Application of the Hot Wire Method to Measure the Thermal Conductivity Coefficient of a Gypsum Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Composite in the Form of a Homogeneous Mixture
- Water was added to the raw gypsum in a weight ratio of 0.35:0.65.
- Liquid paraffins were added to the prepared gypsum in a weight ratio of 0.8:0.2.
- The polymer was added to the prepared composite in three weight percentages: 0.1%; 0.5%; 1%.
- The mixture prepared in this way was mixed on a magnetic stirrer for about 15 s until obtaining the appropriate consistency.
2.2. Compressive Strength Test
2.3. Testing the Temperature Increase during Solidification by the Hot Wire Method
2.4. Testing the Thermal Conductivity by the Hot Wire Method
- Q—the amount of heat emitted by the heat source (W);
- L—heating element length (m);
- t—time (s); and
- T—temperature of the heating element (°C).
- U—voltage (A); and
- I—current intensity (V).
3. Results
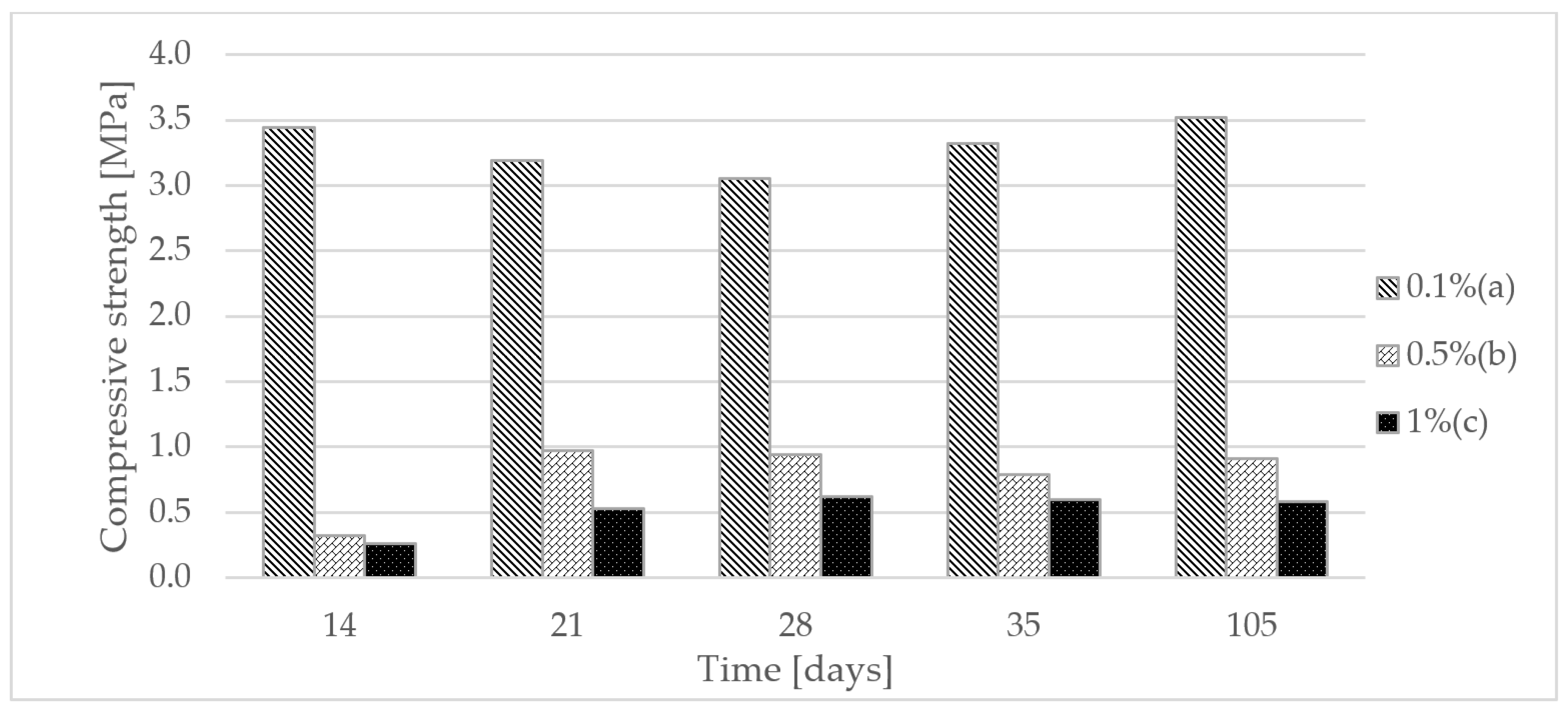
3.1. Compressive Strength Test of a Homogeneous Gypsum Composite
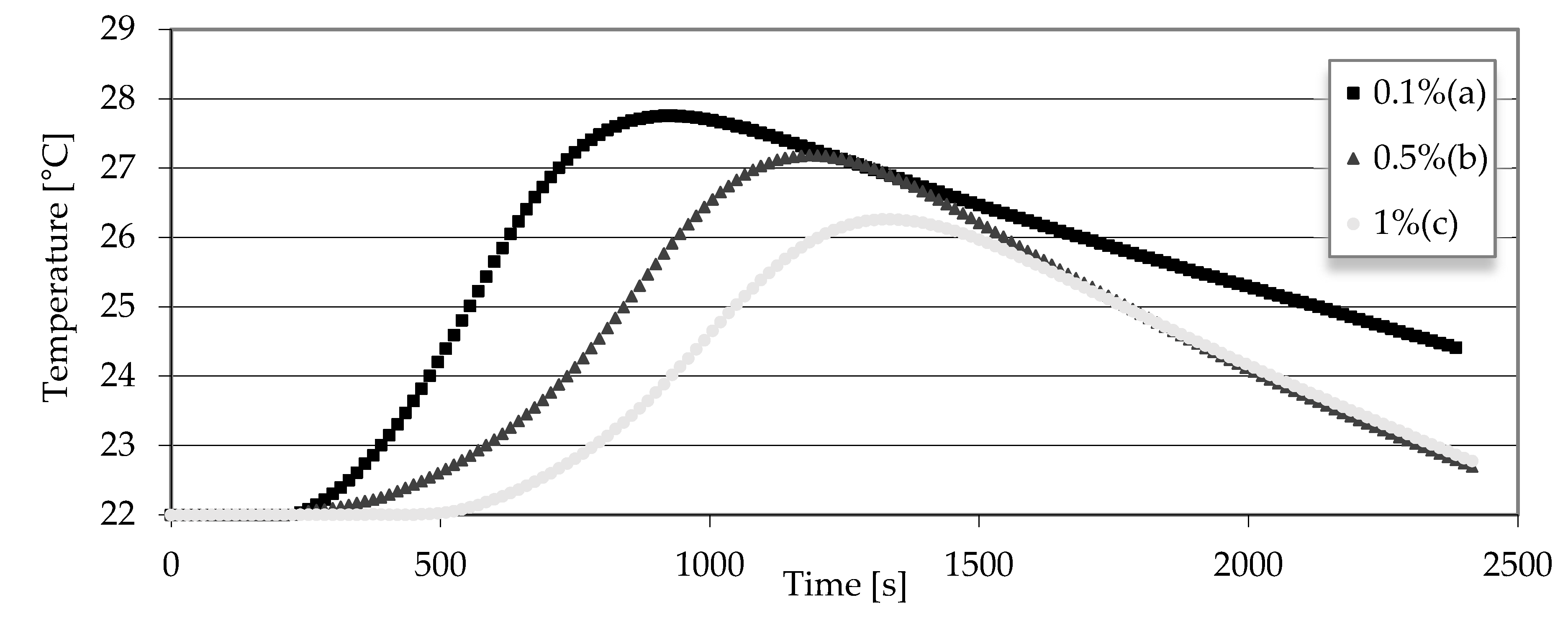
3.2. Testing the Temperature Increase during Solidification by the Hot Wire Method
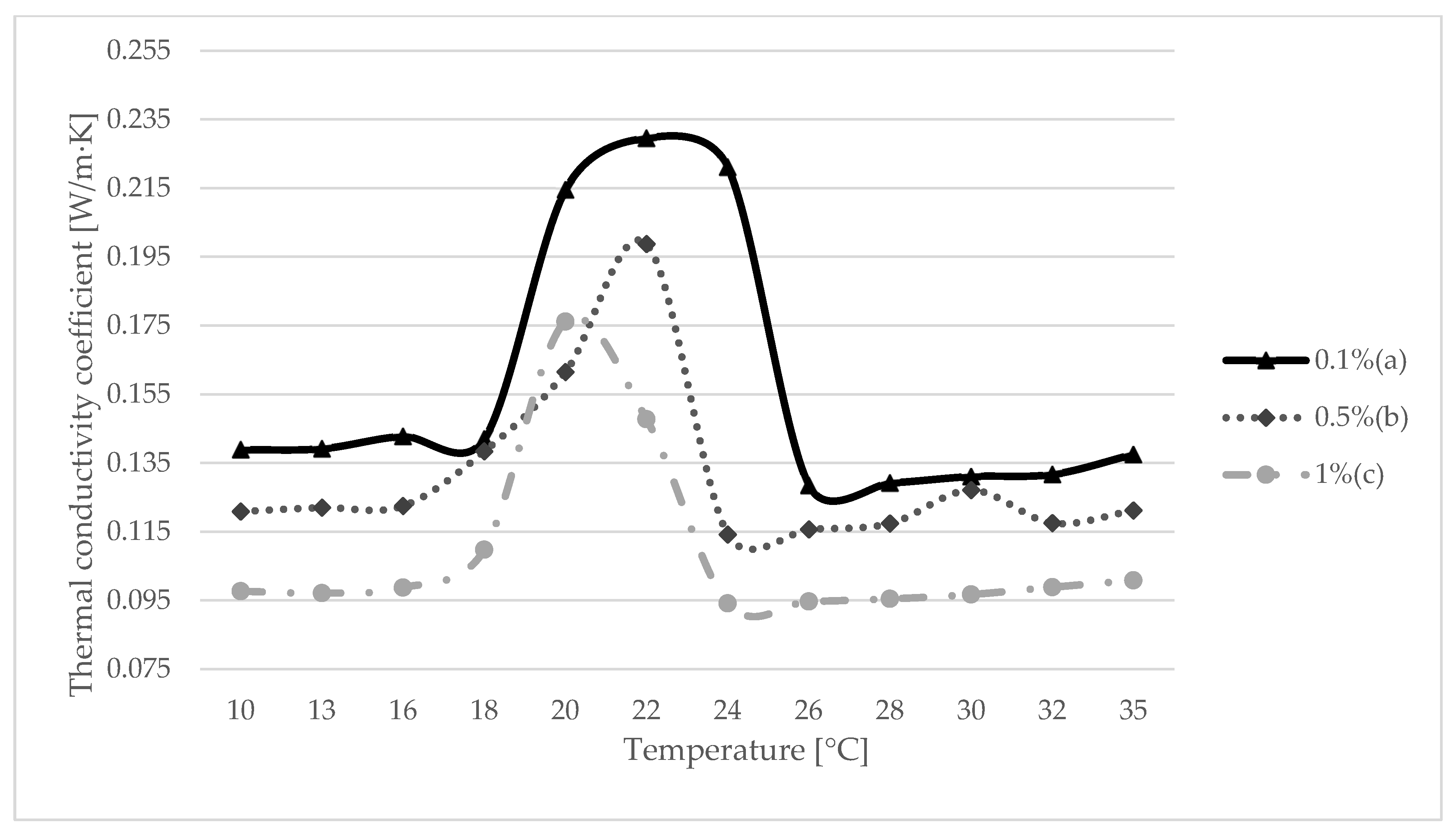
3.3. Testing the Thermal Conductivity by the Hot Wire Method
4. Discussion
5. Conclusions
- The addition of the polymer influenced the compressive strength of the gypsum samples. Looking at the results, a sample with 0.1% polymer gave favorable strength results. In the first studies, between days 14 and 28, endurance decreased, while at day 105, it reached the highest value. This means that the gypsum is mature enough to meet all the regulations that allow the use of gypsum composite in construction.
- Measurement of temperature changes as a function of time using the hot wire method showed that the proportion of polymer reduces the maximum temperature and lengthens the time to reach it. Moreover, it also coincides with the results from the Vicat apparatus, which were presented in another article.
- A hot wire heat conductivity study confirmed that the material used gave the best results when the phase transition point was reached. This means that when thermal comfort is achieved in the room, the composite that will be used to cover the wall layer will be activated and will store energy.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ürge-Vorsatz, D.; Cabeza, L.F.; Serrano, S.; Barreneche, C.; Petrichenko, K. Heating and cooling energy trends and drivers in buildings. Renew. Sustain. Energy Rev. 2015, 41, 85–98. [Google Scholar] [CrossRef]
- Ciemnicka, J.; Prałat, K.; Koper, A.; Makomaski, G.; Majewski, Ł.; Wójcicka, K.; Buczkowska, K.E. Changes in the strength properties and phase transition of gypsum modified with microspheres, aerogel and HEMC polymer. Materials 2021, 14, 3486. [Google Scholar] [CrossRef] [PubMed]
- Heim, D.; Wieprzkowicz, A. Attenuation of temperature fluctuations on an external surface of thewall by a phase change material-activated layer. Appl. Sci. 2017, 8, 11. [Google Scholar] [CrossRef]
- Rostami, R.; Klemm, A.J.; Almeida, F.C.R. Reduction of shrinkage by Superabsorbent polymers (SAP) in fibre reinforced mortars. Constr. Build. Mater. 2021, 288, 123109. [Google Scholar] [CrossRef]
- Munarim, U.; Ghisi, E. Environmental feasibility of heritage buildings rehabilitation. Renew. Sustain. Energy Rev. 2016, 58, 235–249. [Google Scholar] [CrossRef]
- Rio Merino, M. del Aplicaciones del yeso y la escayola en la edificación. Nuevas publicaciones. Inf. Construcción 2004, 56, 53–60. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, Y.; Chen, X.; Song, X.; Tang, K. Experimental Study of an Enhanced Phase Change Material of Para ffi n / Expanded Graphite / Nano-Metal Particles for a Personal Cooling System. Materials 2020, 13, 980. [Google Scholar] [CrossRef]
- Frigione, M.; Lettieri, M. Phase Change Materials for Energy E ffi ciency in Buildings and Their Use in Mortars. Materials 2019, 12, 1260. [Google Scholar] [CrossRef]
- Hawlader, M.N.A.; Uddin, M.S.; Khin, M.M. Microencapsulated PCM thermal-energy storage system. Appl. Energy 2003, 74, 195–202. [Google Scholar] [CrossRef]
- Boh, B.; Sumiga, B. Microencapsulation technology and its applications in building construction materials. RMZ—Mater. Geoenviron. 2008, 55, 329–344. [Google Scholar]
- Wang, H.; Wang, J.P.; Wang, X.; Li, W.; Zhang, X. Preparation and properties of microencapsulated phase change materials containing two-phase core materials. Ind. Eng. Chem. Res. 2013, 52, 14706–14712. [Google Scholar] [CrossRef]
- Alkan, C.; Sari, A.; Karaipekli, A.; Uzun, O. Preparation, characterization, and thermal properties of microencapsulated phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2009, 93, 143–147. [Google Scholar] [CrossRef]
- Cellat, K.; Tezcan, F.; Beyhan, B.; Kardaş, G.; Paksoy, H. A comparative study on corrosion behavior of rebar in concrete with fatty acid additive as phase change material. Constr. Build. Mater. 2017, 143, 490–500. [Google Scholar] [CrossRef]
- Hadiya, J.P.; Shukla, A.K.N. Thermal energy storage using phase change materials: A way forward. Int. J. Glob. Energy Issues 2018, 41, 108–127. [Google Scholar] [CrossRef]
- Kalnæs, S.E.; Jelle, B.P. Phase change materials and products for building applications: A state-of-the-art review and future research opportunities. Energy Build. 2015, 94, 150–176. [Google Scholar] [CrossRef]
- Silakhori, M.; Naghavi, M.S.; Simon, H.; Metselaar, C.; Meurah, T.; Mahlia, I.; Fauzi, H.; Mehrali, M. Accelerated Thermal Cycling Test of Microencapsulated Paraffin Wax/Polyaniline Made by Simple Preparation Method for Solar Thermal Energy Storage. Materials 2013, 6, 1608–1620. [Google Scholar] [CrossRef]
- Xin, C.; Lu, S.F.; Shen, T.W.; Xiao, C.P.; Zhang, Y.S. Preparation and properties of polyurethane microencapsulated phase change materials. Xiandai Huagong/Mod. Chem. Ind. 2019, 39, 100–102. [Google Scholar] [CrossRef]
- Qiu, X.; Lu, L.; Tang, G.; Song, G. Preparation and thermal properties of microencapsulated paraffin with polyurea/acrylic resin hybrid shells as phase change energy storage materials. J. Therm. Anal. Calorim. 2020, 143, 3023–3032. [Google Scholar] [CrossRef]
- Li, C.; Yu, H.; Song, Y.; Liu, Z. Novel hybrid microencapsulated phase change materials incorporated wallboard for year-long year energy storage in buildings. Energy Convers. Manag. 2019, 183, 791–802. [Google Scholar] [CrossRef]
- Silva, N.; Aguiar, J.B.; Bragança, L.M.; Freire, T.; Cardoso, I. Properties of gypsum-PCM based mortars for interior plastering of construction systems. Mater. Sci. Forum 2008, 587–588, 913–917. [Google Scholar] [CrossRef]
- Powała, K.; Heim, D. Paraffin Permeability of Synthetic Gypsum Binders Modified by Individual Polymers. Latv. J. Phys. Tech. Sci. 2019, 56, 47–56. [Google Scholar] [CrossRef]
- Powała, K.; Obraniak, A.; Heim, D. Mechanical Properties of Gypsum-PCM Composite Refined with the Acrylic Copolymer. Period. Polytech. Civ. Eng. 2022, 66, 235–243. [Google Scholar] [CrossRef]
- Heim, D.; Mrowiec, A.; Prałat, K. Udoskonalona Metoda Pomiarów Przewodności Cieplnej Płynów—Stanowisko Badawcze Małych Mocy. Fiz. Budowli W Teor. I Prakt. 2011, VI, 35–40. [Google Scholar]
- Powała, K.; Obraniak, A.; Heim, D. Testing a Gypsum Composite Based on Raw Gypsum with a Direct Admixture of Paraffin and Polymer to Improve Thermal Properties. Materials 2021, 14, 3241. [Google Scholar] [CrossRef] [PubMed]
- Errebai, F.B.; Chikh, S.; Derradji, L.; Amara, M.; Younsi, Z. Optimum mass percentage of microencapsulated PCM mixed with gypsum for improved latent heat storage. J. Energy Storage 2021, 33, 101910. [Google Scholar] [CrossRef]

| Parameter | Unit | Result |
|---|---|---|
| Melting area Congealing area | °C °C | 20–23 23–20 |
| Heat storage capacity | kJ/kg | 190 |
| Specific heat capacity Density solid Density liquid | kJ/kg·K kg/L kg/L | 2 0.76 0.7 |
| Parameter | Unit | Result |
|---|---|---|
| Calcium sulfate hemihydrate content | % | >95 |
| (β-CaSO4·0.5H2O) | ||
| Crystallization water | % | 5.6–6.0 |
| Mechanical strength after drying to constant weight | ||
| - for bending | >5.0 | |
| - for compression | MPa | >12.0 |
| 0.1% (a) | 0.5% (b) | 1% (c) | ||||
|---|---|---|---|---|---|---|
| Day | Average | Standard Deviation | Average | Standard Deviation | Average | Standard Deviation |
| - | (MPa) | (MPa) | (MPa) | |||
| 14 | 3.44 | 0.57 | 0.32 | 0.12 | 0.26 | 0.05 |
| 21 | 3.19 | 0.52 | 0.97 | 0.53 | 0.53 | 0.14 |
| 28 | 3.05 | 0.58 | 0.94 | 0.65 | 0.62 | 0.10 |
| 35 | 3.32 | 0.82 | 0.79 | 0.42 | 0.60 | 0.12 |
| 105 | 3.52 | 0.70 | 0.91 | 0.39 | 0.58 | 0.12 |
| Percentage of Polymer | Drying Time |
|---|---|
| 0.1% | 19 min |
| 0.5% | 38 min 42 s |
| 1% | 55 min 30 s |
| 0.1% (a) | 0.5% (b) | 1% (c) | |
|---|---|---|---|
| Temperature | Average | ||
| (ºC) | (W/m·K) | ||
| 10 | 0.138872 | 0.120914 | 0.09772 |
| 13 | 0.139181 | 0.122027 | 0.097125 |
| 16 | 0.142789 | 0.122518 | 0.09873 |
| 18 | 0.141993 | 0.138395 | 0.109752 |
| 20 | 0.214557 | 0.161498 | 0.176168 |
| 22 | 0.229555 | 0.198744 | 0.147769 |
| 24 | 0.22113 | 0.114198 | 0.09413 |
| 26 | 0.128469 | 0.115705 | 0.094686 |
| 28 | 0.129188 | 0.117409 | 0.09549 |
| 30 | 0.131072 | 0.127101 | 0.096732 |
| 32 | 0.131698 | 0.117528 | 0.098869 |
| 35 | 0.137517 | 0.121184 | 0.100865 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Powała, K.; Obraniak, A.; Heim, D.; Mrowiec, A. Application of the Hot Wire Method to Measure the Thermal Conductivity Coefficient of a Gypsum Composite. Materials 2022, 15, 6564. https://doi.org/10.3390/ma15196564
Powała K, Obraniak A, Heim D, Mrowiec A. Application of the Hot Wire Method to Measure the Thermal Conductivity Coefficient of a Gypsum Composite. Materials. 2022; 15(19):6564. https://doi.org/10.3390/ma15196564
Chicago/Turabian StylePowała, Krzysztof, Andrzej Obraniak, Dariusz Heim, and Andrzej Mrowiec. 2022. "Application of the Hot Wire Method to Measure the Thermal Conductivity Coefficient of a Gypsum Composite" Materials 15, no. 19: 6564. https://doi.org/10.3390/ma15196564
APA StylePowała, K., Obraniak, A., Heim, D., & Mrowiec, A. (2022). Application of the Hot Wire Method to Measure the Thermal Conductivity Coefficient of a Gypsum Composite. Materials, 15(19), 6564. https://doi.org/10.3390/ma15196564











