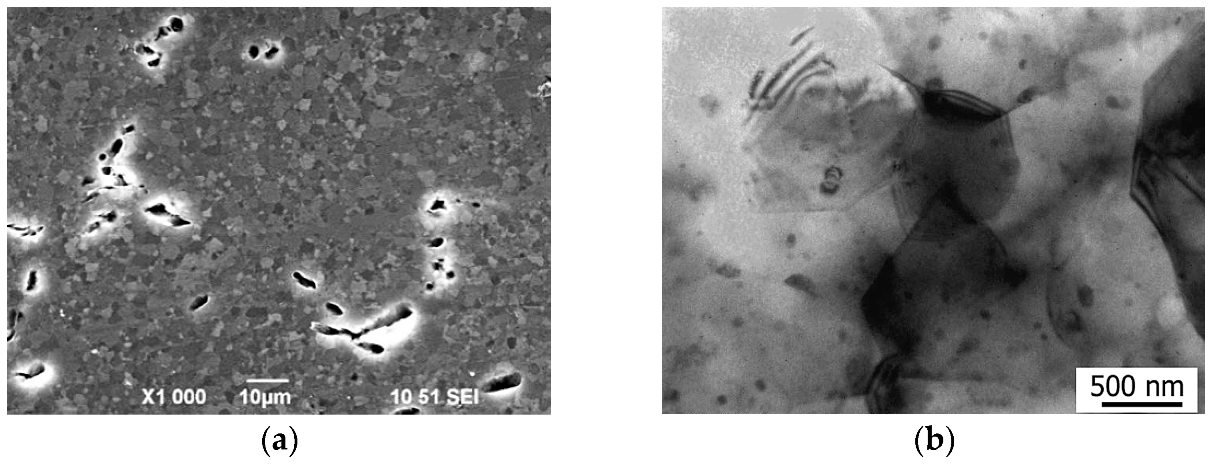
3.2. Results of Microstructural Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhou, J.; Chen, Q.; Detsch, R.; Cui, X.; Jin, G.; Virtanen, S.; Boccaccini, A.R. In vitro osteocompatibility and enhanced biocorrosion resistance of diammonium hydrogen phosphate-pretreated/poly (ether imide) coatings on magnesium for orthopedic application. ACS Appl. Mater. Interfaces 2019, 11, 29667–29680. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y. Magnesium Alloys as Degradable Biomaterials; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Yin, D.-S.; Zhang, E.-L.; Zeng, S.-Y. Effect of Zn on mechanical property and corrosion property of extruded Mg-Zn-Mn alloy. Trans. Nonferrous Met. Soc. China 2008, 18, 763–768. [Google Scholar] [CrossRef]
- Ding, W. Opportunities and challenges for the biodegradable magnesium alloys as next-generation biomaterials. Regen. Biomater. 2016, 3, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Atrens, A. Understanding magnesium corrosion: A framework for improved alloy performance. Adv. Eng. Mater. 2003, 5, 837–858. [Google Scholar] [CrossRef]
- Raman, R.S.K.; Choudhary, L. Cracking of magnesium-based biodegradable implant alloys under the combined action of stress and corrosive body fluid: A review. Emerg. Mater. Res. 2013, 2, 219–228. [Google Scholar] [CrossRef]
- Choudhary, L.; Raman, R.S.K. Magnesium alloys as body implants: Fracture mechanism under dynamic and static loadings in a physiological environment. Acta Biomater. 2012, 8, 916–923. [Google Scholar] [CrossRef]
- Song, G.; Song, S. A possible biodegradable magnesium implant material. Adv. Eng. Mater. 2007, 9, 298–302. [Google Scholar] [CrossRef]
- Gu, X.; Zheng, Y.; Cheng, Y.; Zhong, S.; Xi, T. In vitro corrosion and biocompatibility of binary magnesium alloys. Biomaterials 2009, 30, 484–498. [Google Scholar] [CrossRef]
- Hofstetter, J.; Rüedi, S.; Baumgartner, I.; Kilian, H.; Mingler, B.; Povoden-Karadeniz, E.; Pogatscher, S.; Uggowitzer, P.J.; Löffler, J.F. Processing and microstructure–property relations of high-strength low-alloy (HSLA) Mg-Zn-Ca alloys. Acta Mater. 2015, 98, 423–432. [Google Scholar] [CrossRef]
- Seong, J.W.; Kim, W.J. Mg-Ca binary alloy sheets with Ca contents of ≤1 wt.% with high corrosion resistance and high toughness. Corros. Sci. 2015, 98, 372–381. [Google Scholar] [CrossRef]
- Jin, Y.; Blawert, C.; Feyerabend, F.; Bohlen, J.; Silva, C.M.; Gavras, S.; Willumeit, R.S.R. Time-sequential corrosion behaviour observation of micro-alloyed Mg0.5Zn-0.2Ca alloy via a quasi-in situ approach. Corros. Sci. 2019, 158, 108096. [Google Scholar] [CrossRef]
- Li, Z.; Gu, X.; Lou, S.; Zheng, Y. The development of binary Mg–Ca alloys for use as biodegradable materials within bone. Biomaterials 2008, 29, 1329–1344. [Google Scholar] [CrossRef] [PubMed]
- Merson, D.; Brilevski, A.; Myagkikh, P.; Tarkova, A.; Prokhorikhin, A.; Kretov, E.; Frolova, T.; Vinogradov, A. The functional properties of Mg–Zn–X biodegradable magnesium alloys. Materials 2020, 13, 544. [Google Scholar] [CrossRef]
- Bakhsheshi, R.H.R.; Abdul, K.M.R.; Idris, M.H.; Farahany, S. Relationship between the corrosion behavior and the thermal characteristics and microstructure of Mg–0.5Ca–xZn alloys. Corros. Sci. 2012, 64, 184–197. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Islamgaliev, R.K.; Alexandrov, I.V. Bulk nanostructured materials from severe plastic deformation. Prog. Mater. Sci. 2000, 45, 103–189. [Google Scholar] [CrossRef]
- Lowe, T.C.; Valiev, R.Z. Frontiers of bulk nanostructured metals in biomedical applications. In Advanced Biomaterials and Biodevices; Tiwari, A., Nordin, A.N., Eds.; Wiley-Scrivener Publ.: Beverly, MA, USA, 2014. [Google Scholar]
- Kulyasova, O.B.; Islamgaliev, R.K.; Zhao, Y.; Valiev, R.Z. Enhancement of the mechanical properties of an Mg–Zn–Ca alloy using high-pressure torsion. Adv. Eng. Mater. 2015, 17, 1738–1741. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Straumal, B.; Langdon, T.G. Using severe plastic deformation to produce nanostructured materials with superior properties. Annu. Rev. Mater. Res. 2022, 52, 357–382. [Google Scholar] [CrossRef]
- Zhang, C.; Guan, S.; Wang, L.; Zhu, S.; Chang, L. The microstructure and corrosion resistance of biological Mg–Zn–Ca alloy processed by high-pressure torsion and subsequently annealing. J. Mater. Res. 2017, 32, 1061–1072. [Google Scholar] [CrossRef]
- Zhang, C.Z.; Zhu, S.J.; Wang, L.G.; Guo, R.M.; Yue, G.C.; Guan, S.K. Microstructures and degradation mechanism in simulated body fluid of biomedical Mg–Zn–Ca alloy processed by high pressure torsion. Mater. Des. 2016, 96, 54–62. [Google Scholar] [CrossRef] [Green Version]
- Parfenov, E.V.; Kulyasova, O.B.; Mukaeva, V.R.; Mingo, B.; Farrakhov, R.G.; Cherneikina, Y.V.; Yerokhin, A.; Zheng, Y.F.; Valiev, R.Z. Influence of ultra-fine grain structure on corrosion behaviour of biodegradable Mg-1Ca alloy. Corros. Sci. 2020, 163, 108303. [Google Scholar] [CrossRef]
- Kulyasova, O.B.; Islamgaliev, R.K.; Lin, H.C.; Yilmazer, H. Microstructure and Mechanical Properties of the UFG Magnesium Alloy Mg-1%Ca. Mater. Sci. Forum 2021, 1016, 768–773. [Google Scholar] [CrossRef]
- Mukaeva, V.R.; Kulyasova, O.B.; Farrakhov, R.G.; Parfenov, E.V. Mechanical properties and corrosion behavior of Mg-1Zn-0.2Ca alloy with various grain size. IOP Conf. Ser.: Mater. Sci. Eng. 2019, 479, 012075. [Google Scholar] [CrossRef]
- Hirsch, P.; Howie, A.; Nicholson, R.B.; Pashley, D.W.; Whelan, M.J. Electron Microscopy of Thin Crystals; Krieger: New York, NY, USA, 1977. [Google Scholar]
- Fultz, B.; Howe, J.M. Transmission Electron Microscopy and Diffractometry of Materials; Springer-Verlag: Berlin, Germany, 2013. [Google Scholar]
- Leoni, M.; Confente, T.; Scardi, P. PM2K: A flexible program implementing Whole Powder Pattern Modelling. Z. Krist. Suppl. 2006, 23, 249–254. [Google Scholar] [CrossRef]
- Rigaku, C. Integrated X-Ray Powder Diffraction Software PDXL. Rigaku J. 2010, 26, 23–27. [Google Scholar]
- ASTM G1-03; Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. ASTM International: West Conshohocken, PA, USA, 2003.
- Li, H.X.; Qin, S.K.; Yang, C.L.; Ying, Z.M.; Wang, J.; Yun, J.L.; Zhang, J.S. Influence of Ca addition on microstructure, mechanical properties and corrosion behavior of Mg-2Zn alloy. China Foundry 2018, 15, 363–371. [Google Scholar] [CrossRef]
- Zhang, E.; Yang, L. Microstructure, mechanical properties and bio-corrosion properties of Mg–Zn–Mn–Ca alloy for biomedical application. Mater. Sci. Eng. A 2008, 497, 111–118. [Google Scholar] [CrossRef]
- Tong, L.B.; Zheng, M.Y.; Cheng, L.R.; Zhang, D.P.; Kamado, S.; Meng, J.; Zhang, H.J. Influence of deformation rate on microstructure, texture and mechanical properties of indirect extruded Mg–Zn–Ca alloy. Mater. Charact. 2015, 104, 66–72. [Google Scholar] [CrossRef]
- Williams, G.; McMurray, H.N.; Grace, R. Inhibition of magnesium localised corrosion in chloride containing electrolyte. Electrochim. Acta 2010, 55, 7824–7833. [Google Scholar] [CrossRef]
- Miyamoto, H. Corrosion of ultrafine-grained materials by severe plastic deformation, an overview. Mater. Trans. 2016, 57, 559–572. [Google Scholar] [CrossRef]
- Zander, D.; Zumdick, N.A. Influence of Ca and Zn on the microstructure and corrosion of biodegradable Mg–Ca–Zn alloys. Corros. Sci. 2015, 93, 222–233. [Google Scholar] [CrossRef]
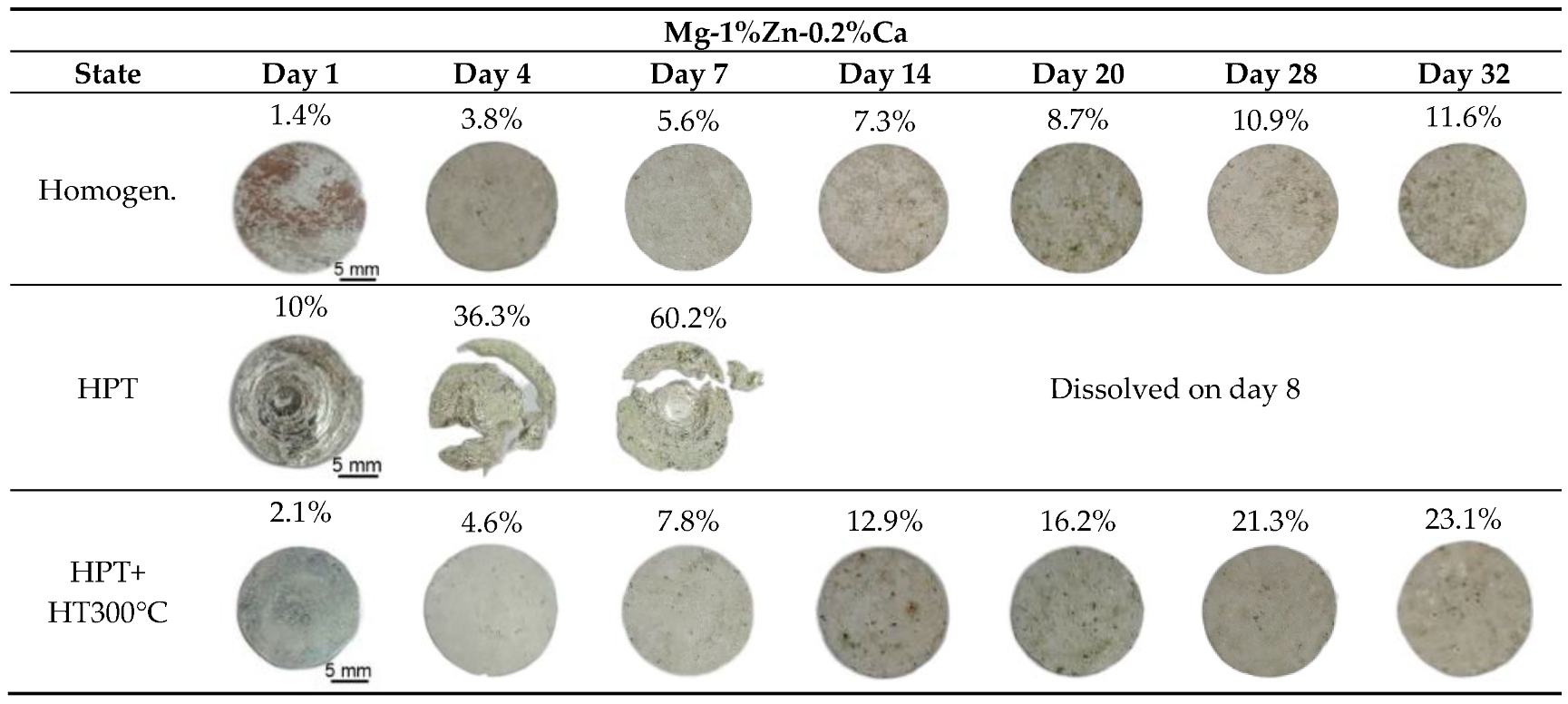
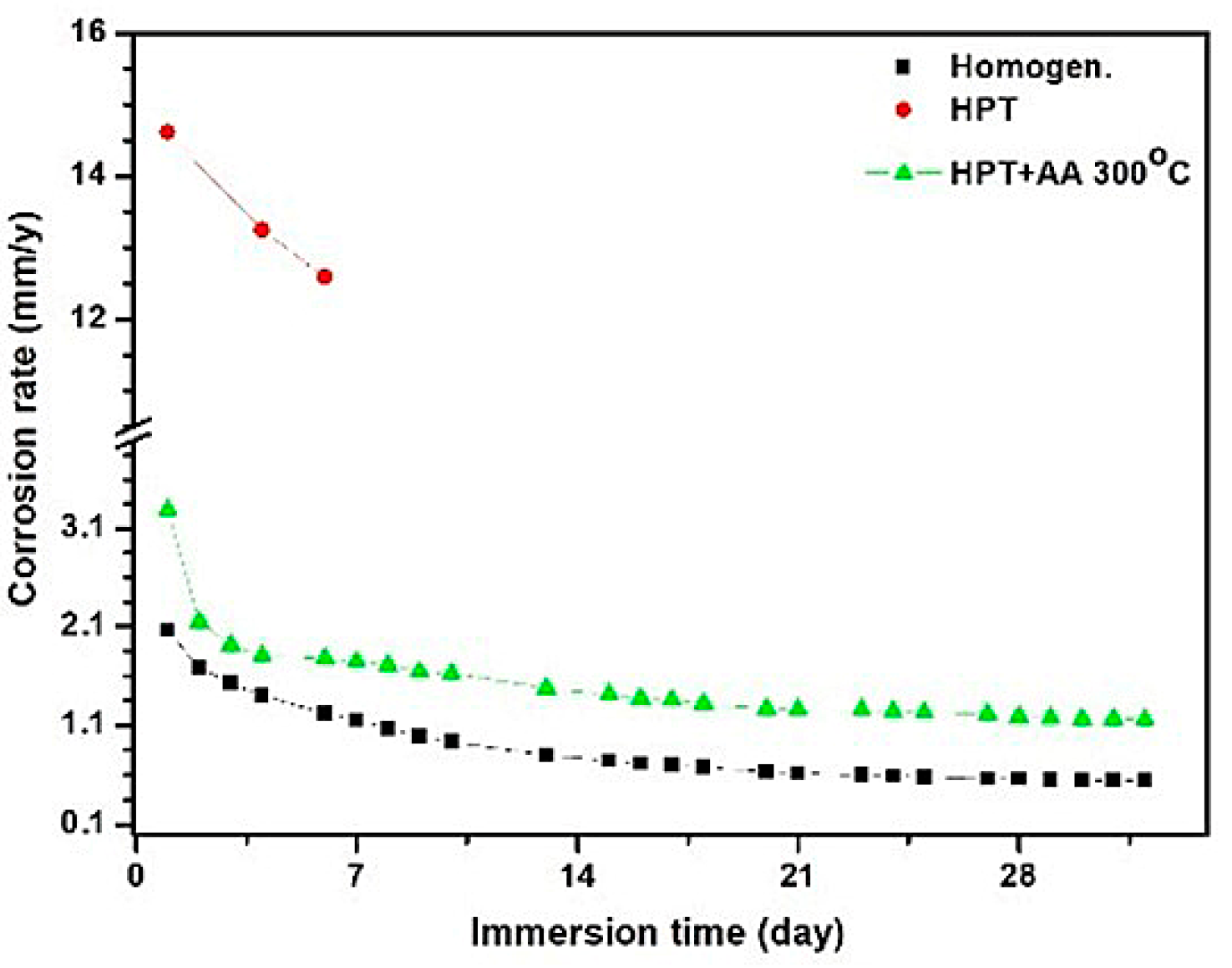

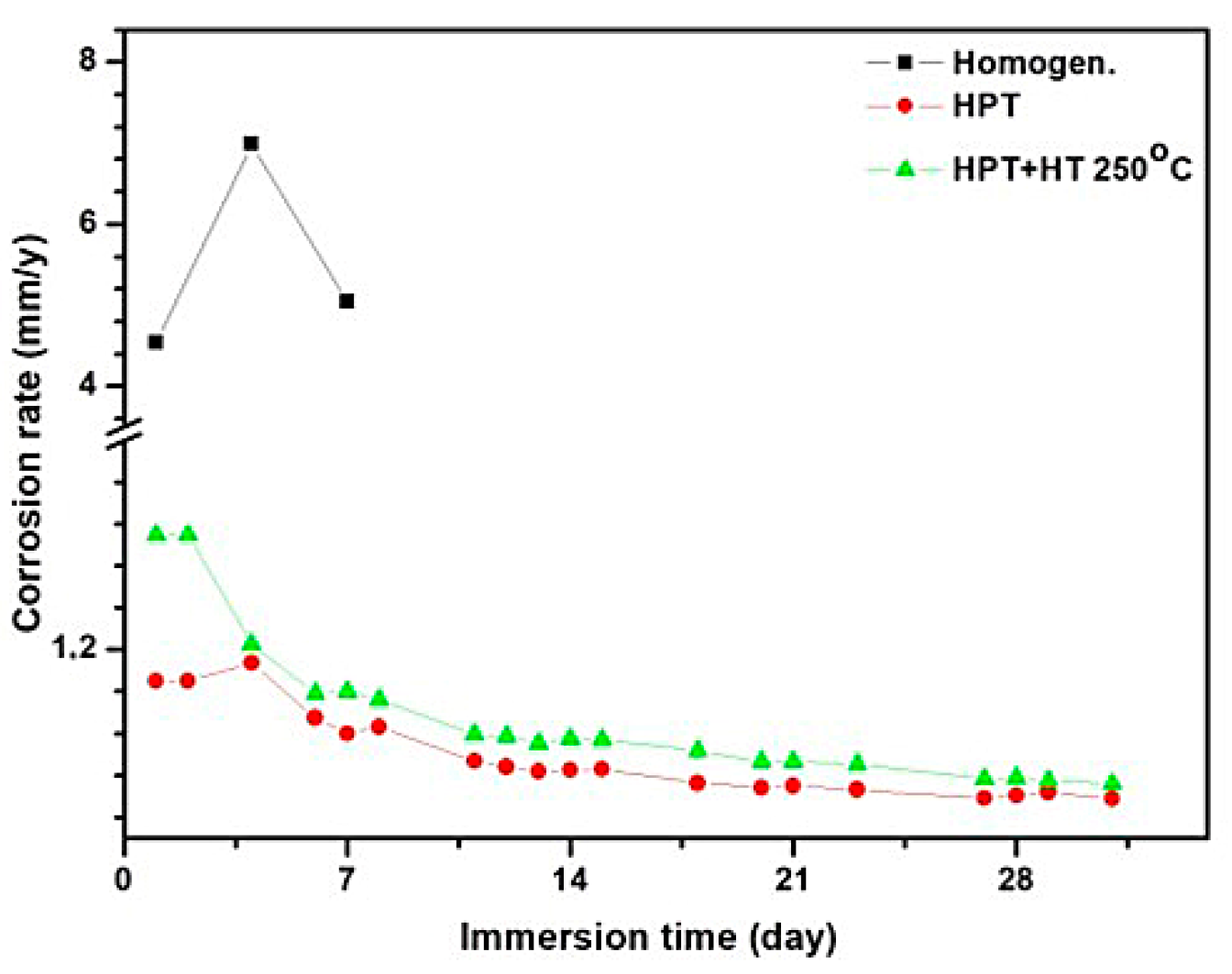



| Immersion Time (day) | Mass Loss (%) | Corrosion Rate (mm/y) | ||||
|---|---|---|---|---|---|---|
| Homogen. | HPT | HPT+HT300 °C | Homogen. | HPT | HPT+HT300 °C | |
| 1 | 1.4 | 10 | 2.1 | 2.06 | 14.61 | 3.28 |
| 4 | 3.8 | 36.3 | 4.6 | 1.39 | 13.25 | 1.800 |
| 7 | 5.6 | 60.2 | 7.8 | 1.15 | 12.57 | 1.74 |
| 14 | 7.7 | 13.5 | 0.74 | 1.41 | ||
| 20 | 8.7 | 16.2 | 0.63 | 1.26 | ||
| 28 | 10.9 | 21.3 | 0.56 | 1.18 | ||
| 32 | 11.6 | 23.1 | 0.54 | 1.16 | ||
| Immersion Time (day) | Mass Loss (%) | Corrosion Rate (mm/y) | ||||
|---|---|---|---|---|---|---|
| Homogen. | HPT | HPT+HT250 °C | Homogen. | HPT | HPT+HT250 °C | |
| 1 | 7.4 | 1.6 | 2.5 | 4.54 | 1.04 | 1.74 |
| 4 | 45.4 | 7.1 | 7.1 | 7 | 1.13 | 1,22 |
| 7 | 57.3 | 8.7 | 10.2 | 5 | 0.8 | 0.98 |
| 14 | 13.6 | 15.8 | 0.62 | 0.77 | ||
| 20 | 17 | 19.4 | 0.54 | 0.66 | ||
| 28 | 23 | 24.1 | 0.69 | 0.58 | ||
| 32 | 26.4 | 28.1 | 0.57 | 0.67 | ||
| State of the Mg-1%Ca Alloy | Lattice Parameter, A | Volume Fraction of Mg2Ca, % |
|---|---|---|
| Homogenized | a = 3.2116 ± 0.00004 c = 5.2115 ± 0.00007 | 5.8 ± 0.3 |
| Homogenized + HPT | a = 3.21183 ± 0.00004 c = 5.21197 ± 0.00006 | 4.1 ± 0.2 |
| Homogenized + HPT + HT at 250 °C | a = 3.21039 ± 0.00006 c = 5.21148 ± 0.00009 | 5.1 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulyasova, O.B.; Khudododova, G.D.; Dyakonov, G.S.; Zheng, Y.; Valiev, R.Z. Effect of Microstructure Refinement on the Corrosion Behavior of the Bioresorbable Mg-1Zn-0.2Ca and Mg-1Ca Alloys. Materials 2022, 15, 6749. https://doi.org/10.3390/ma15196749
Kulyasova OB, Khudododova GD, Dyakonov GS, Zheng Y, Valiev RZ. Effect of Microstructure Refinement on the Corrosion Behavior of the Bioresorbable Mg-1Zn-0.2Ca and Mg-1Ca Alloys. Materials. 2022; 15(19):6749. https://doi.org/10.3390/ma15196749
Chicago/Turabian StyleKulyasova, Olga B., Ganjina D. Khudododova, Grigory S. Dyakonov, Yufeng Zheng, and Ruslan Z. Valiev. 2022. "Effect of Microstructure Refinement on the Corrosion Behavior of the Bioresorbable Mg-1Zn-0.2Ca and Mg-1Ca Alloys" Materials 15, no. 19: 6749. https://doi.org/10.3390/ma15196749
APA StyleKulyasova, O. B., Khudododova, G. D., Dyakonov, G. S., Zheng, Y., & Valiev, R. Z. (2022). Effect of Microstructure Refinement on the Corrosion Behavior of the Bioresorbable Mg-1Zn-0.2Ca and Mg-1Ca Alloys. Materials, 15(19), 6749. https://doi.org/10.3390/ma15196749










