Thermal Conductivity of Helium and Argon at High Pressure and High Temperature
Abstract
1. Introduction
2. Methods
2.1. Sample Preparation
2.2. Thermal Conductivity Measurements
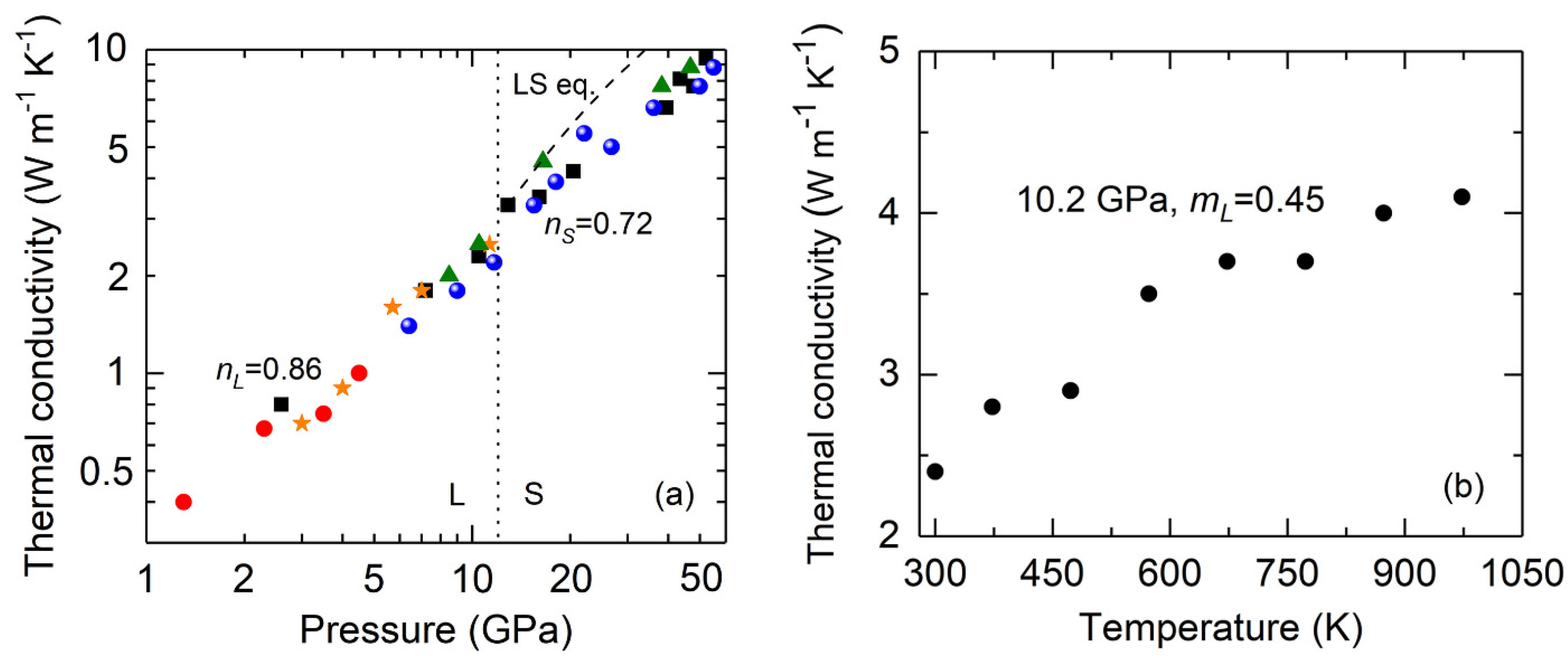
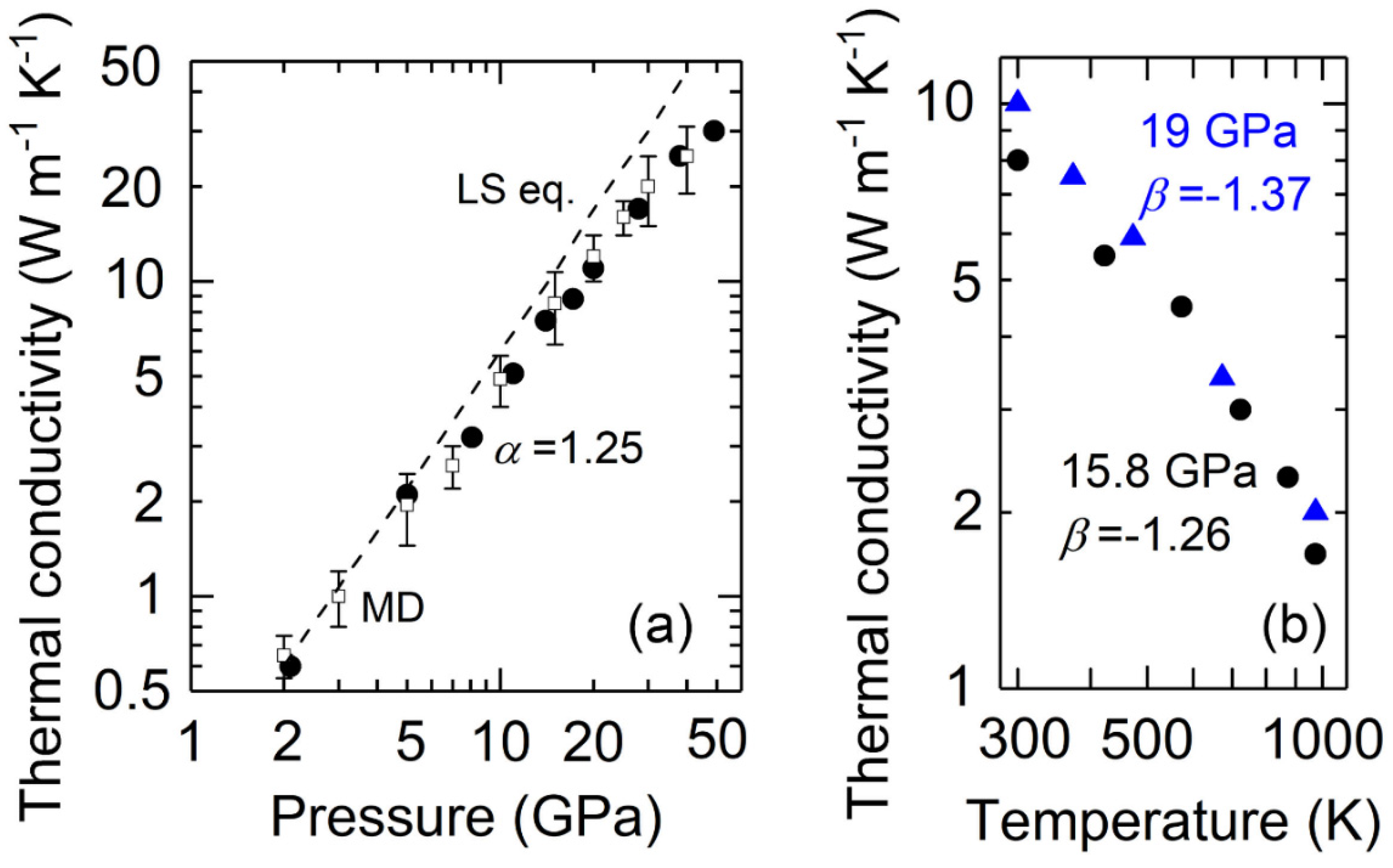
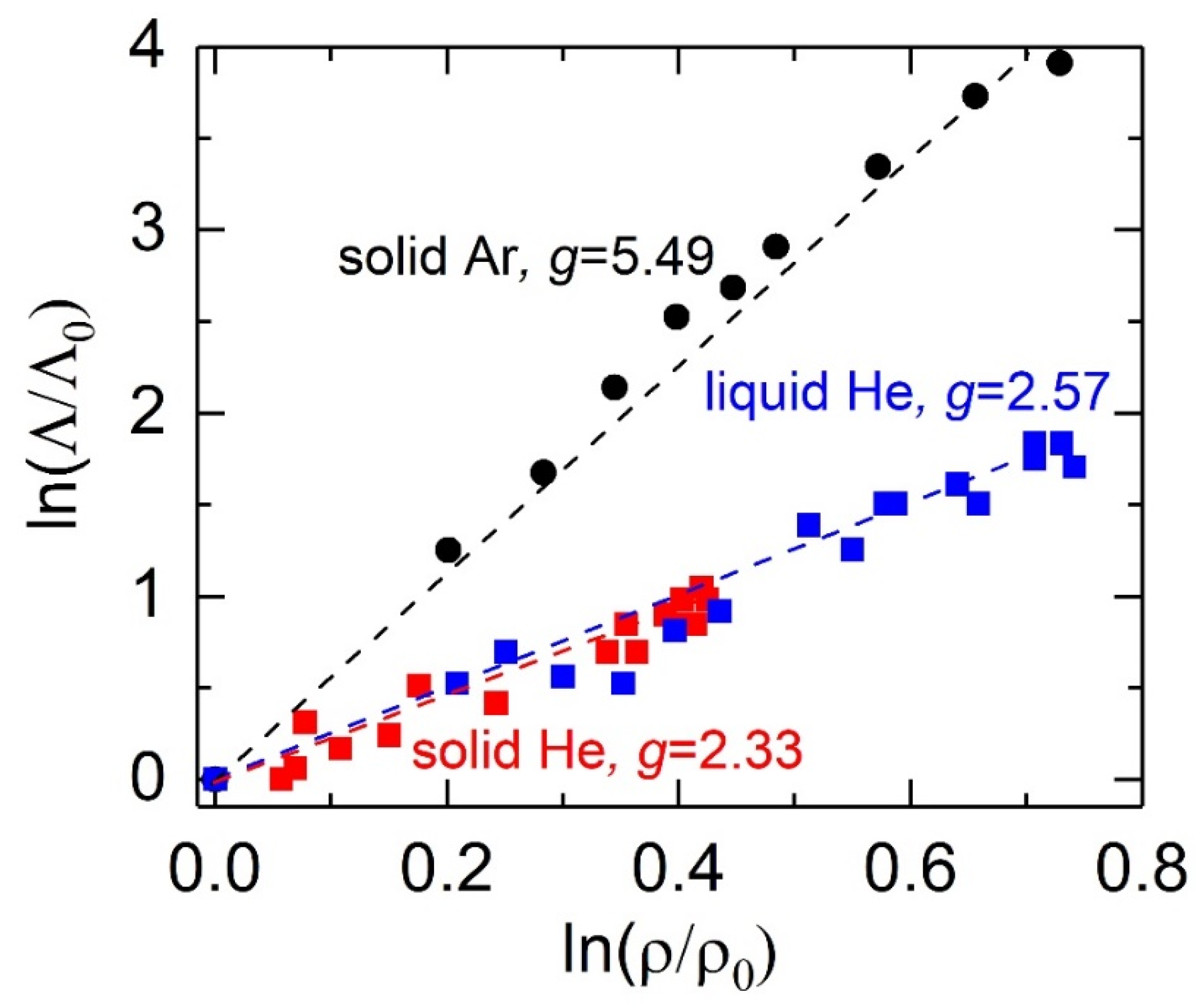
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McMahon, J.M.; Morales, M.A.; Pierleoni, C.; Ceperley, D.M. The properties of hydrogen and helium under extreme conditions. Rev. Mod. Phys. 2012, 84, 1607. [Google Scholar] [CrossRef]
- Loubeyre, P.; Letoullec, R.; Pinceaux, J.P. Equation of state and phase diagram of solid He from single-crystal. Phys. Rev. Lett. 1993, 71, 2272–2275. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.K.; Hemley, R.J.; Wu, Y.; Jephcoat, A.P.; Finger, L.W.; Zha, C.S.; Bassett, W.A. High-Pressure Phase Diagram and Equation of State of Solid Helium from Single-Crystal X-Ray Diffraction to 23.3 GPa. Phys. Rev. Lett. 1988, 60, 2649. [Google Scholar] [CrossRef]
- Klotz, S.; Chervin, J.-C.; Munsch, P.; Le Marchand, G. Hydrostatic limits of 11 pressure transmitting media. J. Phys. D. Appl. Phys. 2009, 42, 075413. [Google Scholar] [CrossRef]
- Lesar, R. Equation of state of dense helium. Phys. Rev. Lett. 1988, 61, 2121–2124. [Google Scholar] [CrossRef]
- Young, D.A.; McMahan, A.K.; Ross, M. Equation of state and melting curve of helium to very high pressure. Phys. Rev. B 1981, 24, 5119–5127. [Google Scholar] [CrossRef]
- Santamaría-Pérez, D.; Mukherjee, G.D.; Schwager, B.; Boehler, R. High-pressure melting curve of helium and neon: Deviations from corresponding states theory. Phys. Rev. B—Condens. Matter Mater. Phys. 2010, 81, 214101. [Google Scholar] [CrossRef]
- Datchi, F.; Loubeyre, P.; LeToullec, R. Extended and accurate determination of the melting curves of argon, helium, ice and hydrogen. Phys. Rev. B 2000, 61, 6535–6546. [Google Scholar] [CrossRef]
- Zha, C.S.; Mao, H.K.; Hemley, R.J. Elasticity of dense helium. Phys. Rev. B—Condens. Matter Mater. Phys. 2004, 70, 174107. [Google Scholar] [CrossRef]
- Freiman, Y.A.; Grechnev, A.; Tretyak, S.M.; Goncharov, A.F.; Zha, C.S.; Hemley, R.J. Sound velocities of hexagonal close-packed H2 and He under pressure. Phys. Rev. B 2013, 88, 214501. [Google Scholar] [CrossRef]
- Grechnev, A.; Tretyak, S.M.; Freiman, Y.A.; Goncharov, A.F.; Gregoryanz, E. Elastic anisotropy and Poisson’s ratio of solid helium under pressure. Phys. Rev. B 2015, 92, 024102. [Google Scholar] [CrossRef]
- Polian, A.; Grimsditch, M. Elastic properties and density of helium up to 20 gpa. Eur. Lett. 1986, 2, 849–855. [Google Scholar] [CrossRef]
- Herrero, C.P. Compressibility of solid helium. J. Phys. Condens. Matter 2008, 20, 295230. [Google Scholar] [CrossRef][Green Version]
- Dewaele, A.; Eggert, J.H.; Loubeyre, P.; Le Toullec, R. Measurement of refractive index and equation of state in dense He, H2, H2O, and Ne under high pressure in a diamond anvil cell. Phys. Rev. B 2003, 67, 094112. [Google Scholar] [CrossRef]
- Watson, G.H.; Daniels, W.B. Raman scattering from solid helium at high pressure. Phys. Rev. B 1985, 31, 4705–4707. [Google Scholar] [CrossRef]
- Chase, C.E. Thermal conduction in liquid helium II. I. Temperature dependence. Phys. Rev. 1962, 127, 361–370. [Google Scholar] [CrossRef]
- Arp, V.; McCarty, R. Thermophysical properties of helium-4 from 0.8 to 1500 K with pressures to 2000 MPa. NIST Tech. Note 1989, 1334, 101–131. [Google Scholar]
- Blais, N.C.; Mann, J.B. Thermal conductivity of helium and hydrogen at high temperatures. J. Chem. Phys. 1960, 32, 1459–1465. [Google Scholar] [CrossRef]
- Hsieh, W.-P. High-pressure thermal conductivity and compressional velocity of NaCl in B1 and B2 phase. Sci. Rep. 2021, 11, 21321. [Google Scholar] [CrossRef]
- Hsieh, W.-P. Thermal conductivity of methanol-ethanol mixture and silicone oil at high pressures. J. Appl. Phys. 2015, 117, 235901. [Google Scholar] [CrossRef]
- Ross, M.; Mao, H.K.; Bell, P.; Xu, J.A. The equation of state of dense argon: A comparison of shock and static studies. J. Chem. Phys. 1986, 85, 1028. [Google Scholar] [CrossRef]
- Grimsditch, M.; Loubeyre, P.; Polian, A. Brillouin scattering and three-body forces in argon at high pressures. Phys. Rev. B 1986, 33, 7192–7200. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Tashiro, H.; Kume, T.; Sasaki, S. High-pressure elastic properties of solid argon to 70 GPa. Phys. Rev. Lett. 2001, 86, 4568–4571. [Google Scholar] [CrossRef] [PubMed]
- Tretiakov, K.V.; Scandolo, S. Thermal conductivity of solid argon at high pressure and high temperature: A molecular dynamics study. J. Chem. Phys. 2004, 121, 11177–11182. [Google Scholar] [CrossRef]
- Goncharov, A.F.; Wong, M.; Allen Dalton, D.; Ojwang, J.G.O.; Struzhkin, V.V.; Konôpková, Z.; Lazor, P. Thermal conductivity of argon at high pressures and high temperatures. J. Appl. Phys. 2012, 111, 112609. [Google Scholar] [CrossRef]
- Gewurtz, S.; Stoicheff, B. Elastic constant of Ar single crystal by Brillouin scattering. Phys. Rev. B 1974, 10, 3487. [Google Scholar] [CrossRef]
- Freiman, Y.A.; Goncharov, A.F.; Tretyak, S.M.; Grechnev, A.; Tse, J.S.; Errandonea, D.; Mao, H.K.; Hemley, R.J. Raman scattering in hcp rare gas solids under pressure. Phys. Rev. B 2008, 78, 014301. [Google Scholar] [CrossRef]
- Finger, L.; Hazen, R.; Zou, G.; Mao, H.K.; Bell, P. Structure and compression of crystalline argon and neon at high pressure and room temperature. Appl. Phys. Lett. 1981, 39, 892. [Google Scholar] [CrossRef]
- Christen, D.; Pollack, G. Thermal conductivity of solid argon. Phys. Rev. B 1975, 12, 3380. [Google Scholar] [CrossRef]
- Chernatynskiy, A.; Phillpot, S.R. Thermal conductivity of argon at high pressure from first principles calculations. J. Appl. Phys. 2013, 114, 064902. [Google Scholar] [CrossRef]
- Dewaele, A.; Loubeyre, P.; Mezouar, M. Equations of state of six metals above 94 GPa. Phys. Rev. B 2004, 70, 094112. [Google Scholar] [CrossRef]
- Akahama, Y.; Kawamura, H. High-pressure Raman spectroscopy of diamond anvils to 250 GPa: Method for pressure determination in the multimegabar pressure range. J. Appl. Phys. 2004, 96, 3748–3751. [Google Scholar] [CrossRef]
- Hsieh, W.-P.; Chen, B.; Li, J.; Keblinski, P.; Cahill, D.G. Pressure tuning of the thermal conductivity of the layered muscovite crystal. Phys. Rev. B 2009, 80, 180302. [Google Scholar] [CrossRef]
- Lai, X.; Zhu, F.; Zhang, J.S.; Zhang, D.; Tkachev, S.; Prakapenka, V.B.; Chen, B. An externally-heated diamond anvil cell for synthesis and single-crystal elasticity determination of Ice-VII at high pressure-temperature conditions. J. Vis. Exp. 2020, 160, e61389. [Google Scholar] [CrossRef] [PubMed]
- Cahill, D.G.; Ford, W.K.; Goodson, K.E.; Ford, W.K.; Maris, H.J. Nanoscale thermal transport. J. Appl. Phys. 2003, 93, 793. [Google Scholar] [CrossRef]
- Cahill, D.G.; Braun, P.V.; Chen, G.; Clarke, D.R.; Fan, S.; Goodson, K.E.; Keblinski, P.; King, W.P.; Mahan, G.D.; Majumdar, A.; et al. Nanoscale thermal transport. II. 2003–2012. Appl. Phys. Rev. 2014, 1, 011305. [Google Scholar] [CrossRef]
- Hsieh, W.P.; Goncharov, A.F.; Labrosse, S.; Holtgrewe, N.; Lobanov, S.S.; Chuvashova, I.; Deschamps, F.; Lin, J.F. Low thermal conductivity of iron-silicon alloys at Earth’s core conditions with implications for the geodynamo. Nat. Commun. 2020, 11, 3332. [Google Scholar] [CrossRef]
- Hsieh, W.-P.; Deschamps, F.; Okuchi, T.; Lin, J.-F. Effects of iron on the lattice thermal conductivity of Earth’s deep mantle and implications for mantle dynamics. Proc. Natl. Acad. Sci. USA 2018, 115, 4099. [Google Scholar] [CrossRef]
- Zhou, Y.; Dong, Z.Y.; Hsieh, W.P.; Goncharov, A.F.; Chen, X.J. Thermal conductivity of materials under pressure. Nat. Rev. Phys. 2022, 4, 319–335. [Google Scholar] [CrossRef]
- Kang, K.; Koh, Y.K.; Chiritescu, C.; Zheng, X.; Cahill, D.G. Two-tint pump-probe measurements using a femtosecond laser oscillator and sharp-edged optical filters. Rev. Sci. Instrum. 2008, 79, 114901. [Google Scholar] [CrossRef]
- Cahill, D.G. Analysis of heat flow in layered structures for time-domain thermoreflectance. Rev. Sci. Instrum. 2004, 75, 5119–5122. [Google Scholar] [CrossRef]
- Chen, B.; Hsieh, W.-P.; Cahill, D.G.; Trinkle, D.R.; Li, J. Thermal conductivity of compressed H2O to 22 GPa: A test of the Leibfried-Schlömann equation. Phys. Rev. B 2011, 83, 132301. [Google Scholar] [CrossRef]
- Cahill, D.G.; Watanabe, F. Thermal conductivity of isotopically pure and Ge-doped Si epitaxial layers from 300 to 550 K. Phys. Rev. B 2004, 70, 235322. [Google Scholar] [CrossRef]
- Zheng, X.; Cahill, D.G.; Krasnochtchekov, P.; Averback, R.S.; Zhao, J.C. High-throughput thermal conductivity measurements of nickel solid solutions and the applicability of the Wiedemann-Franz law. Acta Mater. 2007, 55, 5177–5185. [Google Scholar] [CrossRef]
- Tikunoff, D.; Spera, F. Thermal conductivity of molten and glassy NaAlSi3O8, CaMgSi2O6, and Mg2SiO4 by non-equilibrium molecular dynamics at elevated temperature and pressure. Am. Mineral. 2014, 99, 2328–2336. [Google Scholar] [CrossRef]
- Roufosse, M.; Klemens, P.G. Thermal Conductivity of Complex Dielectric Crystals. Phys. Rev. B 1973, 7, 5379. [Google Scholar] [CrossRef]
- Shieh, S.R.; Hsieh, W.P.; Tsao, Y.C.; Crisostomo, C.; Hsu, H. Low Thermal Conductivity of Carbon Dioxide at High Pressure: Implications for Icy Planetary Interiors. J. Geophys. Res. Planets 2022, 127, e2022JE007180. [Google Scholar] [CrossRef]
- Meyer, D.W.; Hsieh, W.P.; Hsu, H.; Kuo, C.Y.; Lin, J.F. Thermal Conductivity and Compressional Velocity of Methane at High Pressure: Insights Into Thermal Transport Properties of Icy Planet Interiors. J. Geophys. Res. Planets 2022, 127, e2021JE007059. [Google Scholar] [CrossRef]
- Hsieh, W.-P.; Losego, M.D.; Braun, P.V.; Shenogin, S.; Keblinski, P.; Cahill, D.G. Testing the minimum thermal conductivity model for amorphous polymers using high pressure. Phys. Rev. B 2011, 83, 174205. [Google Scholar] [CrossRef]
- de Koker, N. Thermal conductivity of MgO periclase at high pressure: Implications for the D″ region. Earth Planet. Sci. Lett. 2010, 292, 392–398. [Google Scholar] [CrossRef]
- Dalton, D.A.; Hsieh, W.-P.; Hohensee, G.T.; Cahill, D.G.; Goncharov, A.F. Effect of mass disorder on the lattice thermal conductivity of MgO periclase under pressure. Sci. Rep. 2013, 3, 2400. [Google Scholar] [CrossRef]
- Le Toullec, R.; Loubeyre, P.; Pinceaux, J.P. Refractive-index measurements of dense helium up to 16 GPa at T=298 K: Analysis of its thermodynamic and electronic properties. Phys. Rev. B 1989, 40, 2368–2378. [Google Scholar] [CrossRef]
- Slack, G.A. Solid State Physics; Academic: New York, NY, USA, 1979; Volume 34, p. 35. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, W.-P.; Tsao, Y.-C.; Lin, C.-H. Thermal Conductivity of Helium and Argon at High Pressure and High Temperature. Materials 2022, 15, 6681. https://doi.org/10.3390/ma15196681
Hsieh W-P, Tsao Y-C, Lin C-H. Thermal Conductivity of Helium and Argon at High Pressure and High Temperature. Materials. 2022; 15(19):6681. https://doi.org/10.3390/ma15196681
Chicago/Turabian StyleHsieh, Wen-Pin, Yi-Chi Tsao, and Chun-Hung Lin. 2022. "Thermal Conductivity of Helium and Argon at High Pressure and High Temperature" Materials 15, no. 19: 6681. https://doi.org/10.3390/ma15196681
APA StyleHsieh, W.-P., Tsao, Y.-C., & Lin, C.-H. (2022). Thermal Conductivity of Helium and Argon at High Pressure and High Temperature. Materials, 15(19), 6681. https://doi.org/10.3390/ma15196681




