Effect of Fluoride Content of Mouthwashes on Superelastic Properties of NiTi Orthodontic Archwires
Abstract
1. Introduction
2. Materials and Methods
2.1. Ion Release
2.2. Calorimetric Tests
2.3. Mechanical Tests
2.4. Electronic Microcopy
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paúl, A.; Abalos, C.; Jimenez-Planas, A.; Solano, E.; Gil, F.J. Relationship between the surface defects and the manufacturing process of orthodontic NiTi archwires. Mater. Lett. 2011, 65, 3358–3361. [Google Scholar] [CrossRef]
- Arends, J.; Christoffersen, J. Nature and role of loosely bound fluoride in dental caries. J. Dent. Res. 1990, 69, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, J.D.B. Prevention and reversal of dental caries: Role of low level fluoride. Community Dent. Oral Epidemiol. 1999, 27, 31–40. [Google Scholar] [CrossRef]
- Thylstrup, A. Clinical evidence of the role of pre-eruptive fluoride in caries prevention. J. Dent. Res. 1990, 69, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Juanito, G.M.P.; Morsch, C.S.; Benfatti, C.A.; Fredel, M.C.; Magini, R.S.; Souza, J.C.M. Effect of Fluoride and Bleaching Agents on the Degradation of Ti: Literature Review. Dentistry 2015, 5, 273. [Google Scholar] [CrossRef]
- Nakagawa, M.; Shigeki, M.; Koich, U. Corrosion behavior of pure Ti and Ti alloys in fluoride-containing solutions. Dent. Mater. J. 2001, 20, 305–314. [Google Scholar] [CrossRef]
- Nakagawa, M.; Shigeki, M.; Koichi, U. Effects of fluoride and dissolved oxygen concentrations on the corrosion behavior of pure Ti and Ti alloys. Dent. Mater. J. 2002, 21, 83–92. [Google Scholar] [CrossRef]
- Yamazoe, J.; Nakagawa, M.; Matono, Y.; Takeuchi, A.; Ishikawa, K. The development of Ti alloys for dental implant with high corrosion resistance and mechanical strength. Dent. Mater. J. 2007, 26, 260–267. [Google Scholar] [CrossRef]
- Matono, Y.; Nakagawa, M.; Matsuya, S.; Ishikawa, K.; Terada, Y. Corrosion behavior of pure Ti and Ti alloys in various concentrations of acidulated phosphate fluoride (APF) solutions. Dent. Mater. J. 2006, 25, 104–112. [Google Scholar] [CrossRef]
- Suárez, C.; Vilar, T.; Sevilla, P.; Gil, J. In vitro corrosion behavior of lingual orthodontic archwires. Int. J. Corros. 2011, 5, 2011–2013. [Google Scholar] [CrossRef]
- Suárez, C.; Vilar, T.; Gil, J.; Sevilla, P. In vitro evaluation of surface topographic changes and nickel release of lingual orthodontic archwires. J. Mater. Sci. Mater. Med. 2010, 21, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Pulikkottil, V.J.; Chidambaram, S.; Bejoy, P.U.; Femin, P.K.; Paul, P.; Rishad, M. Corrosion resistance of stainless steel. nickel-Ti. Ti molybdenum alloy. and ion-implanted Ti molybdenum alloy archwires in acidic fluoride-containing artificial saliva: An in vitro study. J. Pharm. Bioallied Sci. 2016, 8 (Suppl. 1), S96. [Google Scholar]
- Alfonso, M.V.; Espinar, E.; Llamas, J.M.; Rupérez, E.; Manero, J.M.; Barrera, J.M.; Gil, F.J. Friction coefficients and wear rates of different orthodontic archwires in artificial saliva. J. Mater. Sci. Mater. Med. 2013, 24, 1327–1332. [Google Scholar] [CrossRef]
- Kim, T.I.; Han, J.H.; Lee, I.S.; Lee, K.H.; Shin, M.C.; Choi, B.B. New Ti alloys for biomaterials: A study of mechanical and corrosion properties and cytotoxicity. Bio-Med. Mater. 1997, 7, 253–263. [Google Scholar]
- Schmalz, G.; Garhammer, P. Biological interactions of dental cast alloys with oral tissues. Dent Mater. 2002, 18, 396–406. [Google Scholar] [CrossRef]
- Staerkjaer, L.; Menné, T. Nickel allergy and orthodontic treatment. Eur. J. Orthodont. 1990, 12, 284–289. [Google Scholar] [CrossRef]
- Wang, J.J.; Sanderson, B.J.; Wang, H. Cyto- and genotoxicity of ultrafine TiO2 particles in culture human lymphobasltoid cells. Mutat Res. 2007, 86, 320–329. [Google Scholar]
- Picas, J.A.; Forn, A.; Gil, F.J. Optimization of the Ti–0.2 Pd alloy properties through heat treatments. J. Light Met. 2002, 2, 57–64. [Google Scholar] [CrossRef]
- Gil, F.J.; Planell, J.A. Behaviour of normal grain growth kinetics in single phase Ti and Ti alloys. Mater. Sci. Eng. A 2000, 283, 17–24. [Google Scholar] [CrossRef]
- Cabot, P.L.; Forn, A.; Vilarrasa, M.; Picas, J.A.; Gil, F.J.; Costa, J.M. Effect of heat treatment on the anodic oxidation of Ti–0.2 Pd alloys in chloride solutions. J. Appl. Electrochem. 2000, 30, 717–722. [Google Scholar] [CrossRef]
- Wang, X.L.; Zhou, Q.; Yang, K.; Zou, C.H.; Wang, L. Performance of surface on ultrafine grained Ti-0.2 Pd in simulated body fluid. Appl. Surf. Sci. 2018, 434, 957–966. [Google Scholar] [CrossRef]
- Arciniegas, M.; Peña, J.; Manero, J.M.; Paniagua, J.C.; Gil, F.J. Quantum parameters for guiding the design of Ti alloys with shape memory and /or low elastic modulus. Philos. Mag. 2008, 88, 2529–2548. [Google Scholar] [CrossRef]
- Arciniegas, M.; Manero, J.M.; Peña, J.; Gil, F.J.; Planell, J.A. Study of new multifunctional shape memory and low elastic modulus Ni-free Ti alloys. Metall. Mater. Trans. A 2008, 39, 742–751. [Google Scholar] [CrossRef]
- Kusy, R.P. A review of contemporary archwires: Their properties and characteristics. Angle Orthod. 1997, 67, 197–207. [Google Scholar] [PubMed]
- Miura, F.; Mogi, M.; Ohura, Y.; Hamanaka, H. The super-elastic property of the Japanese NiTi alloy wire for use in orthodontics. Am. J. Orthod. Dentofac. Orthop. 1986, 90, 1–10. [Google Scholar] [CrossRef]
- Gil, F.J.; Planell, J.A. Effect of copper addition on the superelastic behavior of Ni-Ti shape memory alloys for orthodontic applications. J. Biomed. Mater. Res. 1999, 48, 682–688. [Google Scholar] [CrossRef]
- Gil, F.J.; Solano, E.; Peña, J.; Engel, E.; Mendoza, A.; Planell, J.A. Microstructural. Mechanical and citotoxicity evaluation of different NiTi and NiTiCu shape memory alloys. J. Mater. Sci. Mater. Med. 2004, 15, 1181–1185. [Google Scholar] [CrossRef]
- Gil, F.J.; Solano, E.; Mendoza, A.; Peña, J. Inhibition of Ni release from NiTi and NiTiCu orthodontic archwires by nitrogen diffusion treatment. J. Appl. Biomater. Biomech. 2004, 2, 151–155. [Google Scholar]
- Sarangi, D.; Pattanaik, S. Nanoparticles in dentistry. In Advanced Nanomaterials for Point of Care Diagnosis and Therapy; Elsevier: London, UK, 2022; pp. 335–358. [Google Scholar]
- Carrouel, F.; Viennot, S.; Ottolenghi, L.; Gaillard, C.; Bourgeois, D. Nanoparticles as anti-microbial. anti-inflammatory. and remineralizing agents in oral care cosmetics: A review of the current situation. Nanomaterials 2020, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Borg, W.; Cassar, G. Surface microstructural changes and release of ions from dental metal alloy removable prostheses inpatients suffering from acid reflux. J. Prosthodontic. 2016, 27, 115–119. [Google Scholar] [CrossRef]
- Valdivia, S.; Tapia, A.; Astrid, C. Fluoride concentration in mouth rinses marketed in Chile and Brazil and a discussion regarding their legislations. Braz. Oral Res. 2021, 35, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Park, H.G.; Yeo, M.K. Nanomaterial regulatory policy for human health and environment. Mol. Cell. Toxicol. 2016, 12, 223–236. [Google Scholar] [CrossRef]
- Møretrø, T.; Langsrud, S. Effects of materials containing antimicrobial compounds on food hygiene. J. Food Prot. 2011, 74, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Peña, J.; Solano, E.; Mendoza, A.; Casals, J.; Planell, J.A.; Gil, F.J. Effect of the Ms transformation temperature on the wear behaviour of NiTi shape memory alloys for articular prosthesis. BioMed. Mater. Eng. 2005, 15, 289–293. [Google Scholar] [PubMed]
- Michiardi, A.; Aparicio, C.; Planell, J.A.; Gil, F.J. New oxidation treatment of NiTi shape memory alloys to obtain Ni-free surfaces and to improve biocompatibility. J. Biomed. Mater. Res. B. Appl. Biomater. 2006, 77, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Gil, F.J.; Manero, J.M.; Arcas, R.; Planell, J.A. Grain growth in austenite NiTi shape memory alloys. Scripta Met. Mater. 1994, 31, 483–486. [Google Scholar] [CrossRef]
- Gil, F.J.; Espinar, E.; Llamas, J.M.; Manero, J.M.; Ginebra, M.P. Variation of the superelastic properties and nickel release from original and reused NiTi orthodontic archwires. J. Mech. Behav. Biomed. Mater. 2012, 6, 113–119. [Google Scholar] [CrossRef]
- Gil, F.J.; Cenizo, M.; Espinar, E.; Rodriguez, A.; Rúperez, E.; Manero, J.M. NiTi superelastic orthodontic wires with variable stress obtained by ageing treatments. Mater. Lett. 2013, 104, 5–7. [Google Scholar] [CrossRef]
- Briceño, J.; Romeu, A.; Espinar, E.; Llamas, J.M.; Gil, F.J. Influence of the microstructure on electrochemical corrosion and nickel release in NiTi orthodontic archwires. Mater. Sci. Eng. 2013, 33, 4989–4993. [Google Scholar] [CrossRef]
- Wu, S.; Liu, X.; Chan, Y.L.; Chu, P.K.; Chung, C.Y.; Chu, C.; Luk, K.D. Nickel release behavior and surface characteristics of porous NiTi shape memory alloy modified by different chemical processes. J. Biomed. Mater. Res. Part A 2009, 89, 483–489. [Google Scholar] [CrossRef]
- Michiardi, A.; Aparicio, C.; Planell JAGil, F.J. Electrochemical behaviour of oxidized NiTi shape memory alloys for biomedical applications. Surf. Coat. Technol. 2007, 201, 6484–6488. [Google Scholar] [CrossRef]
- Gil, F.J.; Guilemany, J.M. Energetic evaluation for inducing the thermoelastic martensitic transformation by mechanical stress in Cu-Zn-Al single crystals. Intermetal 1999, 7, 699–704. [Google Scholar] [CrossRef]
- Peña, J.; Gil, F.J.; Guilemany, J.M. Effect of the microstructure on dry sliding wear of CuZnAl shape memory alloys. Acta Mater. 2002, 50, 3115–3124. [Google Scholar] [CrossRef]
- Jafari, K.; Rahimzadeh, S.; Hekmatfar, S. Nickel ion release from dental alloys in two different mouthwashes. J. Dent. Res. Dent. Clin. Dent. Prospect. 2019, 13, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Muris, J.; Goossens, A. Sensitization to palladium and nickelin Europe and the relationship with oral disease and dental alloys. Contact Dermat. 2015, 72, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Mlinaric, M.; Kanizaj, L. Effect of oral antiseptics on the corrosion stability of nickel-Ti orthodontic alloys. Mater. Corros. 2018, 6, 510–518. [Google Scholar] [CrossRef]
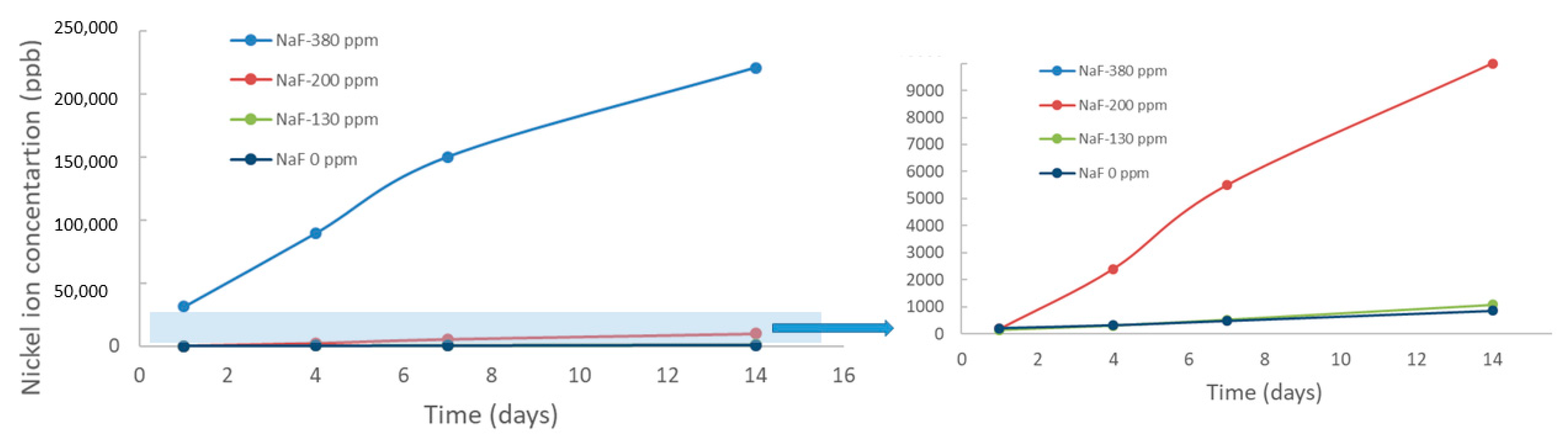
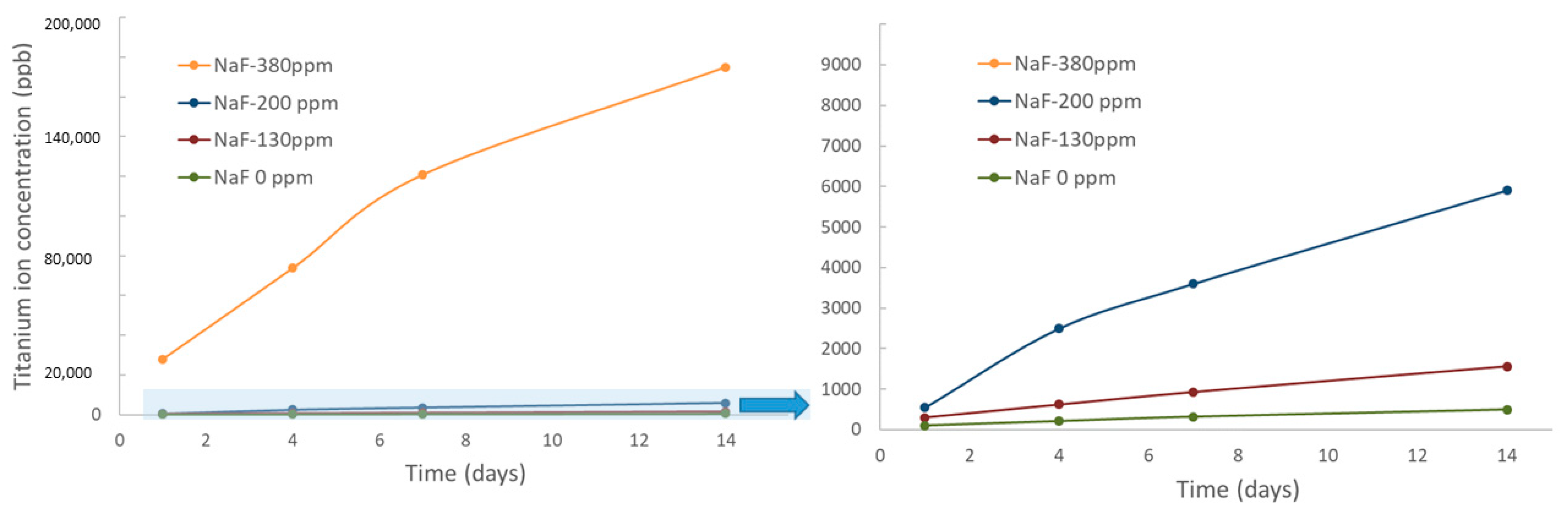

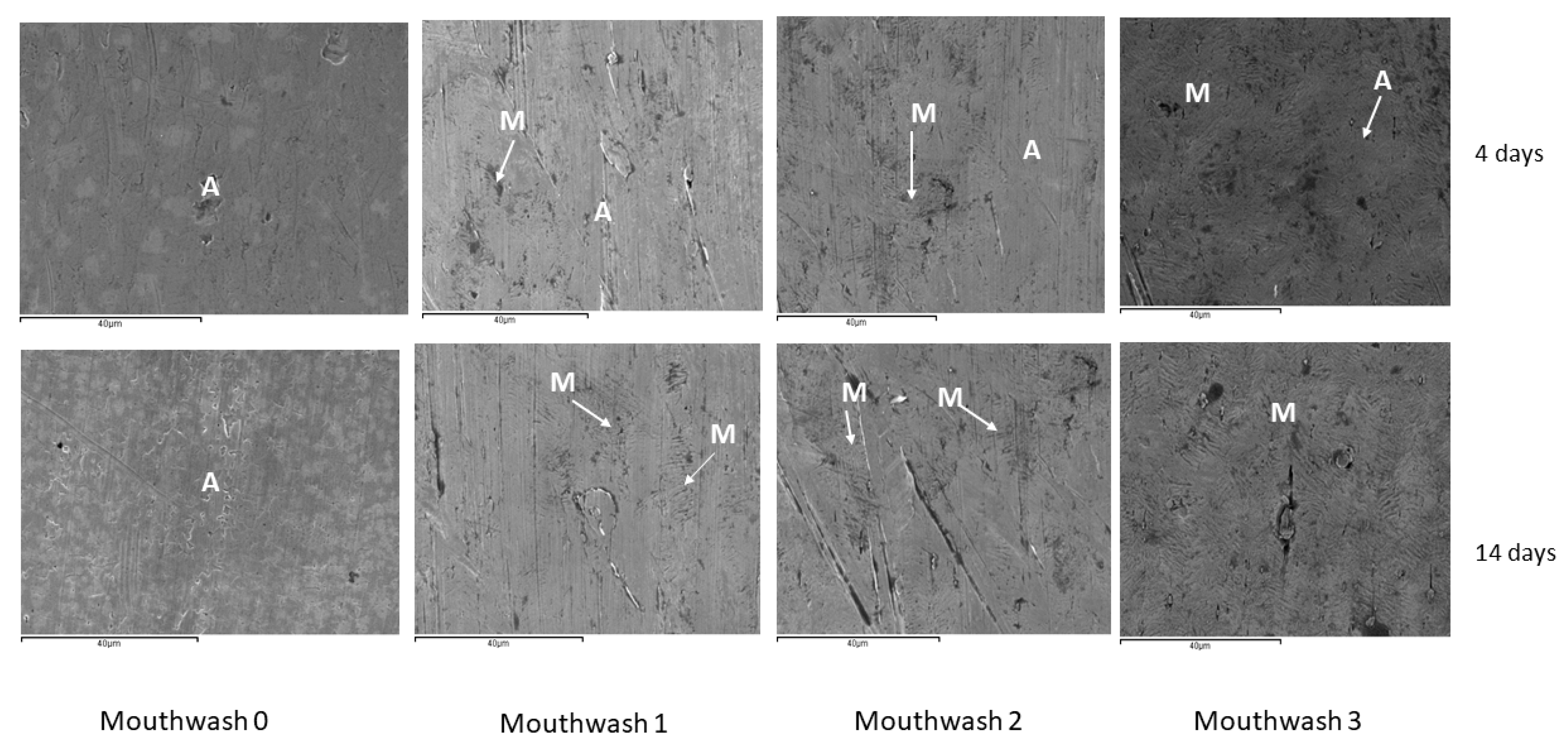

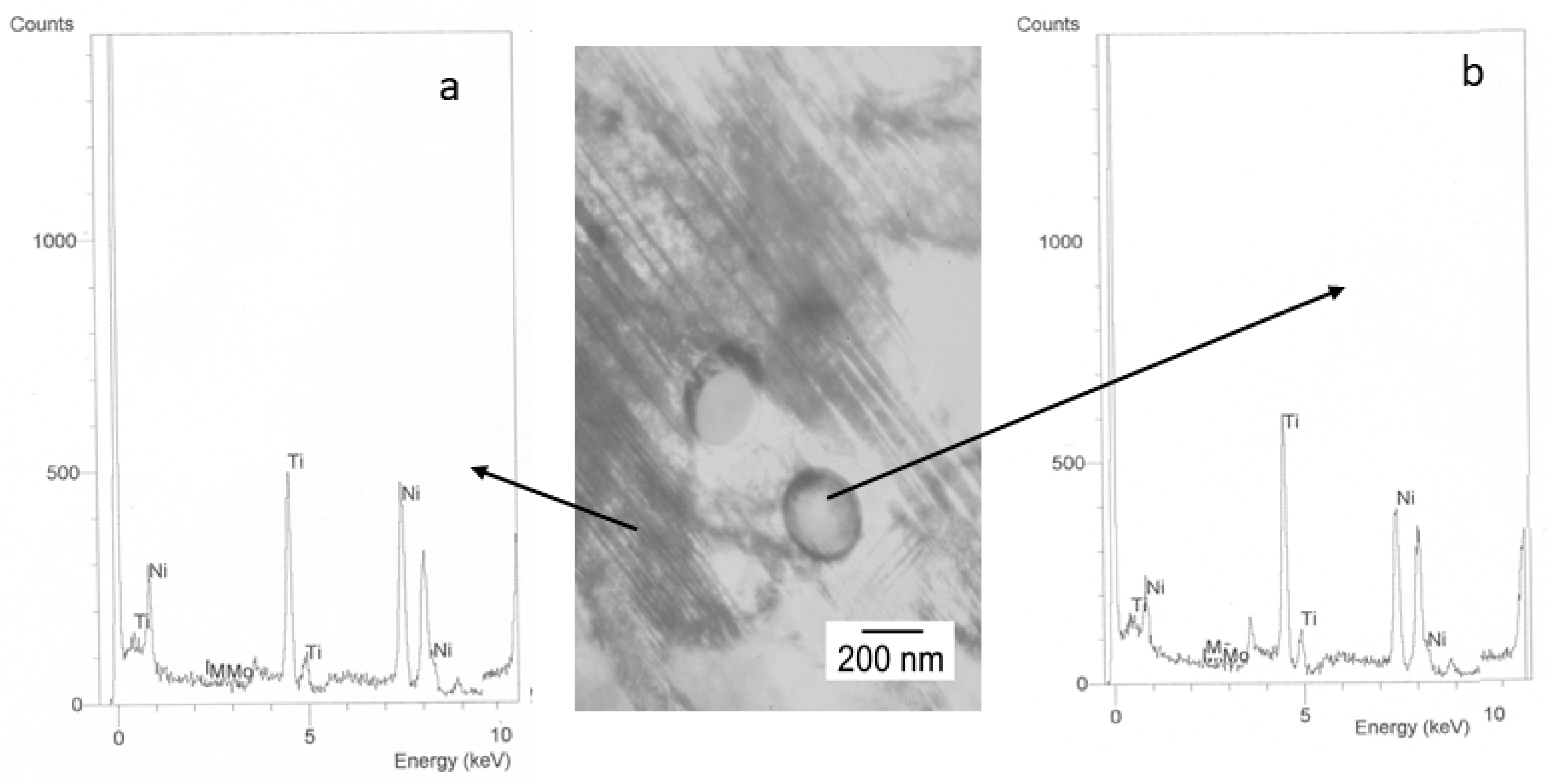
| Mouthwahes | NaF (ppm) |
|---|---|
| 0 | 0 |
| 1 | 130 |
| 2 | 200 |
| 3 | 380 |
| Mouthwash | Time (Days) | %Austenite | %Martensite |
|---|---|---|---|
| Original | 0 | 100 | 0 |
| 0 | 1 | 100 | 0 |
| 0 | 4 | 100 | 0 |
| 0 | 7 | 100 | 0 |
| 0 | 14 | 100 | 0 |
| 1 | 1 | 100 | 0 |
| 1 | 4 | 98 | 2 |
| 1 | 7 | 87 | 13 |
| 1 | 14 | 80 | 20 |
| 2 | 1 | 100 | 0 |
| 2 | 4 | 94 | 6 |
| 2 | 7 | 78 | 22 |
| 2 | 14 | 61 | 39 |
| 3 | 1 | 81 | 19 |
| 3 | 4 | 43 | 57 |
| 3 | 7 | 0 | 100 |
| 3 | 14 | 0 | 100 |
| Mouthwash | Time (Days) | Ms | Mf | As | Af |
|---|---|---|---|---|---|
| Original | 0 | 27.2 ± 0.3 | 16.1 ± 0.4 | 20.0 ± 0.1 | 32.3 ± 0.7 |
| 0 | 1 | 27.0 ± 0.5 | 16.9 ± 0.9 | 22.0 ± 0.9 | 34.5± 0.5 |
| 0 | 4 | 26.9 ± 0.4 | 16.0 ± 0.5 | 21.0 ± 0.5 | 32.0 ± 0.9 |
| 0 | 7 | 27.2 ± 0.3 | 16.1 ± 0.4 | 20.0 ± 0.1 | 32.3 ± 0.8 |
| 0 | 14 | 27.3 ± 0.3 | 16.0 ± 0.4 | 20.1 ± 0.1 | 32.3 ± 0.7 |
| 1 | 1 | 27.9 ± 0.6 | 16.1 ± 0.5 | 21.0 ± 0.1 | 40.3 ± 0.7 |
| 1 | 4 | 27.8 ± 0.3 | 15.8 ± 1.0 | 21.3 ± 0.9 | 40.4 ± 0.5 |
| 1 | 7 | 30.3 ± 0.2 | 9.2 ± 0.3 | 17.1 ± 0.4 | 48.4 ± 0.5 |
| 1 | 14 | 37.4 ± 1.2 | 17.4 ± 1.3 | 28.1 ± 1.9 | 57.6 ± 1.2 |
| 2 | 1 | 30.6 ± 2.2 | 10.3 ± 0.6 | 14.5 ± 1.4 | 38.4 ± 0.9 |
| 2 | 4 | 33.6 ± 0.3 | 1.4 ± 0.3 | 16.1 ± 0.4 | 38.1 ± 0.6 |
| 2 | 7 | 36.6 ± 1.3 | 9.4 ± 1.2 | 19.1 ± 0.9 | 39.3 ± 1.2 |
| 2 | 14 | 38.9 ± 0.9 | 10.0 ± 0.8 | 24.2 ± 2.3 | 47.9 ± 0.9 |
| 3 | 1 | 32.4 ± 0.4 | 13.4 ± 0.1 | 27.3 ± 0.5 | 36.2 ± 0.9 |
| 3 | 4 | 36.4 ± 0.4 | 14.4 ± 0.3 | 34.2 ± 0.5 | 47.3 ±1,0 |
| 3 | 7 | 39.7 ± 1.4 | 16.4 ± 0.1 | 43.2 ± 0.5 | 56.2 ± 2.2 |
| 3 | 14 | 45.4 ± 0.9 | 19.6 ± 1.1 | 54.1 ± 1.4 | 72.3 ± 2.7 |
| Mouthwash | Time (Days) | σβ→SIM (MPa) | σSIM→β (MPa) |
|---|---|---|---|
| Original | 0 | 270 ± 15 | 151 ± 19 |
| 0 | 1 | 276 ± 10 | 141 ± 20 |
| 0 | 4 | 260 ± 14 | 138 ± 18 |
| 0 | 7 | 257 ± 16 | 155 ± 14 |
| 0 | 14 | 278 ± 19 | 150 ± 16 |
| 1 | 1 | 279 ± 20 | 143 ± 17 |
| 1 | 4 | 278 ± 13 | 140 ± 15 |
| 1 | 7 | 180 ± 20 | 88 ± 10 |
| 1 | 14 | 137 ± 12 | 72 ± 10 |
| 2 | 1 | 230 ± 22 | 130 ± 22 |
| 2 | 4 | 198 ± 13 | 98 ± 16 |
| 2 | 7 | 60 ± 15 | 29 ± 10 |
| 2 | 14 | 12 ± 9 | 4 ± 2 |
| 3 | 1 | 82 ± 23 | 26 ± 9 |
| 3 | 4 | 13 ± 6 | 4 ± 3 |
| 3 | 7 | 0 | 0 |
| 3 | 14 | 0 | 0 |
| Mouthwash | Time (Days) | Hysteresis |
|---|---|---|
| Original | 0 | 3.9 |
| 0 | 1 | 5.0 |
| 0 | 4 | 5.1 |
| 0 | 7 | 4.9 |
| 0 | 14 | 4.9 |
| 1 | 1 | 4.9 |
| 1 | 4 | 6.5 |
| 1 | 7 | 7.9 |
| 1 | 14 | 10.7 |
| 2 | 1 | 4.2 |
| 2 | 4 | 14.7 |
| 2 | 7 | 9.7 |
| 2 | 14 | 14.2 |
| 3 | 1 | 13.9 |
| 3 | 4 | 19.8 |
| 3 | 7 | 26.8 |
| 3 | 14 | 34.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastor, F.; Rodríguez, J.C.; Barrera, J.M.; Delgado García-Menocal, J.A.; Brizuela, A.; Puigdollers, A.; Espinar, E.; Gil, J. Effect of Fluoride Content of Mouthwashes on Superelastic Properties of NiTi Orthodontic Archwires. Materials 2022, 15, 6592. https://doi.org/10.3390/ma15196592
Pastor F, Rodríguez JC, Barrera JM, Delgado García-Menocal JA, Brizuela A, Puigdollers A, Espinar E, Gil J. Effect of Fluoride Content of Mouthwashes on Superelastic Properties of NiTi Orthodontic Archwires. Materials. 2022; 15(19):6592. https://doi.org/10.3390/ma15196592
Chicago/Turabian StylePastor, Francisco, Juan Carlos Rodríguez, José María Barrera, José Angel Delgado García-Menocal, Aritza Brizuela, Andreu Puigdollers, Eduardo Espinar, and Javier Gil. 2022. "Effect of Fluoride Content of Mouthwashes on Superelastic Properties of NiTi Orthodontic Archwires" Materials 15, no. 19: 6592. https://doi.org/10.3390/ma15196592
APA StylePastor, F., Rodríguez, J. C., Barrera, J. M., Delgado García-Menocal, J. A., Brizuela, A., Puigdollers, A., Espinar, E., & Gil, J. (2022). Effect of Fluoride Content of Mouthwashes on Superelastic Properties of NiTi Orthodontic Archwires. Materials, 15(19), 6592. https://doi.org/10.3390/ma15196592









