Effect of the Synthesis Conditions of MIL-100(Fe) on Its Catalytic Properties and Stability under Reaction Conditions
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
| Phase | h,k,l | 2θ (°) | d-Spacing | dNO F | dHF | dHF:KF | dKF |
|---|---|---|---|---|---|---|---|
| MIL-100 | 4,2,2 | 5.90 | 14.97 | - | 15.07 | 15.33 | - |
| 5,1,1 | 6.26 | 14.11 | - | 14.22 | 14.55 | 14.27 | |
| 8,2,2 | 10.22 | 8.64 | - | 8.70 | 8.79 | 8.72 | |
| 9,1,1 | 10.98 | 8.05 | 8.10 | 8.10 | 8.18 | 8.09 | |
| 15,5,5 | 20.06 | 4.42 | 4.44 | 4.43 | 4.45 | 4.43 | |
| 1,0,−2 | 24.12 | 3.69 | 3.70 | - | - | 3.69 | |
| 1,0,4 | 33.12 | 2.70 | 2.71 | - | - | 2.71 | |
| 2,−1,0 | 35.62 | 2.51 | 2.52 | - | - | 2.52 | |
| α-Fe2O3 | 1,0,−2 | 24.12 | 3.69 | 3.70 | - | - | 3.69 |
| 1,0,4 | 33.12 | 2.70 | 2.71 | - | - | 2.71 | |
| 2,−1,0 | 35.62 | 2.51 | 2.52 | - | - | 2.52 |
| Entry | Catalyst | Ep./Catalyst (mmol/mg) | Conditions | Solvent | Conv. (%) | Sel. (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | SBA-15 | 18/100 | 4 h, 120 °C, 6.9 bar | Acetonitrile | 1.5 | 9.0 | [54] |
| 2 | MFI | 10/100 | 4 h, 140 °C, 20 bar | No solvent | 90.1 | 95.0 | [55] |
| 3 | TS-1 | 18/100 | 4 h, 120 °C, 6.9bar | Methylene chloride | 70.8 | 73.0 | [56] |
| 4 | MCM-41 | 18/50 | 3 h, 120 °C, 6.9 bar | Acetonitrile | 99.0 | 80.0 | [57] |
| 5 | ZIF-8 | 18/100 | 4 h, 80 °C, 7 bar | No solvent | 84.0 | 52.0 | [58] |
| 6 | HKUST-1 | 18/100 | 4 h, 100 °C, 7 bar | No solvent | 64.0 | 52.0 | [59] |
| 7 | MIL-125 | 5/20 | 6 h, 100 °C, 20 bar | No solvent | 64.0 | 99.0 | [60] |
| 8 | MIL-101(Cr) | 9.2/57 | 24 h, 35 °C, 1.5 bar | No solvent | 99.0 | 99.0 | [61] |
| 9 | MIL-100(Cr) | 20/25 | 12 h,60 °C, 10 bar | No solvent | 11.0 | 99.0 | [62] |
| 10 | MIL-100(Fe) 1 | 203/1627 | 4 h, 60 °C, 15 bar | No solvent | 21.0 | 99.0 | This work |
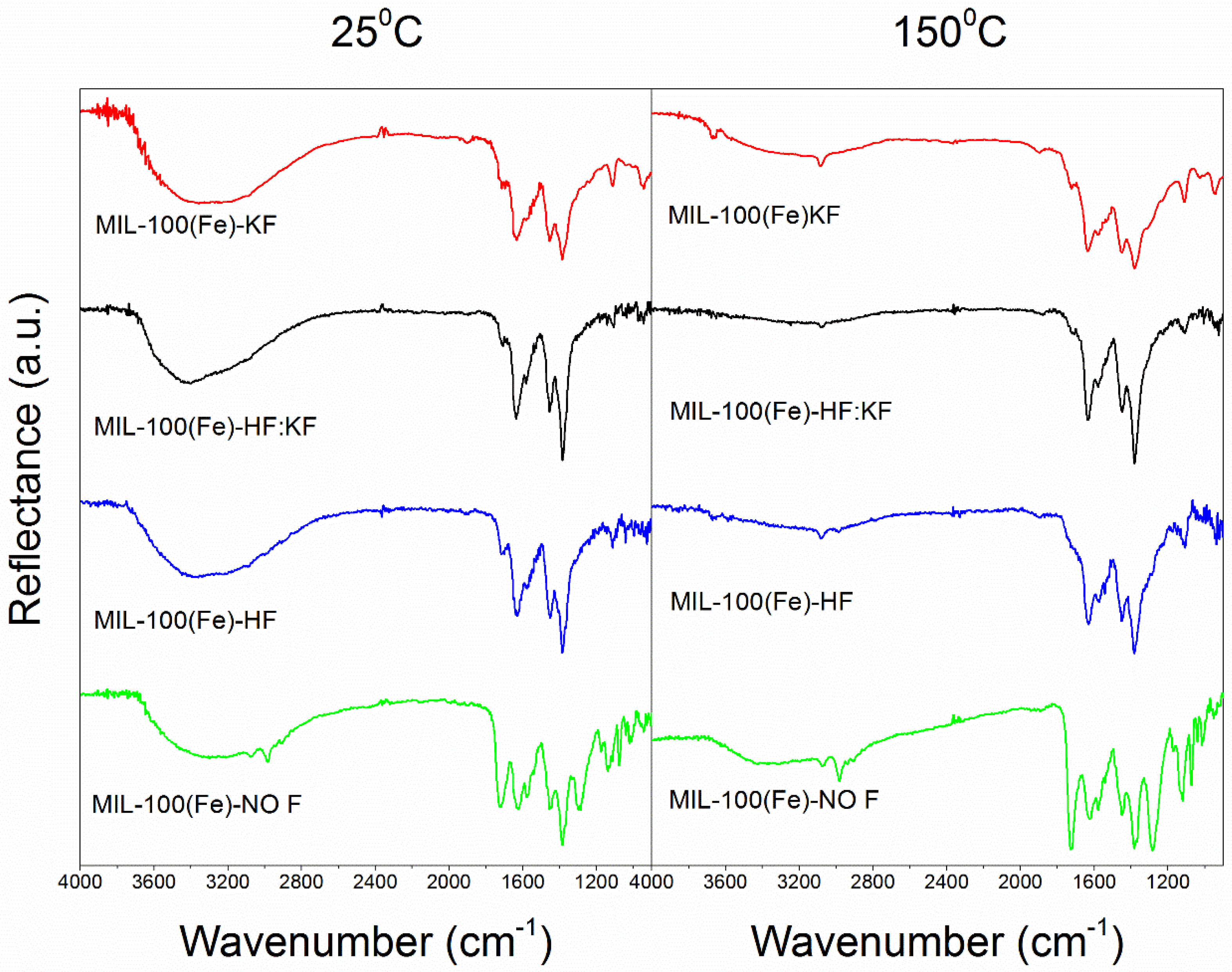
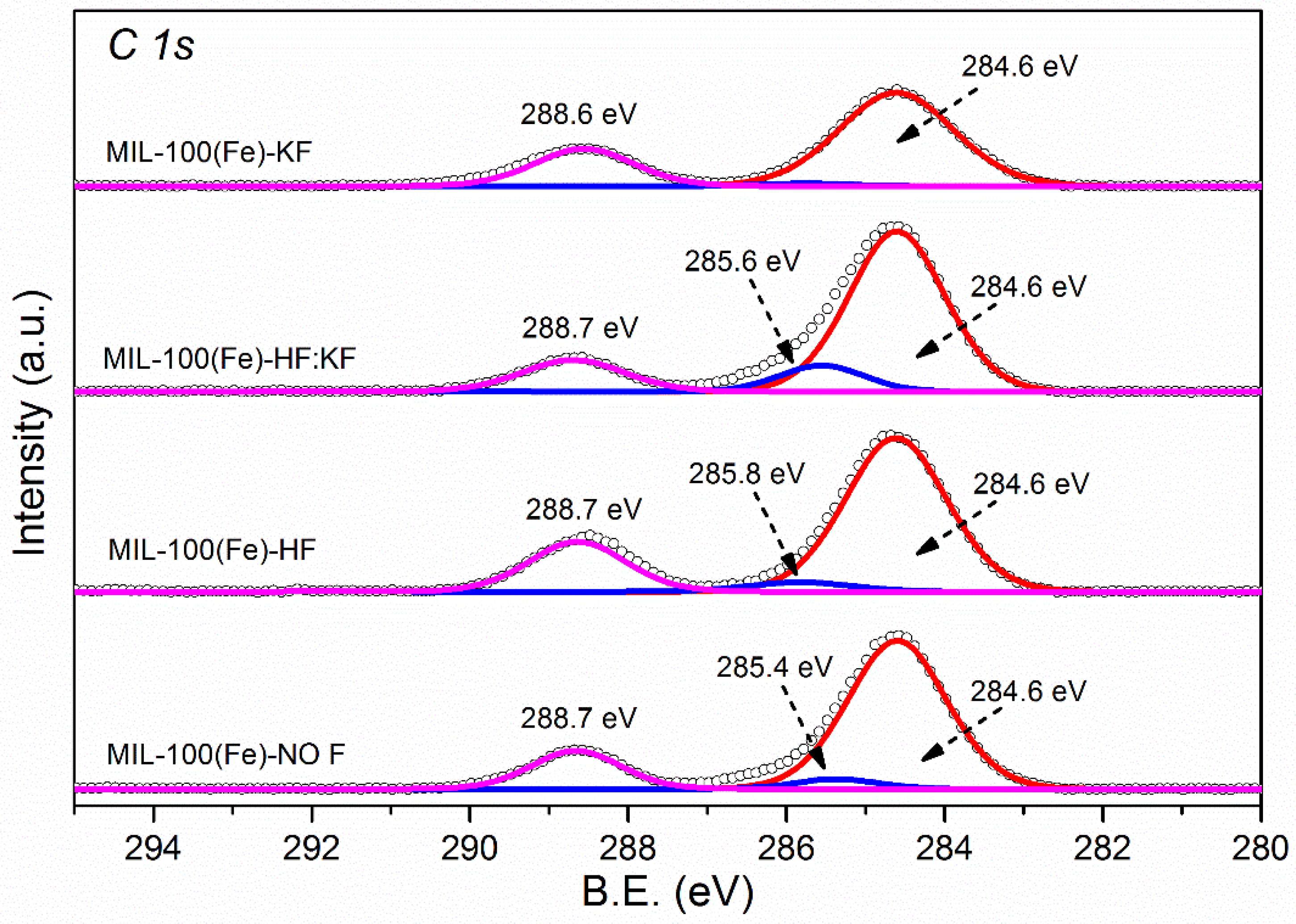
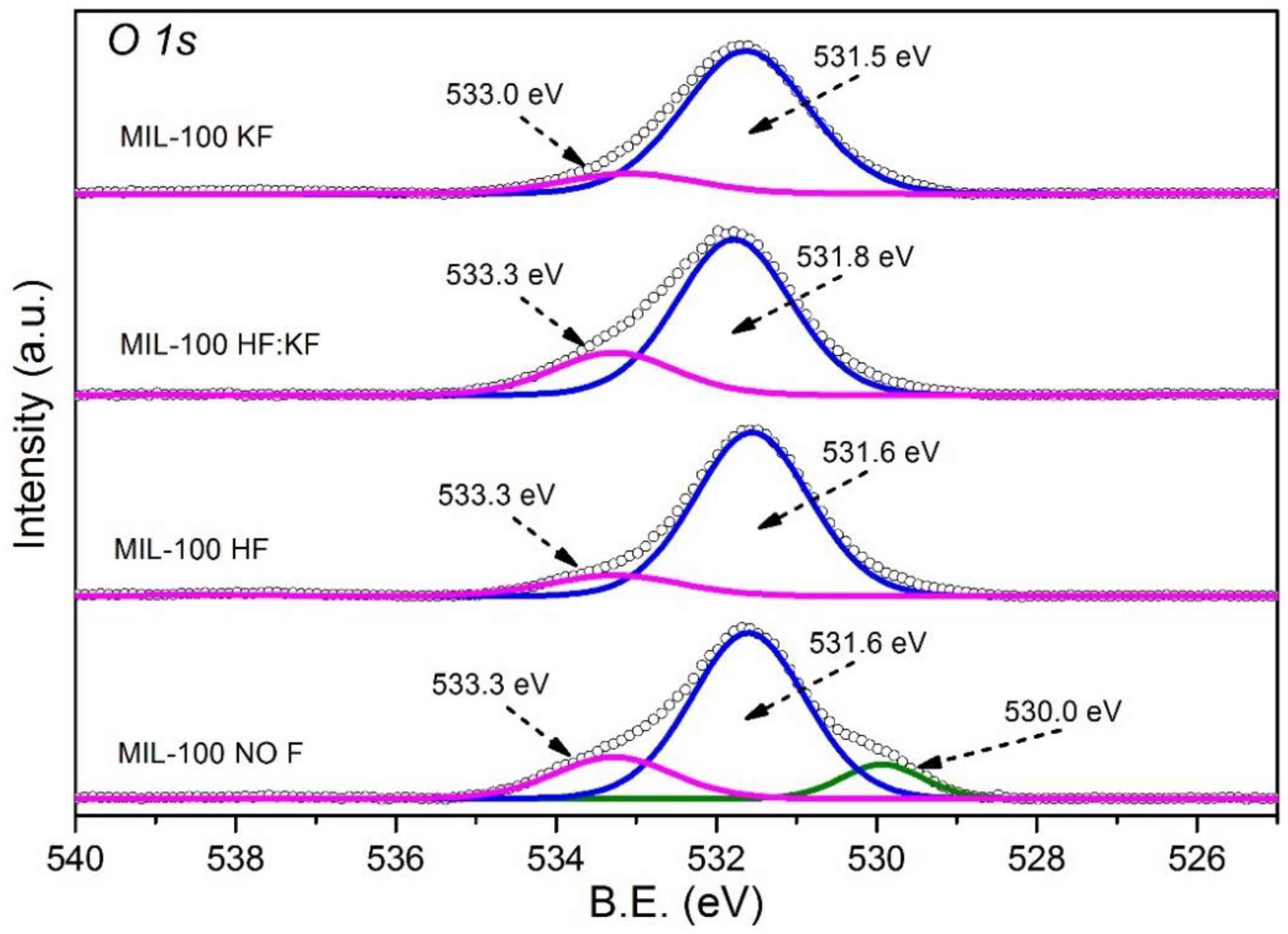
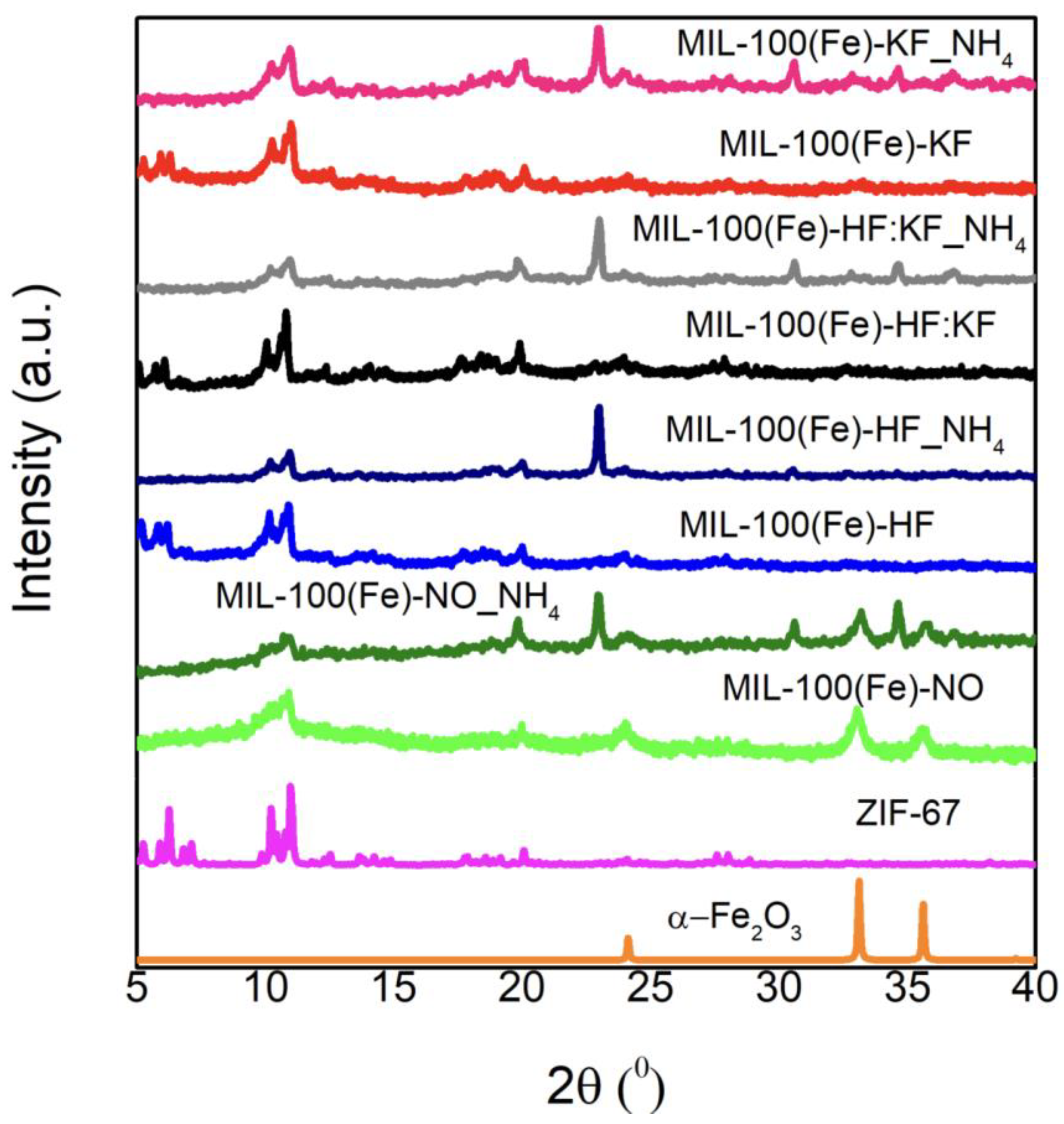

References
- Zanon, A.; Chaemchuen, S.; Mousavi, B.; Verpoort, F. 1 Zn-doped ZIF-67 as catalyst for the CO2 fixation into cyclic carbonates. J. CO2 Util. 2017, 20, 282–291. [Google Scholar] [CrossRef]
- An, B.; Zhang, J.; Cheng, K.; Ji, P.; Wang, C.; Lin, W. Confinement of Ultrasmall Cu/ZnOx Nanoparticles in Metal–Organic Frameworks for Selective Methanol Synthesis from Catalytic Hydrogenation of CO2. J. Am. Chem. Soc. 2017, 139, 3834–3840. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Feng, J.; Zhao, Y.; Gu, S.; Liu, J. MOF derived mesoporous K-ZrO2 with enhanced basic catalytic performance for Knoevenagel condensations. RSC Adv. 2017, 7, 55920–55926. [Google Scholar] [CrossRef]
- Xu, D.; Pan, Y.; Chen, M.; Pan, Q.; Zhu, L.; Xue, M.; Zhang, D.; Fang, Q.; Qiu, S. Synthesis and application of a MOF-derived Ni@C catalyst by the guidance from an in situ hot stage in TEM. RSC Adv. 2017, 7, 26377–26383. [Google Scholar] [CrossRef]
- Ramos-Fernández, E.V.; Serrano-Ruiz, J.C.; Sepúlveda-Escribano, A.; Narciso, J.; Ferrando-Soria, J.; Pardo, E. CHAPTER 9. Metal Organic Frameworks: From Material Chemistry to Catalytic Applications. In Heterogeneous Catalysis for Energy Applications; Reina, T.R., Odriozola, J.A., Eds.; The Royal Society of Chemistry: London, UK, 2020; pp. 235–303. ISBN 978-1-78801-718-3. [Google Scholar]
- Fang, X.; Zong, B.; Mao, S. Metal–Organic Framework-Based Sensors for Environmental Contaminant Sensing. Nano-Micro Lett. 2018, 10, 64. [Google Scholar] [CrossRef]
- Chuang, C.; Kung, C. Metal−Organic Frameworks toward Electrochemical Sensors: Challenges and Opportunities. Electroanalysis 2020, 32, 1885–1895. [Google Scholar] [CrossRef]
- Zhao, F.; Sun, T.; Geng, F.; Chen, P.; Gao, Y. Metal-Organic Frameworks-Based Electrochemical Sensors and Biosensors. Int. J. Electrochem. Sci. 2016, 14, 5287–5304. [Google Scholar] [CrossRef]
- Liu, W.; Yin, X.-B. Metal–organic frameworks for electrochemical applications. TrAC Trends Anal. Chem. 2016, 75, 86–96. [Google Scholar] [CrossRef]
- Peralta, D.; Chaplais, G.; Simon-Masseron, A.; Barthelet, K.; Pirngruber, G.D. Synthesis and adsorption properties of ZIF-76 isomorphs. Microporous Mesoporous Mater. 2011, 153, 1–7. [Google Scholar] [CrossRef]
- Isaeva, V.I.; Kustov, L.M. Metal-organic frameworks—New materials for hydrogen storage. Russ. J. Gen. Chem. 2007, 77, 721–739. [Google Scholar] [CrossRef]
- Liu, Y.; Kasik, A.; Linneen, N.; Liu, J.; Lin, Y. Adsorption and diffusion of carbon dioxide on ZIF-68. Chem. Eng. Sci. 2014, 118, 32–40. [Google Scholar] [CrossRef]
- Zhang, Z.; Xian, S.; Xi, H.; Wang, H.; Li, Z. Improvement of CO2 adsorption on ZIF-8 crystals modified by enhancing basicity of surface. Chem. Eng. Sci. 2011, 66, 4878–4888. [Google Scholar] [CrossRef]
- Li, Y.; Yang, R.T. Gas Adsorption and Storage in Metal−Organic Framework MOF-177. Langmuir 2007, 23, 12937–12944. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Fernandez, E.V.; Redondo-Murcia, A.; Grau-Atienza, A.; Sepúlveda-Escribano, A.; Narciso, J. Clean production of Zeolitic Imidazolate Framework 8 using Zamak residues as metal precursor and substrate. J. Clean. Prod. 2020, 260, 121081. [Google Scholar] [CrossRef]
- Narciso, J.; Ramos-Fernandez, E.V.; Delgado-Marín, J.J.; Affolter, C.W.; Olsbye, U.; Redekop, E.A. New route for the synthesis of Co-MOF from metal substrates. Microporous Mesoporous Mater. 2021, 324, 111310. [Google Scholar] [CrossRef]
- Villalgordo-Hernández, D.; Grau-Atienza, A.; García-Marín, A.A.; Ramos-Fernández, E.V.; Narciso, J. Manufacture of Carbon Materials with High Nitrogen Content. Materials 2022, 15, 2415. [Google Scholar] [CrossRef]
- Delgado-Marín, J.J.; Izan, D.P.; Molina-Sabio, M.; Ramos-Fernandez, E.V.; Narciso, J. New Generation of MOF-Monoliths Based on Metal Foams. Molecules 2022, 27, 1968. [Google Scholar] [CrossRef]
- Ramos-Fernandez, E.V.; Grau-Atienza, A.; Farrusseng, D.; Aguado, S. A water-based room temperature synthesis of ZIF-93 for CO2 adsorption. J. Mater. Chem. A 2018, 6, 5598–5602. [Google Scholar] [CrossRef]
- Horcajada, P.; Surblé, S.; Serre, C.; Hong, D.-Y.; Seo, Y.-K.; Chang, J.-S.; Grenèche, J.-M.; Margiolaki, I.; Férey, G. Synthesis and catalytic properties of MIL-100(Fe), an iron(iii) carboxylate with large pores. Chem. Commun. 2007, 100, 2820–2822. [Google Scholar] [CrossRef]
- Hall, J.N.; Bollini, P. Structure, characterization, and catalytic properties of open-metal sites in metal organic frameworks. React. Chem. Eng. 2019, 4, 207–222. [Google Scholar] [CrossRef]
- Vandichel, M.; Hajek, J.; Vermoortele, F.; Waroquier, M.; De Vos, D.E.; Van Speybroeck, V. Active site engineering in UiO-66 type metal–organic frameworks by intentional creation of defects: A theoretical rationalization. CrystEngComm 2015, 17, 395–406. [Google Scholar] [CrossRef]
- Rogge, S.M.J.; Bavykina, A.; Hajek, J.; Garcia, H.; Olivos-Suarez, A.I.; Sepúlveda-Escribano, A.; Vimont, A.; Clet, G.; Bazin, P.; Kapteijn, F.; et al. Metal–organic and covalent organic frameworks as single-site catalysts. Chem. Soc. Rev. 2017, 46, 3134–3184. [Google Scholar] [CrossRef] [PubMed]
- Eddaoudi, M.; Eubank, J.F. Insight into the Development of Metal-Organic Materials (MOMs): At Zeolite-Like Metal-Organic Frameworks (ZMOFs). In Metal-Organic Frameworks: Design and Application; John Wiley and Sons: Tampa, FL, USA, 2010; pp. 37–89. ISBN 9780470195567. [Google Scholar]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sébrié, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal–organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2010, 9, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.W.; Seo, Y.-K.; Hwang, Y.K.; Chang, J.-S.; Leclerc, H.; Wuttke, S.; Bazin, P.; Vimont, A.; Daturi, M.; Bloch, E.; et al. Controlled Reducibility of a Metal-Organic Framework with Coordinatively Unsaturated Sites for Preferential Gas Sorption. Angew. Chem. Int. Ed. 2010, 49, 5949–5952. [Google Scholar] [CrossRef]
- Jordá, J.L.; Silvestre-Albero, J.; Casco, M.E.; Ramos-Fernández, E.V.; Rodríguez-Reinoso, F.; Fauth, F.; Rudić, S.; Rey, F.; Martínez-Escandell, M.; Jordá, J.L.; et al. Paving the way for methane hydrate formation on metal–organic frameworks (MOFs). Chem. Sci. 2016, 7, 3658–3666. [Google Scholar] [CrossRef]
- Patra, S.; Crespo, T.H.; Permyakova, A.; Sicard, C.; Serre, C.; Chaussé, A.; Steunou, N.; Legrand, L. Design of metal organic framework–enzyme based bioelectrodes as a novel and highly sensitive biosensing platform. J. Mater. Chem. B 2015, 3, 8983–8992. [Google Scholar] [CrossRef]
- Cunha, D.; Ben Yahia, M.; Hall, S.; Miller, S.R.; Chevreau, H.; Elkaïm, E.; Maurin, G.; Horcajada, P.; Serre, C. Rationale of Drug Encapsulation and Release from Biocompatible Porous Metal–Organic Frameworks. Chem. Mater. 2013, 25, 2767–2776. [Google Scholar] [CrossRef]
- Yoon, J.W.; Chang, H.; Lee, S.-J.; Hwang, Y.K.; Hong, D.-Y.; Lee, S.-K.; Lee, J.S.; Jang, S.; Yoon, T.-U.; Kwac, K.; et al. Selective nitrogen capture by porous hybrid materials containing accessible transition metal ion sites. Nat. Mater. 2017, 16, 526–531. [Google Scholar] [CrossRef]
- Goesten, M.G.; Juan-Alcaniz, J.; Ramos-Fernandez, E.V.; Gupta, K.S.S.; Stavitskic, E.; Van Bekkum, H.; Gascon, J.; Kapteijn, F. Sulfation of metal–organic frameworks: Opportunities for acid catalysis and proton conductivity. J. Catal. 2011, 281, 177–187. [Google Scholar] [CrossRef]
- Gaab, M.; Trukhan, N.; Maurer, S.; Gummaraju, R.; Müller, U. The progression of Al-based metal-organic frameworks—From academic research to industrial production and applications. Microporous Mesoporous Mater. 2012, 157, 131–136. [Google Scholar] [CrossRef]
- Mitchell, L.; Gonzalez-Santiago, B.; Mowat, J.P.S.; Gunn, M.E.; Williamson, P.; Acerbi, N.; Clarke, M.L.; Wright, P.A. Remarkable Lewis acid catalytic performance of the scandium trimesate metal organic framework MIL-100(Sc) for C–C and C=N bond-forming reactions. Catal. Sci. Technol. 2013, 3, 606–617. [Google Scholar] [CrossRef]
- Hou, Y.; Mao, H.; Xu, L. MIL-100(V) and MIL-100(V)/rGO with various valence states of vanadium ions as sulfur cathode hosts for lithium-sulfur batteries. Nano Res. 2017, 10, 344–353. [Google Scholar] [CrossRef]
- Lieb, A.; Leclerc, H.; Devic, T.; Serre, C.; Margiolaki, I.; Mahjoubi, F.; Lee, J.S.; Vimont, A.; Daturi, M.; Chang, J.-S. MIL-100(V)—A mesoporous vanadium metal organic framework with accessible metal sites. Microporous Mesoporous Mater. 2012, 157, 18–23. [Google Scholar] [CrossRef]
- Juan-Alcañiz, J.; Goesten, M.G.; Ramos-Fernandez, E.V.; Gascon, J.; Kapteijn, F. Towards efficient polyoxometalate encapsulation in MIL-100(Cr): Influence of synthesis conditions. New J. Chem. 2012, 36, 977–987. [Google Scholar] [CrossRef]
- Zhou, Y.-X.; Chen, Y.-Z.; Hu, Y.; Huang, G.; Yu, S.-H.; Jiang, H.-L. MIL-101-SO3H: A Highly Efficient Brønsted Acid Catalyst for Heterogeneous Alcoholysis of Epoxides under Ambient Conditions. Chem.—Eur. J. 2014, 20, 14976–14980. [Google Scholar] [CrossRef]
- Kholdeeva, O.A.; Skobelev, I.Y.; Ivanchikova, I.D.; Kovalenko, K.; Fedin, V.P.; Sorokin, A.B. Hydrocarbon oxidation over Fe- and Cr-containing metal-organic frameworks MIL-100 and MIL-101–a comparative study. Catal. Today 2014, 238, 54–61. [Google Scholar] [CrossRef]
- Vermoortele, F.; Ameloot, R.; Alaerts, L.; Matthessen, R.; Carlier, B.; Fernandez, E.V.R.; Gascon, J.; Kapteijn, F.; De Vos, D.E. Tuning the catalytic performance of metal–organic frameworks in fine chemistry by active site engineering. J. Mater. Chem. 2012, 22, 10313–10321. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Alvaro, M.; Horcajada, P.; Gibson, E.; Vishnuvarthan, M.; Vimont, A.; Grenèche, J.-M.; Serre, C.; Daturi, M.; Garcia, H. Comparison of Porous Iron Trimesates Basolite F300 and MIL-100(Fe) As Heterogeneous Catalysts for Lewis Acid and Oxidation Reactions: Roles of Structural Defects and Stability. ACS Catal. 2012, 2, 2060–2065. [Google Scholar] [CrossRef]
- Férey, G. The Simplicity of Complexity-Rational Design of Giant Pores. Science 2001, 291, 994–995. [Google Scholar] [CrossRef]
- Liu, D.; Li, G.; Liu, H. Functionalized MIL-101 with imidazolium-based ionic liquids for the cycloaddition of CO2 and epoxides under mild condition. Appl. Surf. Sci. 2018, 428, 218–225. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Bezverkhyy, I.; Weber, G.; Bellat, J.-P. Degradation of fluoride-free MIL-100(Fe) and MIL-53(Fe) in water: Effect of temperature and pH. Microporous Mesoporous Mater. 2016, 219, 117–124. [Google Scholar] [CrossRef]
- Guesh, K.; Caiuby, C.A.D.; Mayoral, A.; Díaz-García, M.; Díaz, I.; Sanchez-Sanchez, M. Sustainable Preparation of MIL-100(Fe) and Its Photocatalytic Behavior in the Degradation of Methyl Orange in Water. Cryst. Growth Des. 2017, 17, 1806–1813. [Google Scholar] [CrossRef]
- Jeffes, J.H.E. Ellingham Diagrams; Buschow, K.H.J., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Veyssière, P., Eds.; Elsevier: Oxford, UK, 2001; pp. 2751–2753. ISBN 978-0-08-043152-9. [Google Scholar]
- Van de Voorde, B.; Boulhout, M.; Vermoortele, F.; Horcajada, P.; Cunha, D.; Lee, J.S.; Chang, J.-S.; Gibson, E.; Daturi, M.; Lavalley, J.-C.; et al. N/S-Heterocyclic Contaminant Removal from Fuels by the Mesoporous Metal–Organic Framework MIL-100: The Role of the Metal Ion. J. Am. Chem. Soc. 2013, 135, 9849–9856. [Google Scholar] [CrossRef] [PubMed]
- Nagano, Y.; Mizuno, H.; Sakiyama, M.; Fujiwara, T.; Kondo, Y. Hydration enthalpy of tetra-n-butylammonium ion. J. Phys. Chem. 1991, 95, 2536–2540. [Google Scholar] [CrossRef]
- Khadim, A.T.; Albayati, T.M.; Saady, N.M.C. Desulfurization of actual diesel fuel onto modified mesoporous material Co/MCM-41. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100635. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 66137, Tetramethylammonium Bromide; National Center for Biotechnology Information: Bethesda, MD, USA, 2005.
- Ali, N.S.; Alismaeel, Z.T.; Majdi, H.S.; Salih, H.G.; Abdulrahman, M.A.; Saady, N.M.C.; Albayati, T.M. Modification of SBA-15 mesoporous silica as an active heterogeneous catalyst for the hydroisomerization and hydrocracking of n-heptane. Heliyon 2022, 8, e09737. [Google Scholar] [CrossRef]
- Campello, I.; Sepúlveda-Escribano, A.; Ramos-Fernández, E.V. Metal–Organic Frameworks (MOFs) for CO2 Cycloaddition Reactions. In Engineering Solutions for CO2 Conversion; Wiley: Hoboken, NJ, USA, 2021; pp. 407–427. ISBN 9783527346523. [Google Scholar]
- Blake, R.L.; Hessevick, R.E.; Zoltai, T.; Finger, L.W. Refinement of the hematite structure. Am. Mineral. 1966, 51, 123–129. [Google Scholar]
- Srivastava, R.; Srinivas, D.; Ratnasamy, P. CO2 activation and synthesis of cyclic carbonates and alkyl/aryl carbamates over adenine-modified Ti-SBA-15 solid catalysts. J. Catal. 2005, 233, 1–15. [Google Scholar] [CrossRef]
- Li, C.-G.; Xu, L.; Wu, P.; Wu, H.; He, M. Efficient cycloaddition of epoxides and carbon dioxide over novel organic–inorganic hybrid zeolite catalysts. Chem. Commun. 2014, 50, 15764–15767. [Google Scholar] [CrossRef]
- Srivastava, R.; Srinivas, D.; Ratnasamy, P. Synthesis of Polycarbonate Precursors over Titanosilicate Molecular Sieves. Catal. Lett. 2003, 91, 133–139. [Google Scholar] [CrossRef]
- Srivastava, R.; Srinivas, D.; Ratnasamy, P. Syntheses of polycarbonate and polyurethane precursors utilizing CO2 over highly efficient, solid as-synthesized MCM-41 catalyst. Tetrahedron Lett. 2006, 47, 4213–4217. [Google Scholar] [CrossRef]
- Miralda, C.M.; Macias, E.E.; Zhu, M.; Ratnasamy, P.; Carreon, M.A. Zeolitic Imidazole Framework-8 Catalysts in the Conversion of CO2 to Chloropropene Carbonate. ACS Catal. 2012, 2, 180–183. [Google Scholar] [CrossRef]
- Macias, E.E.; Ratnasamy, P.; Carreon, M.A. Catalytic activity of metal organic framework Cu3(BTC)2 in the cycloaddition of CO2 to epichlorohydrin reaction. Catal. Today 2012, 198, 215–218. [Google Scholar] [CrossRef]
- Kim, S.-N.; Kim, J.; Kim, H.-Y.; Cho, H.-Y.; Ahn, W.-S. Adsorption/catalytic properties of MIL-125 and NH2-MIL-125. Catal. Today 2013, 204, 85–93. [Google Scholar] [CrossRef]
- Verpoort, F. Reaction Conditions. Stud. Surf. Sci. Catal. 2000, 131, 197–236. [Google Scholar] [CrossRef]
- Saghian, M.; Dehghanpour, S.; Sharbatdaran, M. Amine-functionalized frameworks as highly actives catalysts for chemical fixation of CO2 under solvent and co-catalyst free conditions. J. CO2 Util. 2020, 41, 101253. [Google Scholar] [CrossRef]

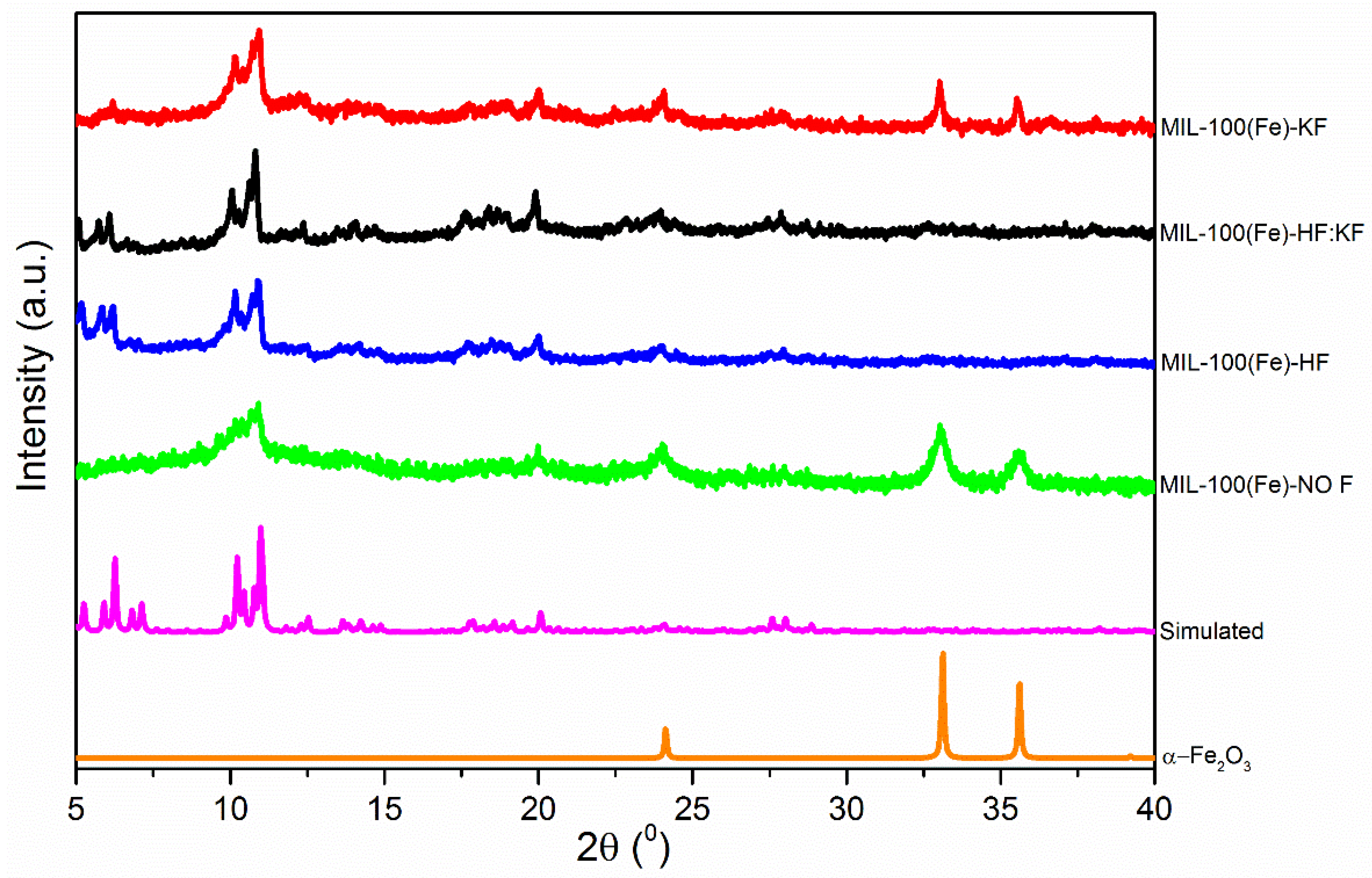
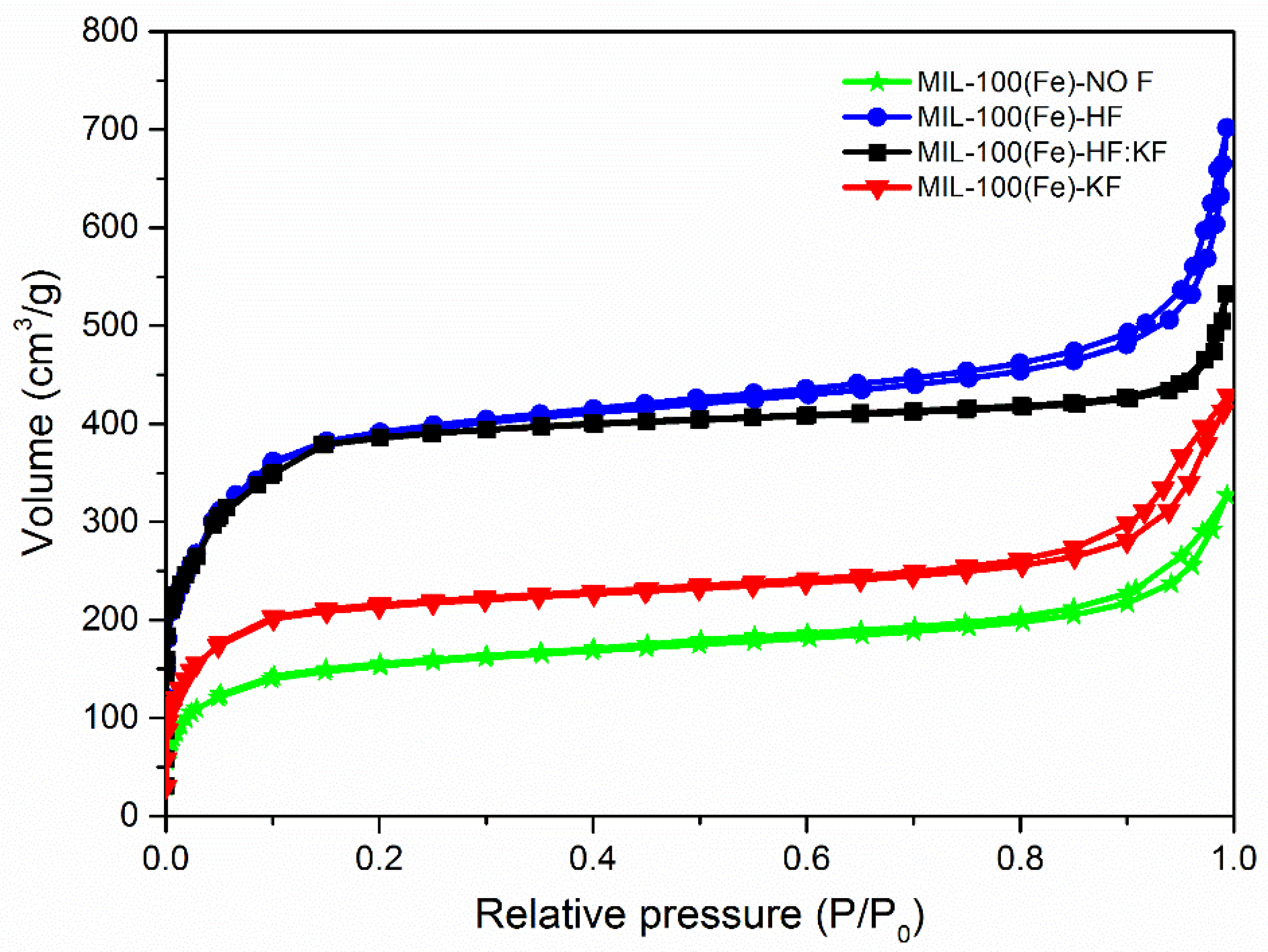
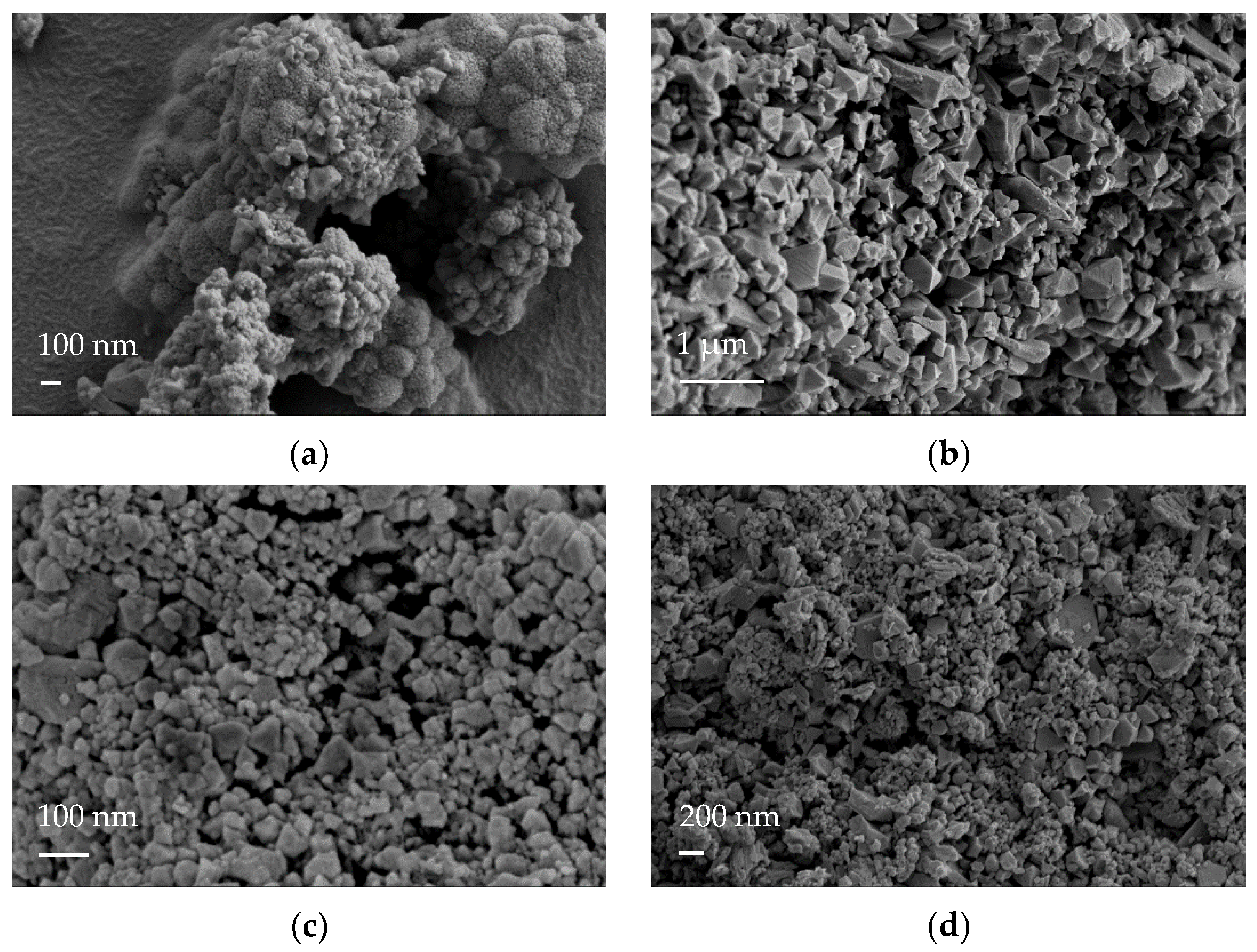
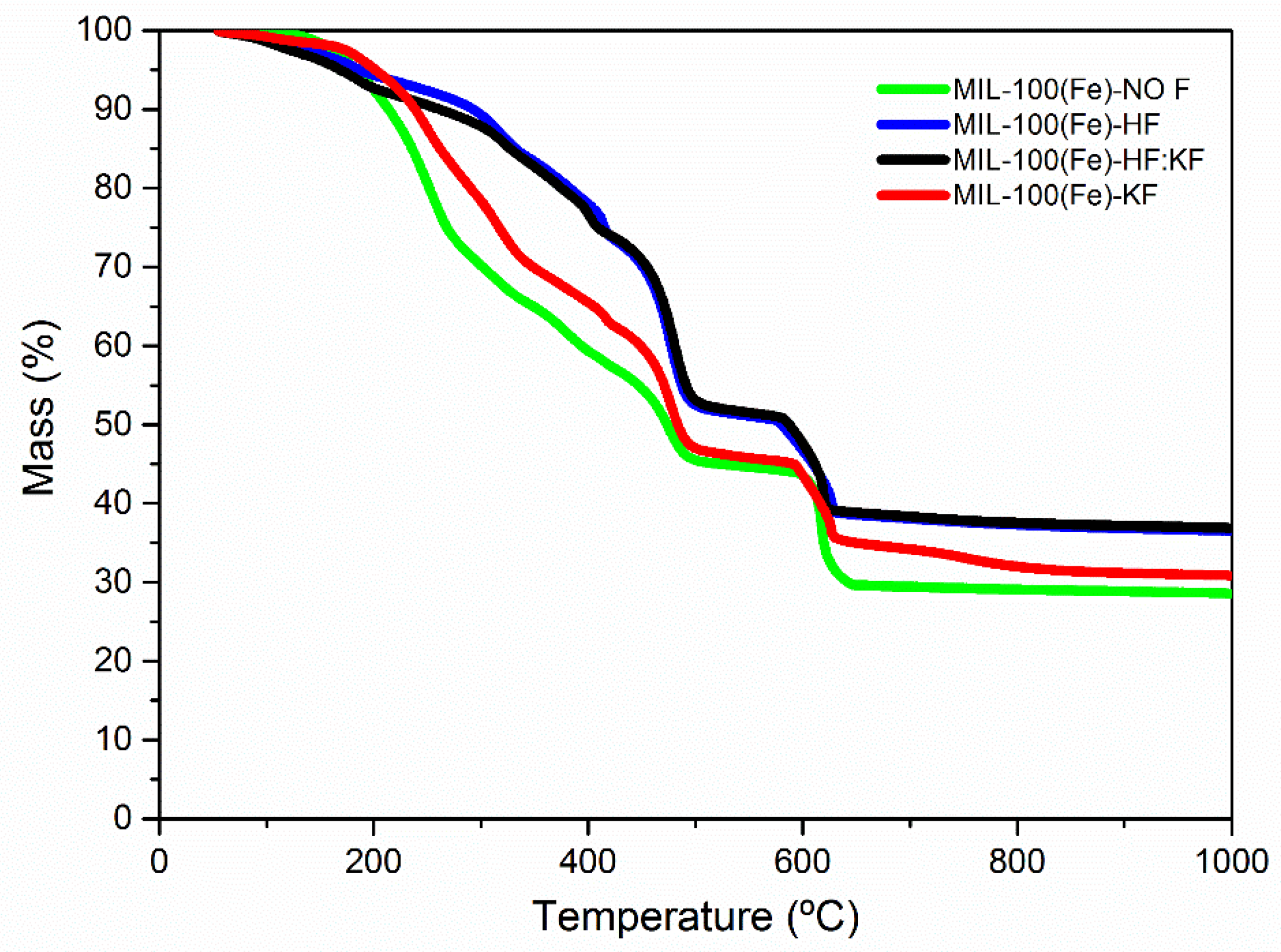
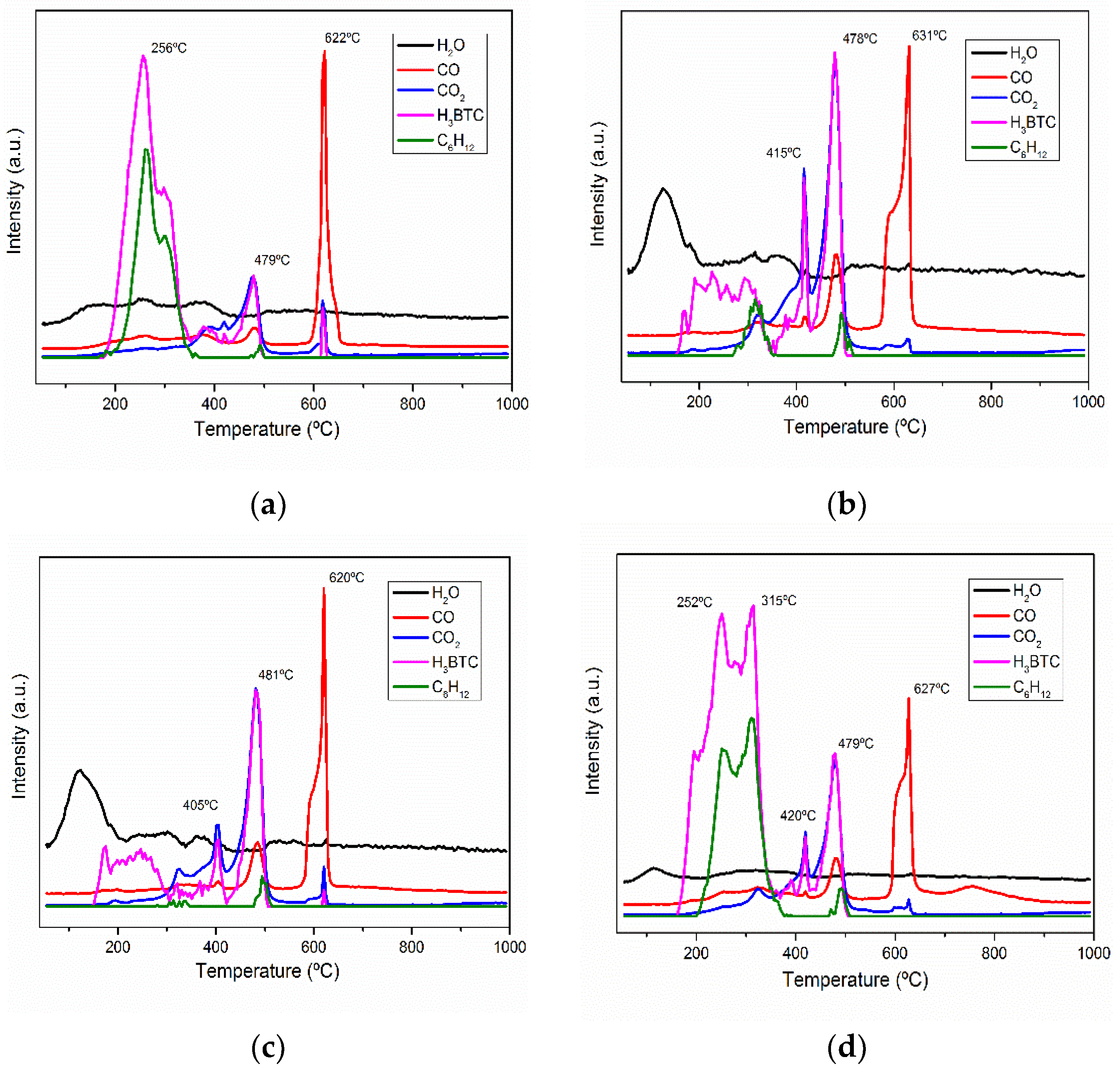
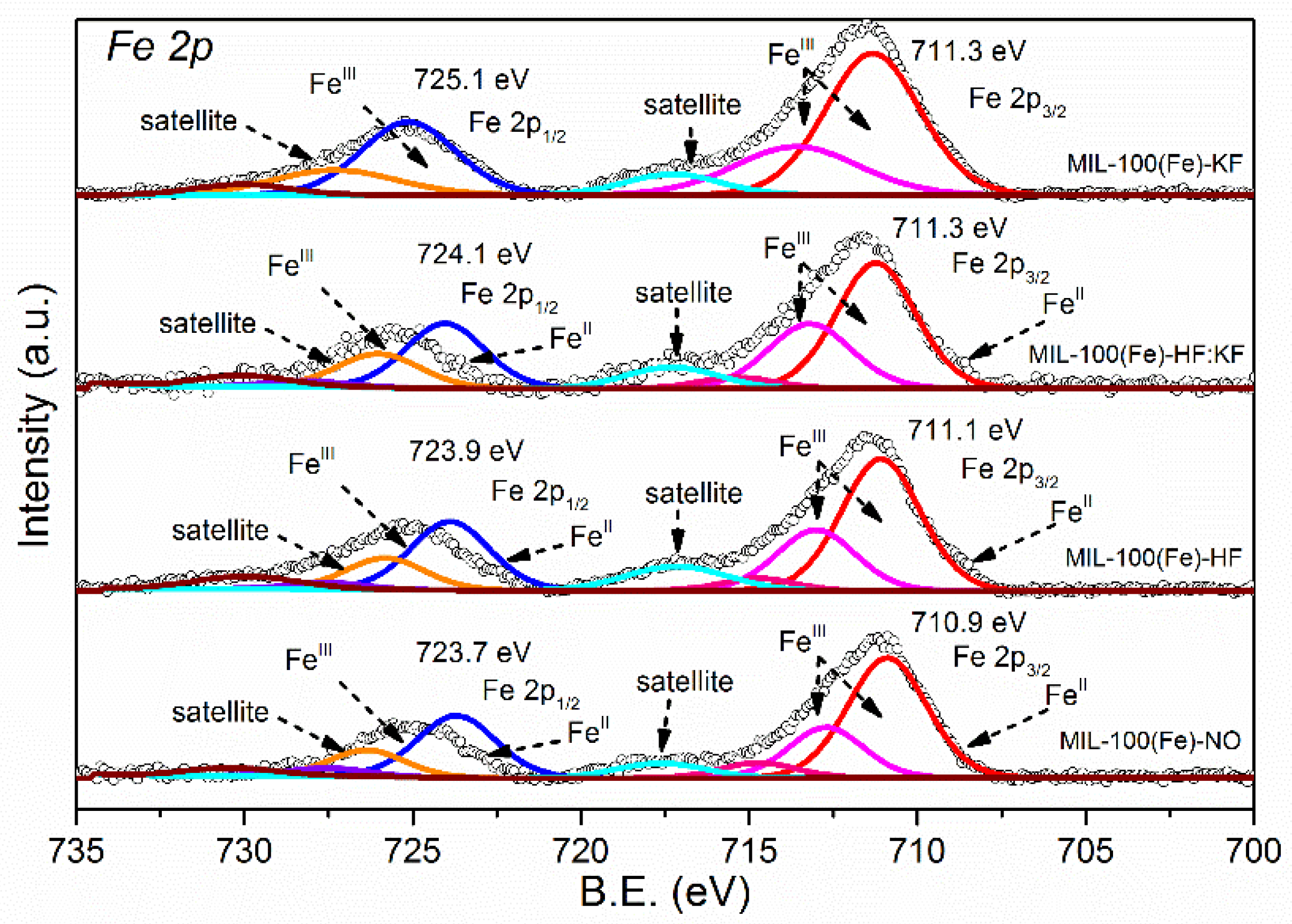
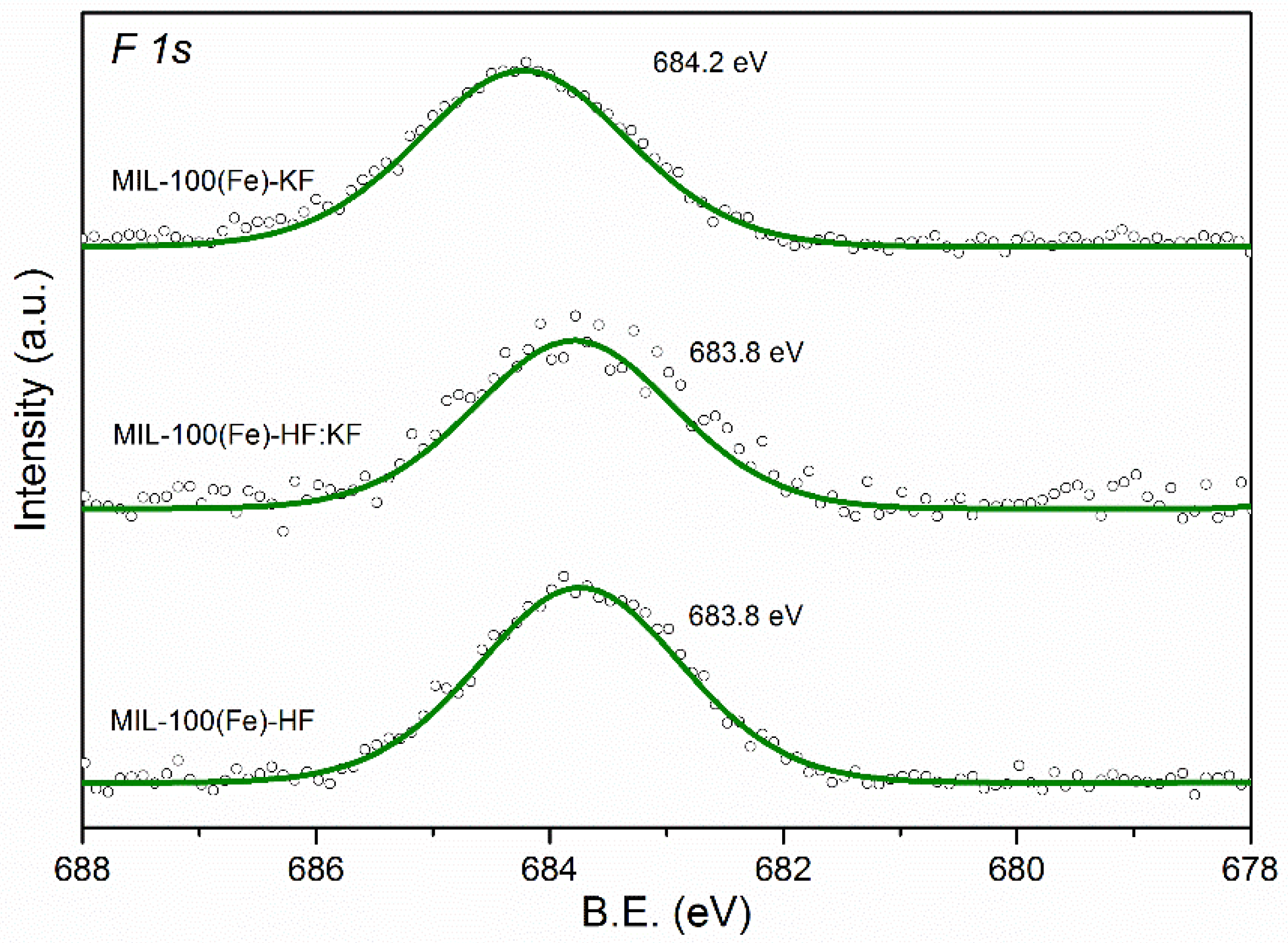
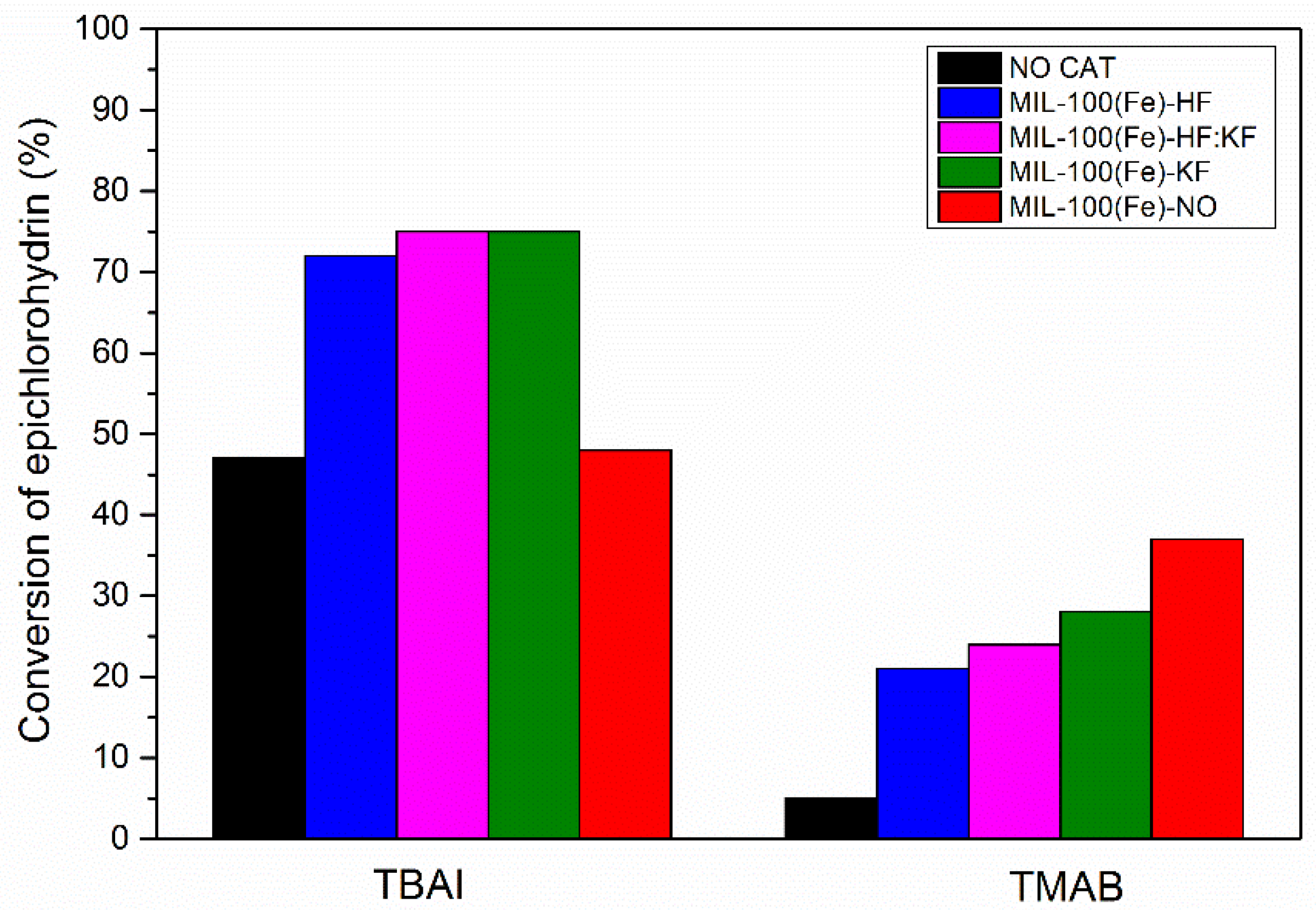

| Samples | Specific Surface Area (m2·g−1) | Vmicro (cm3·g−1) | Vmeso (cm3·g−1) |
|---|---|---|---|
| MIL-100(Fe)-NO F | 453 | 0.24 | 0.13 |
| MIL-100(Fe)-HF | 946 | 0.45 | 0.33 |
| MIL-100(Fe)-HF:KF | 962 | 0.46 | 0.23 |
| MIL-100(Fe)-KF | 576 | 0.32 | 0.11 |
| Sample | Weight Loss wt.% | |
|---|---|---|
| Fe(BTC) | MIL-100(Fe) | |
| MIL-100(Fe)-NO | 64 | 36 |
| MIL-100(Fe)-HF | 31 | 69 |
| MIL-100(Fe)-HF:KF | 32 | 68 |
| MIL-100(Fe)-KF | 55 | 45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado-Marín, J.J.; Narciso, J.; Ramos-Fernández, E.V. Effect of the Synthesis Conditions of MIL-100(Fe) on Its Catalytic Properties and Stability under Reaction Conditions. Materials 2022, 15, 6499. https://doi.org/10.3390/ma15186499
Delgado-Marín JJ, Narciso J, Ramos-Fernández EV. Effect of the Synthesis Conditions of MIL-100(Fe) on Its Catalytic Properties and Stability under Reaction Conditions. Materials. 2022; 15(18):6499. https://doi.org/10.3390/ma15186499
Chicago/Turabian StyleDelgado-Marín, José J., Javier Narciso, and Enrique V. Ramos-Fernández. 2022. "Effect of the Synthesis Conditions of MIL-100(Fe) on Its Catalytic Properties and Stability under Reaction Conditions" Materials 15, no. 18: 6499. https://doi.org/10.3390/ma15186499
APA StyleDelgado-Marín, J. J., Narciso, J., & Ramos-Fernández, E. V. (2022). Effect of the Synthesis Conditions of MIL-100(Fe) on Its Catalytic Properties and Stability under Reaction Conditions. Materials, 15(18), 6499. https://doi.org/10.3390/ma15186499








