High-Performance pH Sensor Electrodes Based on a Hexagonal Pt Nanoparticle Array-Coated Nanoporous Alumina Membrane
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
2.2. Fabrication and Characterization of the PAAM/Pt Sensor Electrode
3. Results and Discussion
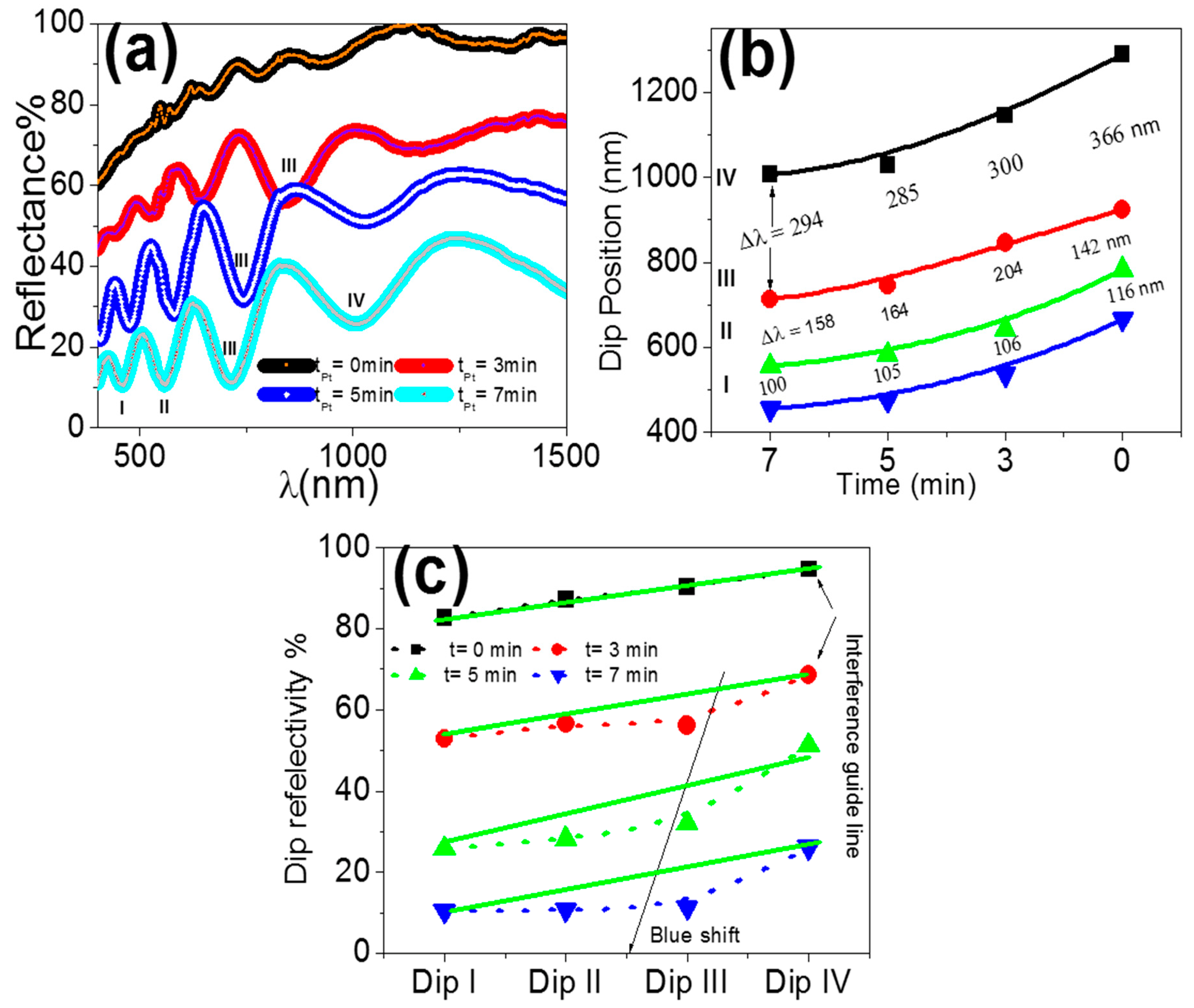
3.1. Characterization of PAAM and PAAM/Pt
3.2. Sensing Properties
3.2.1. Sensitivity of PAAM Sensor to H+ Ions under the Effect of Different Pt Film Thicknesses
3.2.2. PAAM/Pt Sensor Electrode Selectivity
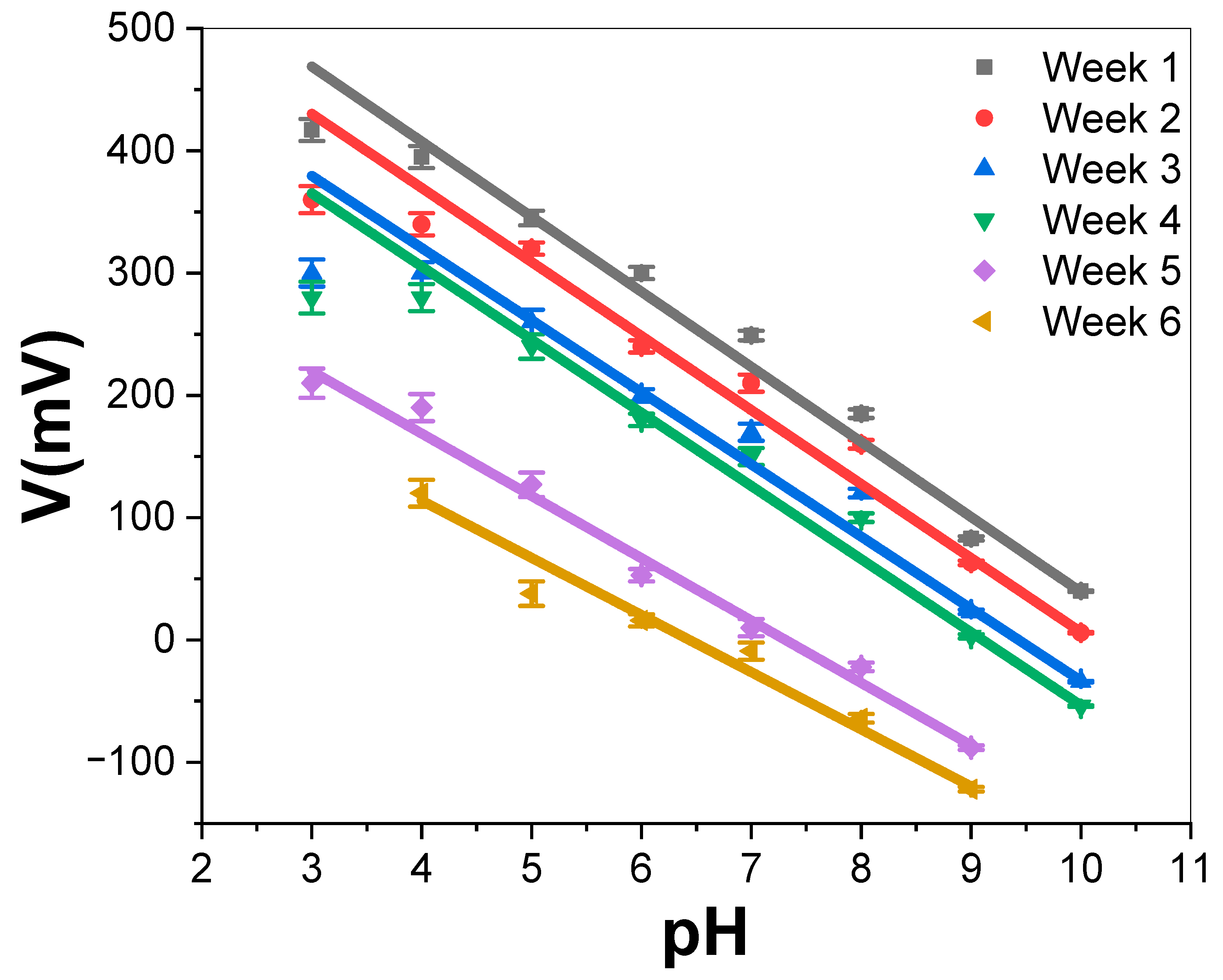
3.2.3. PAAM/Pt Sensor Electrode Stability
3.2.4. Response Time and Reproducibility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rabia, M.; Mohamed, S.H.; Zhao, H.; Shaban, M.; Lei, Y.; Ahmed, A.M. TiO2/TiOxNY hollow mushrooms-like nanocomposite photoanode for hydrogen electrogeneration. J. Porous Mater. 2020, 27, 133–139. [Google Scholar] [CrossRef]
- Shaban, M.; Hamdy, H.; Shahin, F.; Ryu, S.W. Strong surface plasmon resonance of ordered gold nanorod array fabricated in porous anodic alumina template. J. Nanosci. Nanotechnol. 2010, 10, 3034–3037. [Google Scholar] [CrossRef] [PubMed]
- Yanagishita, T.; Kato, K.; Shirano, N.; Masuda, H. Preparation of Ideally Ordered Anodic Porous Alumina by Prepatterning Process Using a Flexible Mold. ECS J. Solid State Sci. Technol. 2022, 11, 013001. [Google Scholar] [CrossRef]
- Bennett, T.D.; Coudert, F.-X.; James, S.L.; Cooper, A.I. The changing state of porous materials. Nat. Mater. 2021, 20, 1179–1187. [Google Scholar] [CrossRef]
- Sun, Y.; Rogge, S.M.J.; Lamaire, A.; Vandenbrande, S.; Wieme, J.; Siviour, C.R.; van Speybroeck, V.; Tan, J.-C. High-rate nanofluidic energy absorption in porous zeolitic frameworks. Nat. Mater. 2021, 20, 1015–1023. [Google Scholar] [CrossRef]
- Saharil, F.; Forsberg, F.; Liu, Y.; Bettotti, P.; Kumar, N.; Niklaus, F.; Haraldsson, T.; van der Wijngaart, W.; Gylfason, K.B. Dry adhesive bonding of nanoporous inorganic membranes to microfluidic devices using the OSTE(+) dual-cure polymer. J. Micromech. Microeng. 2013, 23, 025021. [Google Scholar] [CrossRef]
- Shaban, M.; Rabia, M.; Fathallah, W.; El-Mawgoud, N.A.; Mahmoud, A.; Hussien, H.; Said, O. Preparation and Characterization of Polyaniline and Ag/Polyaniline Composite Nanoporous Particles and Their Antimicrobial Activities. J. Polym. Environ. 2018, 26, 434–442. [Google Scholar] [CrossRef]
- Shaban, M.; Ali, S.; Rabia, M. Design and application of nanoporous graphene oxide film for CO2, H2, and C2H2 gases sensing. J. Mater. Res. Technol. 2019, 8, 4510–4520. [Google Scholar] [CrossRef]
- Li, Z.; Wang, D.; Chen, J.; Wu, C.; Shen, J.; Li, Y.; He, G.; Li, Y. Transactions of the Indian Institute of Metals; Springer: Berlin/Heidelberg, Germany, 2022; Volume 75, pp. 771–781. [Google Scholar]
- Lai, C.W. Surface Morphology and Growth of Anodic Titania Nanotubes Films: Photoelectrochemical Water Splitting Studies. J. Nanomater. 2015, 2015, 820764. [Google Scholar] [CrossRef]
- Shaban, M.; Ali, M.; Abdel-Hady, K.; Hamdy, H. Al2O3 and Sn/Al2O3 nanowires: Fabrication and characterisation. Micro Nano Lett. 2015, 10, 324–329. [Google Scholar] [CrossRef]
- Sousa, C.T.; Leitao, D.C.; Proenca, M.P.; Ventura, J.; Pereira, A.M.; Araujo, J.P. Nanoporous alumina as templates for multifunctional applications. Appl. Phys. Rev. 2014, 1, 031102. [Google Scholar] [CrossRef]
- Safronov, A.P.; Stadler, B.J.H.; Um, J.; Kouhpanji, M.R.Z.; Masa, J.A.; Galyas, A.G.; Kurlyandskaya, G.V. Polyacrylamide Ferrogels with Ni Nanowires. Materials 2019, 12, 2582. [Google Scholar] [CrossRef]
- Tantawi, K.H.; Berdiev, B.; Cerro, R.; Williams, J.D. Porous silicon membrane for investigation of transmembrane proteins. Superlattices Microstruct. 2013, 58, 72–80. [Google Scholar] [CrossRef]
- Kouhpanji, M.R.Z.; Stadler, B.J.H. A Guideline for Effectively Synthesizing and Characterizing Magnetic Nanoparticles for Advancing Nanobiotechnology: A Review. Sensors 2020, 20, 2554. [Google Scholar] [CrossRef]
- García-Arribas, A.; Martínez, F.; Fernández, E.; Ozaeta, I.; Kurlyandskaya, G.V.; Svalov, A.V.; Berganzo, J.; Barandiaran, J.M. GMI detection of magnetic-particle concentration in continuous flow. Sens. Actuators A Phys. 2011, 172, 103–108. [Google Scholar] [CrossRef]
- Fatimah, S.; Rahman, A.; Yusof, N.A.; Hashim, U.; Nuzaihan, M.; Nor, M. Design and Fabrication of Silicon Nanowire based Sensor. Int. J. Electrochem. Sci. 2013, 8, 10946–10960. [Google Scholar]
- Werner, J.; Belz, M.; Klein, K.-F.; Sun, T.; Grattan, K.T.V. Fiber optic sensor designs and luminescence-based methods for the detection of oxygen and pH measurement. Measurement 2021, 178, 109323. [Google Scholar] [CrossRef]
- Ahmed, N.M.; Sabah, F.A.; Al-Hardan, N.H.; Almessiere, M.A.; Mohammad, S.M.; Foong Lim, W.; Jumaah, M.; Islam, A.K.M.S.; Hassan, Z.; Quah, H.J.; et al. Development of EGFET-based ITO pH sensors using epoxy free membrane. Semicond. Sci. Technol. 2021, 36, 045027. [Google Scholar] [CrossRef]
- Rasheed, H.S.; Ahmed, N.M.; Matjafri, M.Z.; Al-Hardan, N.H.; Almessiere, M.A.; Sabah, F.A.; Al-Hazeem, N.Z. Multilayer ZnO/Pd/ZnO Structure as Sensing Membrane for Extended-Gate Field-Effect Transistor (EGFET) with High pH Sensitivity. J. Electron. Mater. 2017, 46, 5901–5908. [Google Scholar] [CrossRef]
- Ahmadi, H.; Keshipour, S.; Ahour, F. New water-soluble colorimetric pH and metal ione sensor based on graphene quantum dot modified with alizarine red S. Sci. Rep. 2020, 10, 14185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dong, Y.; Liu, G.; Zhang, G.; Li, J.; Zhu, Y.; Yu, W.; Liu, H. Using Cu2+ ions as a detection material to verify the synthesis mechanism of Au nanoclusters mediated by wool keratin and silk fibroin resilience network. Text. Res. J. 2022, 92, 2150–2161. [Google Scholar] [CrossRef]
- Flores, J.M.; Maningas, M.B.B.; Sevilla, F.B. Micro-Probe Potentiometric pH Sensor for Detection of Amplification in the LAMP Assay for White Spot Syndrome Virus (WSSV) in Shrimps. IEEE Sens. J. 2022, 22, 9289–9295. [Google Scholar] [CrossRef]
- Boswell, P.G.; Szíjjártó, C.; Jurisch, M.; Gladysz, J.A.; Rábai, J.; Bühlmann, P. Fluorophilic ionophores for potentiometric pH determinations with fluorous membranes of exceptional selectivity. Anal. Chem. 2008, 80, 2084–2090. [Google Scholar] [CrossRef] [PubMed]
- Sayyah, S.M.; Shaban, M.; Rabia, M. m-Toluidine Polymer Film Coated Platinum Electrode as a pH Sensor by Potentiometric Methods. Sens. Lett. 2015, 13, 961–966. [Google Scholar] [CrossRef]
- Kim, D.M.; Cho, S.J.; Cho, C.H.; Kim, K.B.; Kim, M.Y.; Shim, Y.B. Disposable all-solid-state pH and glucose sensors based on conductive polymer covered hierarchical AuZn oxide. Biosens. Bioelectron. 2016, 79, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Ibupoto, Z.H.; Khun, K.; Willander, M. Development of a pH sensor using nanoporous nanostructures of NiO. J. Nanosci. Nanotechnol. 2014, 14, 6699–6703. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kwak, T.J.; Zhang, X.; McClain, R.; Chang, W.-J.; Gunasekaran, S. Digital pH test strips for in-field pH monitoring using iridium oxide-reduced graphene oxide hybrid thin films. ACS Sens. 2016, 1, 1235–1243. [Google Scholar] [CrossRef]
- Manjakkal, L.; Szwagierczak, D.; Dahiy, R. Metal oxides based electrochemical pH sensors: Current progress and future perspectives. Prog. Mater. Sci. 2020, 109, 100635. [Google Scholar] [CrossRef]
- Rao, T.; Li, J.; Cai, W.; Wu, M.; Jiang, J.; Yang, P.; Zhou, Y.; Liao, W. Fabrication of a Mesoporous Multimetallic Oxide-based Ion-Sensitive Field Effect Transistor for pH Sensing. ACS Omega 2021, 6, 32297. [Google Scholar] [CrossRef] [PubMed]
- Young, S.-J.; Chu, Y.-J.; Chen, Y.-L. Enhancing pH Sensors Performance of ZnO Nanorods With Au Nanoparticles Adsorption. IEEE Sens. J. 2021, 21, 13068–13073. [Google Scholar] [CrossRef]
- Chu, Y.-L.; Young, S.-J.; Dai, H.-R.; Lee, Y.-M.; Khosla, A.; Chu, T.-T.; Ji, L.-W. Improved pH-Sensing Characteristics by Pt Nanoparticle-Decorated ZnO Nanostructures. ECS J. Solid State Sci. Technol. 2021, 10, 067001. [Google Scholar] [CrossRef]
- Serry, M.; Gamal, A.; Shaban, M.; Sharaf, A.H. High sensitivity optochemical and electrochemical metal ion sensor. Micro Nano Lett. 2013, 8, 775–778. [Google Scholar] [CrossRef]
- Shaban, M.; Hady, A.G.A.; Serry, M. A new sensor for heavy metals detection in aqueous media. IEEE Sens. J. 2014, 14, 436–441. [Google Scholar] [CrossRef]
- Shaban, M.; Hamdy, H.; Shahin, F.; Ryu, S.W. Optical properties of porous anodic alumina membrane uniformly decorated with ultra-thin porous gold nanoparticles arrays. J. Nanosci. Nanotechnol. 2011, 11, 941–952. [Google Scholar] [CrossRef]
- Shaban, M. Effect of pore thickness and the state of polarization on the optical properties of hexagonal nanoarray of Au/nanoporous anodic alumina membrane. J. Nanomater. 2015, 2015, 347486. [Google Scholar] [CrossRef]
- Wang, G.; Shi, C.; Zhao, N.; Du, X. Synthesis and characterization of Ag nanoparticles assembled in ordered array pores of porous anodic alumina by chemical deposition. Mater. Lett. 2007, 61, 3795–3797. [Google Scholar] [CrossRef]
- Hopper, A.; Beswick-Jones, H.; Brown, A.M. The Nernst equation: Using physico-chemical laws to steer novel experimental design. Adv. Physiol. Educ. 2022, 46, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Ciribelli, B.N.; Colmati, F.; de Souza, E.C. Nernst equation applied to electrochemical systems and centenary of his Nobel Prize in chemistry. Int. J. Innov. Educ. Res. 2020, 8, 670–683. [Google Scholar] [CrossRef]
- Sayyah, E.S.M.; Shaban, M.; Rabia, M. A sensor of m-cresol nanopolymer/Pt-electrode film for detection of lead ions by potentiometric methods. Adv. Polym. Technol. 2018, 37, 1296–1304. [Google Scholar] [CrossRef]
- Sayyah, S.M.; Shaban, M.; Rabia, M. Electropolymerization of m-Toluidin on Platinum Electrode from Aqueous Acidic Solution and Character of the Obtained Polymer. Adv. Polym. Technol. 2018, 37, 126–136. [Google Scholar] [CrossRef]
- Zhuiykov, S. Solid-state sensors monitoring parameters of water quality for the next generation of wireless sensor networks. Sens. Actuators B Chem. 2012, 161, 1–20. [Google Scholar] [CrossRef]
- Zeng, X.Q.; Wang, Y.L.; Deng, H.; Latimer, M.L.; Xiao, Z.L.; Pearson, J.; Xu, T.; Wang, H.H.; Welp, U.; Crabtree, G.W.; et al. Networks of ultrasmall Pd/Cr nanowires as high performance hydrogen sensors. ACS Nano 2011, 5, 7443–7452. [Google Scholar] [CrossRef]
- Sayyah, S.M.; Shaban, M.; Rabia, M. A sensor of m-toluidine/m-cresol polymer film for detection of lead ions by potentiometric methods. Sens. Lett. 2016, 14, 522–529. [Google Scholar] [CrossRef]
- Sayyah, S.M.; Shaban, M.; Rabia, M. A High-Sensitivity Potentiometric Mercuric Ion Sensor Based on m-Toluidine Films. IEEE Sens. J. 2016, 16, 1541–1548. [Google Scholar] [CrossRef]
- Avolio, R.; Grozdanov, A.; Avella, M.; Barton, J.; Cocca, M.; de Falco, F.; Dimitrov, A.T.; Errico, M.E.; Fanjul-Bolado, P.; Gentile, G.; et al. Review of pH sensing materials from macro- to nano-scale: Recent developments and examples of seawater applications. Crit. Rev. Environ. Sci. Technol. 2022, 52, 979–1021. [Google Scholar] [CrossRef]
- Shi, E.; Zhang, W.; Yang, B. Effect of Tb(III) on the unfolding of ciliate Euplotes octocarinatus centrin induced by guanidine hydrochloride. J. Rare Earths 2018, 36, 203–208. [Google Scholar] [CrossRef]
- Hegarty, C.; Kirkwood, S.; Cardosi, M.F.; Lawrence, C.L.; Taylor, C.M.; Smith, R.B.; Davis, J. Disposable solid state pH sensor based on flavin polymer-ferrocyanide redox couples. Microchem. J. 2018, 139, 210–215. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Q.; Guo, Z.; Wang, S. CoA-dependent coordination polymer as a novel electrochemical sensing platform for sensitive detection of hydrogen peroxide in biological environments. J. Electroanal. Chem. 2017, 801, 306–314. [Google Scholar] [CrossRef]
- Cisternas, R.; Ballesteros, L.; Valenzuela, M.L.; Kahlert, H.; Scholz, F. Decreasing the time response of calibration-free pH sensors based on tungsten bronze nanocrystals. J. Electroanal. Chem. 2017, 801, 315–318. [Google Scholar] [CrossRef]
- Lee, C.H.; Sun, T.L. Evaluation of postural stability based on a force plate and inertial sensor during static balance measurements. J. Physiol. Anthropol. 2018, 37, 27. [Google Scholar] [CrossRef]
- Vig, J.R.; Walls, F.L. A review of sensor sensitivity and stability. In Proceedings of the 2000 IEEE/EIA International Frequency Control Symposium and Exhibition, Kansas City, MO, USA, 9 June 2000; pp. 30–33. [Google Scholar]
- Anuar, N.A.B.; Nor, N.H.M.; Awang, R.B.; Nakajima, H.; Tunmee, S.; Tripathi, M.; Dalton, A.; Goh, B.T. Low-temperature growth of graphene nanoplatelets by hot-wire chemical vapour deposition. Surf. Coat. Technol. 2021, 411, 126995. [Google Scholar] [CrossRef]
- El-Hossary, F.M.; Ghitas, A.; El-Rahman, A.M.A.; Shahat, M.A.; Fawey, M.H. The effective reduction of graphene oxide films using RF oxygen plasma treatment. Vacuum 2021, 188, 110158. [Google Scholar] [CrossRef]
- Pang, J.; Le, X.; Pang, K.; Dong, H.; Zhang, Q.; Xu, Z.; Gao, C.; Fu, Y.; Xie, J. Highly precision carbon dioxide acoustic wave sensor with minimized humidity interference. Sens. Actuators B Chem. 2021, 338, 129824. [Google Scholar] [CrossRef]
- Chen, P.; Zhao, Z.; Shao, Z.; Tian, Y.; Li, B.; Huang, B.; Zhang, S.; Liu, C.; Shen, X. Highly selective NH3 gas sensor based on polypyrrole/Ti3C2Tx nanocomposites operating at room temperature. J. Mater. Sci. Mater. Electron. 2022, 33, 6168–6177. [Google Scholar] [CrossRef]
- Chiang, J.L.; Chou, J.C.; Chen, Y.C. Study on light and temperature properties of AlN pH-Ion-sensitive field-effect transistor devices. Jpn. J. Appl. Phys. 2005, 44, 4831–4837. [Google Scholar] [CrossRef]
- Sabah, F.A.; Ahmed, N.M.; Hassan, Z.; Almessiere, M.A. Influence of CuS membrane annealing time on the sensitivity of EGFET pH sensor. Mater. Sci. Semicond. Process. 2017, 71, 217–225. [Google Scholar] [CrossRef]
- Isa, I.M.; Hamzah, M.H.; Sabian, I.S.; Ghani, S.A. Chitosan Based Heterogeneous Membrane Sensor for pH—A Prototype. Int. J. Electrochem. Sci. 2012, 7, 12045–12053. [Google Scholar]
- Le, S.H.; Salmassi, B.R.; Burghartz, J.N.; Sarro, P.M. Characterization of ferroelectric thin film materials for FeRAM. In SAFE 2004; Semiconductor Advances for Future Electronics; STW Technology Foundation: Utrecht, The Netherlands, 2004; pp. 710–713. [Google Scholar]




| Time Coating (min.) | -Slope (mV/Decade) | R2 | Response Range | Detection Limit |
|---|---|---|---|---|
| 3 | 49.65 | 0.989 | 3–10 | 11.77 |
| 5 | 56.31 | 0.974 | 3–10 | 11.47 |
| 7 | 54.47 | 0.974 | 3–10 | 11.42 |
| Ions | V (mV) | RSD % |
|---|---|---|
| H+ | 250 | 0 |
| Na+ | 233 | 4.91 |
| K+ | 230 | 4.97 |
| Mg2+ | 225 | 7.44 |
| Lifetime (Week) | -Slope (mV/Decade) | R2 | Response Range |
|---|---|---|---|
| 1 | 56.31 | 0.987 | 3–10 |
| 2 | 52.1 | 0.980 | 3–10 |
| 3 | 49.7 | 0.997 | 3–10 |
| 4 | 49.67 | 0.977 | 3–10 |
| 5 | 51.2 | 0.990 | 3–9 |
| 6 | 44.02 | 0.98 | 4–9 |
| Repeating Times | -Slope (mV/Decade) | R2 | Response Range |
|---|---|---|---|
| 1 | 56.31 | 0.989 | 3–10 |
| 2 | 55.56 | 0.98 | 3–10 |
| 3 | 55.0 | 0.987 | 3–10 |
| pH Sensor Electrode | Slope mV/Decade | Response Range | Ref |
|---|---|---|---|
| Au/poly(m-toluidine) | 57.34 | 6–10 | [25] |
| ZnO/Si | 22.4 | 4–11 | [60] |
| Chitosan | 56.0 | 2–9 | [59] |
| CuS | 27.8 | 2–12 | [58] |
| SiO2 | 53.0 | 1–9 | [57] |
| ZnO/Pd/ZnO | 52.0 | 5–12 | [20] |
| ITO membrane | 36.4 | 4–10 | [19] |
| PAAM/5 min Pt | 56.31 | 3–10 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altowyan, A.S.; Shaban, M.; Gamel, A.; Gamal, A.; Ali, M.; Rabia, M. High-Performance pH Sensor Electrodes Based on a Hexagonal Pt Nanoparticle Array-Coated Nanoporous Alumina Membrane. Materials 2022, 15, 6515. https://doi.org/10.3390/ma15196515
Altowyan AS, Shaban M, Gamel A, Gamal A, Ali M, Rabia M. High-Performance pH Sensor Electrodes Based on a Hexagonal Pt Nanoparticle Array-Coated Nanoporous Alumina Membrane. Materials. 2022; 15(19):6515. https://doi.org/10.3390/ma15196515
Chicago/Turabian StyleAltowyan, Abeer S., Mohamed Shaban, Asmaa Gamel, Ahmed Gamal, Mona Ali, and Mohamed Rabia. 2022. "High-Performance pH Sensor Electrodes Based on a Hexagonal Pt Nanoparticle Array-Coated Nanoporous Alumina Membrane" Materials 15, no. 19: 6515. https://doi.org/10.3390/ma15196515
APA StyleAltowyan, A. S., Shaban, M., Gamel, A., Gamal, A., Ali, M., & Rabia, M. (2022). High-Performance pH Sensor Electrodes Based on a Hexagonal Pt Nanoparticle Array-Coated Nanoporous Alumina Membrane. Materials, 15(19), 6515. https://doi.org/10.3390/ma15196515







