Influence of 40% Cold Working and Annealing on Precipitation in AISI 316L Austenitic Stainless Steel
Abstract
:1. Introduction
2. Materials and Methods
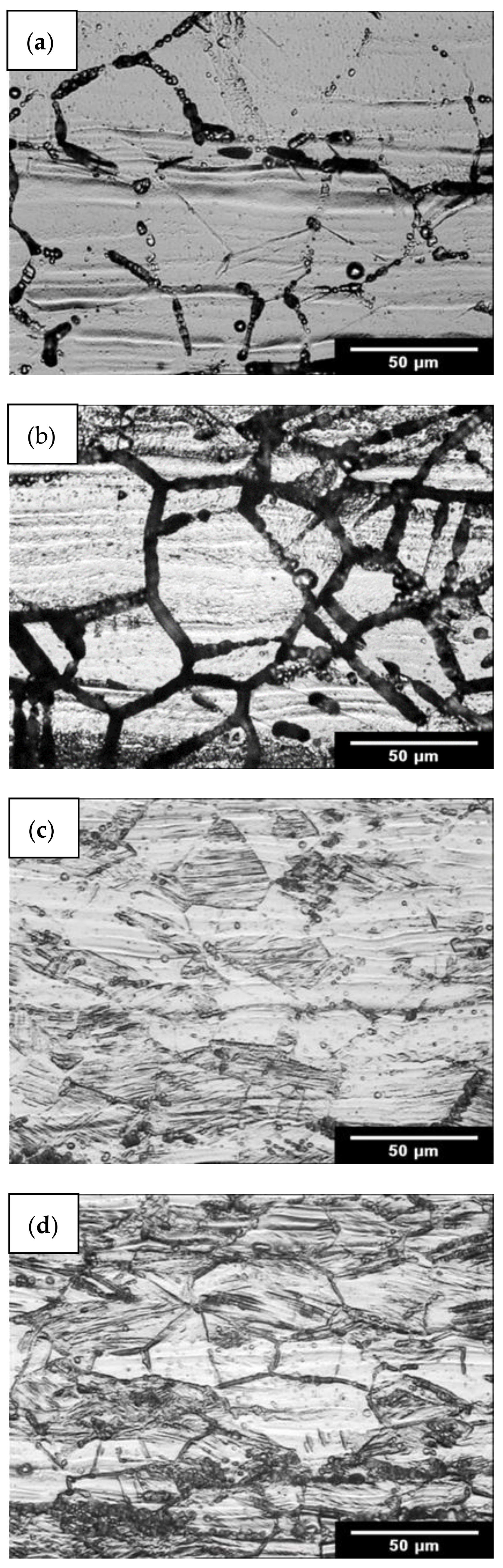
- No corrosion at the grain boundaries (step structure);
- Grains partially surrounded by deeply etched boundaries (dual structure);
- Grains completely surrounded by deeply etched boundaries (ditch structure).
3. Results
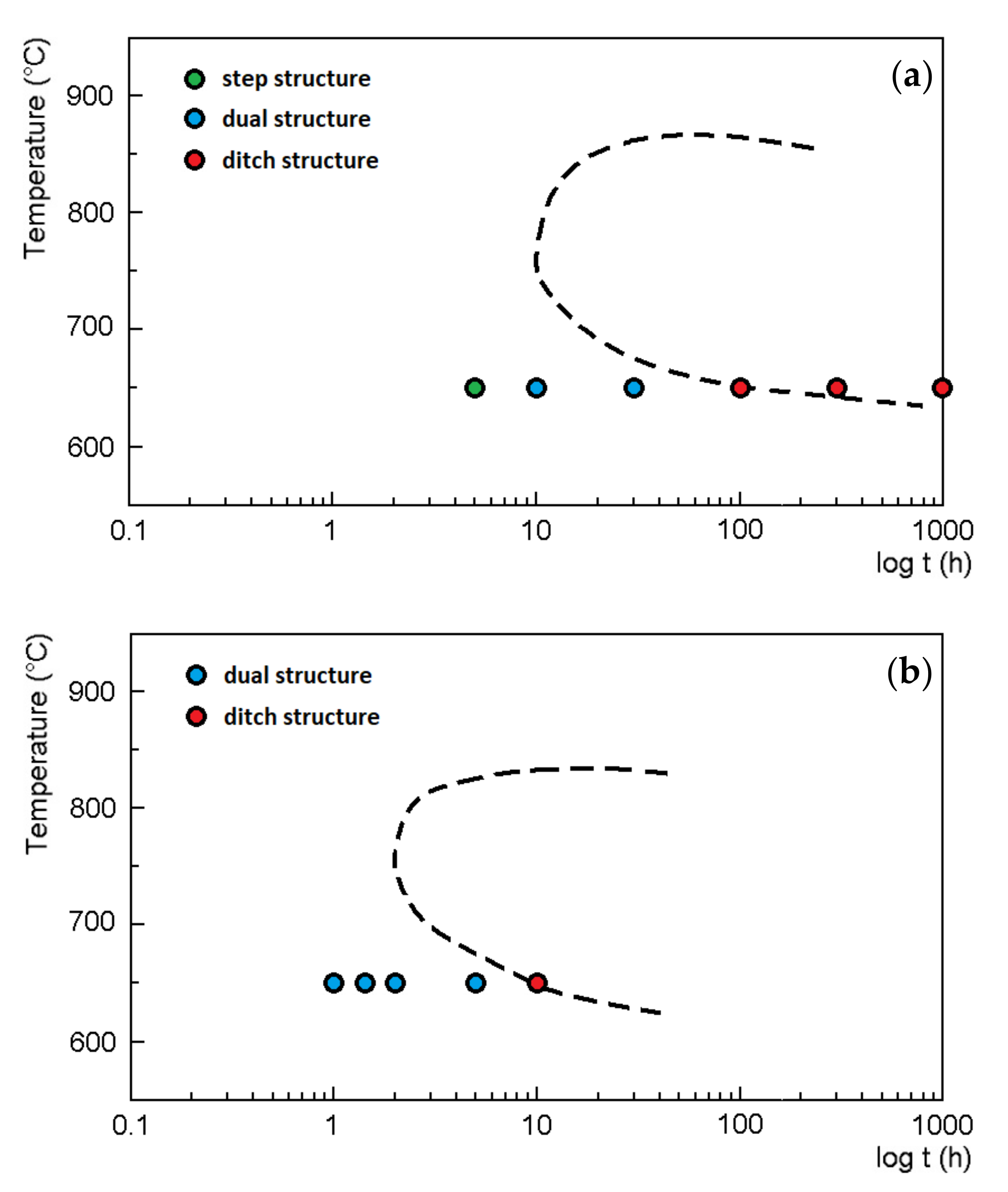
3.1. Oxalic Acid Etch Test
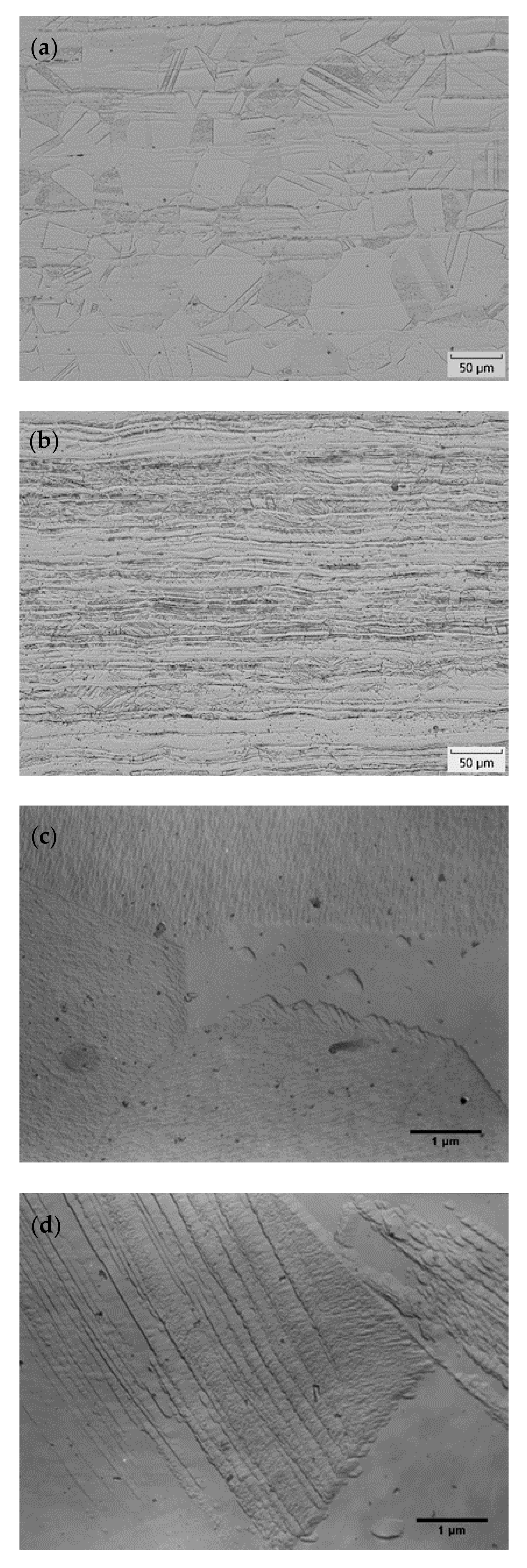
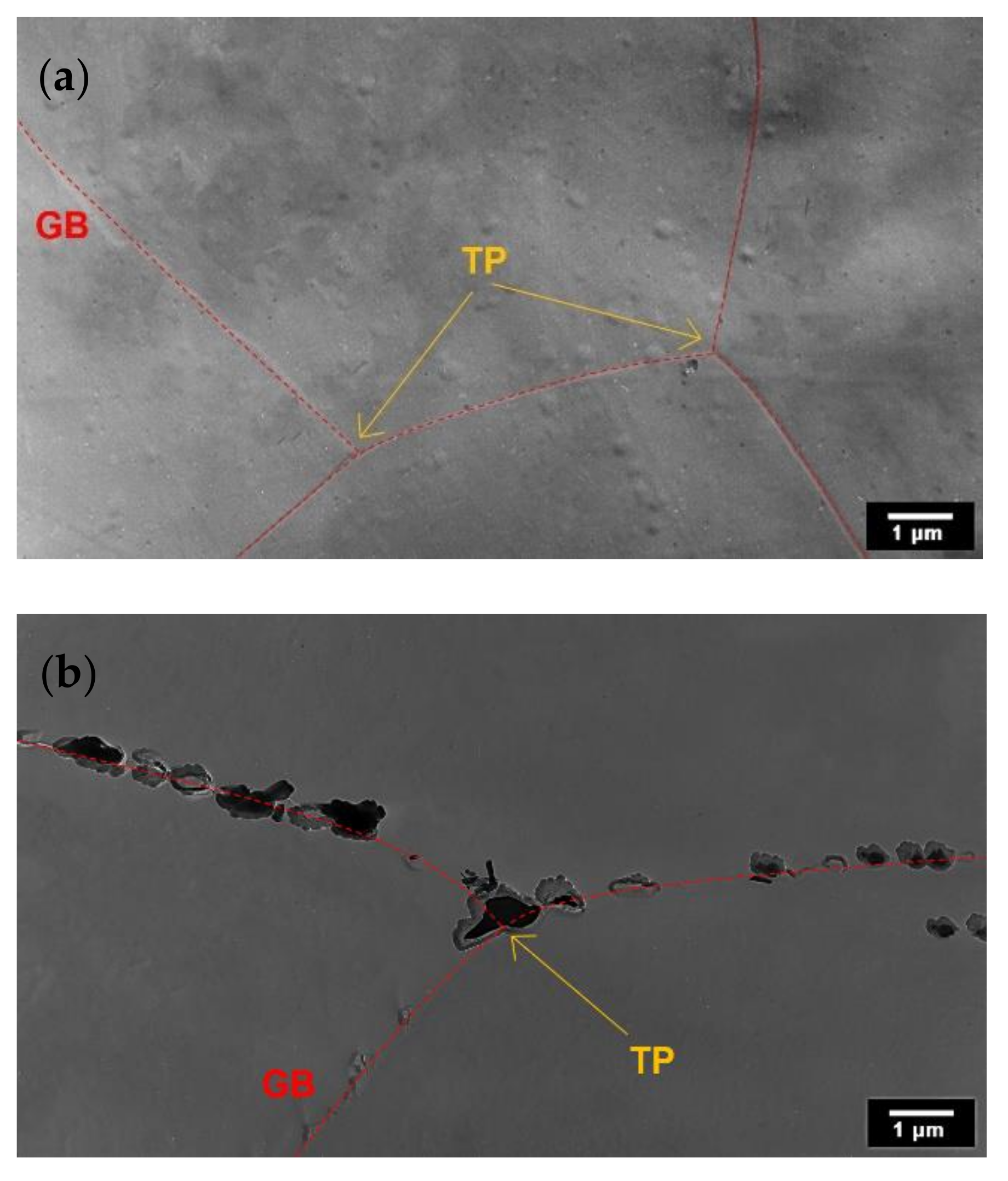
3.2. TEM Analysis of AISI 316L (0% CW)
3.3. TEM Analysis of AISI 316L (40% CW)
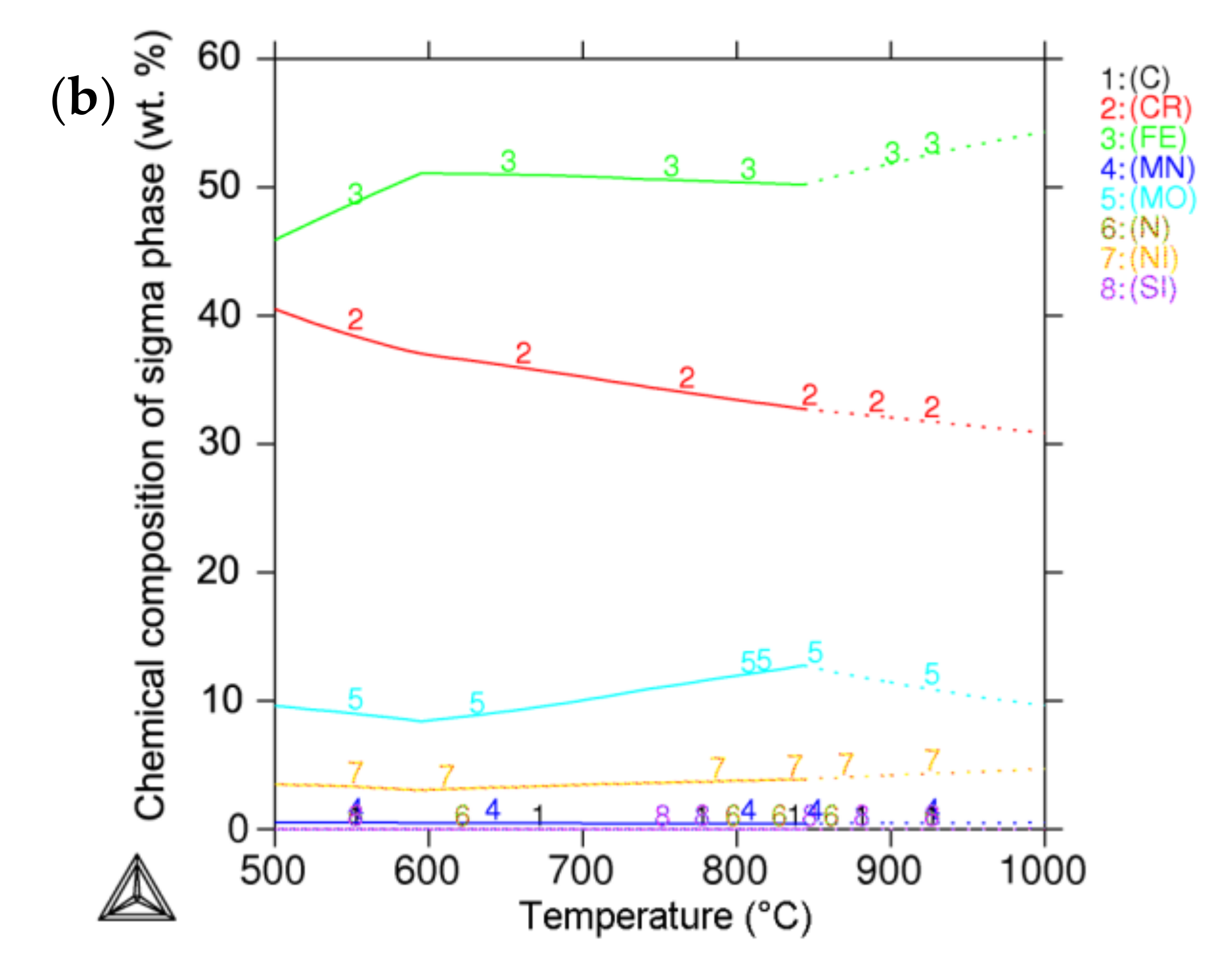
3.4. Thermodynamic Prediction
4. Discussion
5. Conclusions
- Longer annealing times led to the intensive precipitation of secondary phases at the grain boundaries as well as inside the austenitic grains.
- A significant decrease in resistance to intergranular corrosion after the application of 40% CW to AISI 316L steel was observed.
- Four types of secondary phases (M23C6 carbide, sigma phase, chi phase and Laves phase) present in both AISI 316L (0% CW) and AISI 316L (40% CW) steels at a 650 °C temperature were experimentally confirmed by electron diffraction.
- The types of secondary phases could not be recognised based on their morphology
- Cold work of 40% had a significant influence on secondary phases’ precipitation kinetics. The most significant influence was observed on the sigma phase, where the beginning of precipitation was more than 50 times accelerated. A lower influence was observed on the Laves phase and chi phase, where the acceleration was only 5 times higher. The lowest influence of cold work on the precipitation kinetics was observed in the case of M23C6 carbide.
- The influence of the annealing time and 40% CW on the chemical composition of secondary phases was not observed.
- The phase composition predicted by Thermo-Calc software for a 650 °C temperature in the equilibrium state contained austenite, M23C6 carbide, M2N nitride and sigma phase, which is partially in line with experimental results.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parvathavarthini, N.; Dayal, R.K. Influence of chemical composition, prior deformation and prolonged thermal aging on the sensitisation characteristics of austenitic stainless steels. J. Nucl. Mater. 2002, 305, 209–219. [Google Scholar] [CrossRef]
- Parvathavarthini, N.; Dayal, R.K.; Gnanamoorthy, J.B. Influence of prior deformation on sensitisation of AISI type 316LN stainless steel. J. Nucl. Mater. 1994, 208, 209–219. [Google Scholar] [CrossRef]
- Magula, V.; Liao, J.; Ikeuchi, K.; Kuroda, T.; Kikuchi, Y.; Matsuda, F. New aspects of sensitisation behaviour in recent 316 type austenitic stainless steels. Trans. JWRI 1996, 25, 49–58. [Google Scholar]
- Záhumenský, P.; Tuleja, S.; Országová, J.; Janovec, J.; Siládiová, V. Corrosion resistance of 18Cr-12Ni-2.5Mo steel annealed at 500–1050 °C. Corros. Sci. 1999, 41, 1305–1322. [Google Scholar] [CrossRef]
- Radionova, L.V.; Perevozchikov, D.V.; Makoveckii, A.N.; Eremin, V.N.; Akhmedyanov, A.M.; Rushchits, S.V. Study on the Hot Deformation Behavior of Stainless Steel AISI 321. Materials 2022, 15, 4057. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Chen, K.; Yu, L.; Lu, H.; Zhang, L.; Shi, X.; Xu, X. SCC crack growth rate of cold worked 316L stainless steel in PWR environment. J. Nucl. Mater. 2015, 456, 228–234. [Google Scholar] [CrossRef]
- Yang, Y.; Busby, J.T. Thermodynamic modeling and kinetics simulation of precipitate phases in AISI 316 stainless steels. J. Nucl. Mater. 2014, 448, 282–293. [Google Scholar] [CrossRef]
- Panov, D.; Chernichenko, R.; Kudryavtsev, E.; Klimenko, D.; Naumov, S.; Pertcev, A. Effect of Cold Swaging on the Bulk Gradient Structure Formation and Mechanical Properties of a 316-Type Austenitic Stainless Steel. Materials 2022, 15, 2468. [Google Scholar] [CrossRef]
- Dománková, M.; Marek, P.; Moravčík, R. Effect of annealing at 650 °C on precipitation in chosen austenitic stainless steels. Acta Metall. Slovaca 2007, 13, 52–60. [Google Scholar]
- Marek, P.; Dománková, M. Influence of 40% deformation on sensitisation characteristic of 316 and 316L austenitic stainless steels. Acta Metall. Slovaca 2007, 13, 61–67. [Google Scholar]
- Vach, M.; Kuníková, T.; Dománková, M.; Švec, P.; Čaplovič, Ľ.; Gogola, P.; Janovec, J. Evolution of secondary phases in austenitic stainless steels during long-term exposures at 600, 650, and 800 °C. Mater. Charact. 2008, 59, 1792–1798. [Google Scholar] [CrossRef]
- Kain, V.; Chandra, K.; Adhe, K.N.; De, P.K. Effect of cold work on low-temperature sensitisation behaviour of austenitic stainless steels. J. Nucl. Mater. 2004, 334, 115–132. [Google Scholar] [CrossRef]
- Wasnik, D.N.; Dey, G.K.; Kain, V.; Samajdar, I. Precipitation stages in a 316L austenitic stainless steel. Scr. Mater. 2003, 49, 135–141. [Google Scholar] [CrossRef]
- Aydoğdu, G.H.; Aydinol, M.K. Determination of susceptibility to intergranular corrosion and electrochemical reactivation behaviour of AISI 316L type stainless steel. Corros. Sci. 2006, 48, 3565–3583. [Google Scholar] [CrossRef]
- Terada, M.; Escriba, D.M.; Costa, I.; Materna-Morris, E.; Padilha, A.F. Investigation on the intergranular corrosion resistance of the AISI 316L(N) stainless steel after long time creep testing at 600 °C. Mater. Charact. 2008, 59, 663–668. [Google Scholar] [CrossRef]
- Matula, M.; Hyspecka, L.; Svoboda, M.; Vodarek, V.; Dagbert, C.; Galland, J.; Stonawska, Z.; Tuma, L. Intergranular corrosion of AISI 316L steel. Mater. Charact. 2001, 46, 203–210. [Google Scholar] [CrossRef]
- Lescur, A.; Stergar, E.; Lim, J.; Hertelé, S.; Petrov, R.H. Microstructural investigation and identification of intermetallic σ-phase in solution annealed 316L-type austenitic stainless steel. Mater. Charact. 2021, 182, 111524. [Google Scholar] [CrossRef]
- Sahlaoui, H.; Sidhom, H. Experimental Investigation and Analytical Prediction of σ-Phase Precipitation in AISI 316L Austenitic Stainless Steel. Metall. Mater. Trans. A 2013, 44, 3077–3083. [Google Scholar] [CrossRef]
- Cai, B.; Kang, J.-H.; Hong, C.-W.; Kim, S.-J. Effects of N and Cu on the precipitation and the creep life of 316L austenitic stainless steel at 650 °C. Mater. Sci. Eng. A 2016, 662, 198–203. [Google Scholar] [CrossRef]
- Ramírez, L.M.; Almanza, E.; Murr, L.E. Effect of uniaxial deformation to 50% on the sensitisation process in 316 stainless steel. Mater. Charact. 2004, 53, 79–82. [Google Scholar] [CrossRef]
- Padilha, A.F.; Rios, P.R. Decomposition of austenite in austenitic stainless steels. ISIJ Int. 2002, 42, 325–337. [Google Scholar] [CrossRef]
- Fritz, J.D. Effects of Metallurgical Variables on the Corrosion of Stainless Steels. In Corrosion: Fundamentals, Testing and Protection, 1st ed.; Cramer, S.D., Covino, B.S., Jr., Eds.; ASM International: Materials Park, OH, USA, 2003; Volume 13A, pp. 266–286. [Google Scholar]
- Lukas, H.L.; Fries, S.G.; Sundman, B. Computational Thermodynamics: The Calphad Method, 1st ed.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Parvathavarthini, N.; Dayal, R.K.; Khatak, H.S.; Shankar, V.; Shanmugam, V. Sensitization behaviour of modified 316N and 316L stainless steel weld metals after complex annealing and stress relieving cycles. J. Nucl. Mater. 2006, 355, 68–82. [Google Scholar] [CrossRef]
- Sahlaoui, H.; Makhlouf, K.; Sidhom, H.; Philibert, J. Effect of ageing conditions on the precipitates evolution, chromium depletion and intergranular corrosion susceptibility of AISI 316L; experimental and modeling results. Mater. Sci. Eng. 2004, 372, 98–108. [Google Scholar] [CrossRef]
- Kherrouba, N.; Mehdi, B.; Kouba, R.; Badji, R.; Dekik, C.A.; Tounsi, Y.T. Experimental study and simulation of the σ phase precipitation in the stabilized 316Ti austenitic stainless steel. Mater. Chem. Phys. 2021, 266, 124574. [Google Scholar] [CrossRef]
- Ben Rhouma, A.; Amadou, T.; Sidhom, H.; Braham, C. Correlation between microstructure and intergranular corrosion behavior of low delta-ferrite content AISI 316L aged in the range 550–700 °C. J. Alloys Compd. 2017, 708, 871–886. [Google Scholar] [CrossRef]
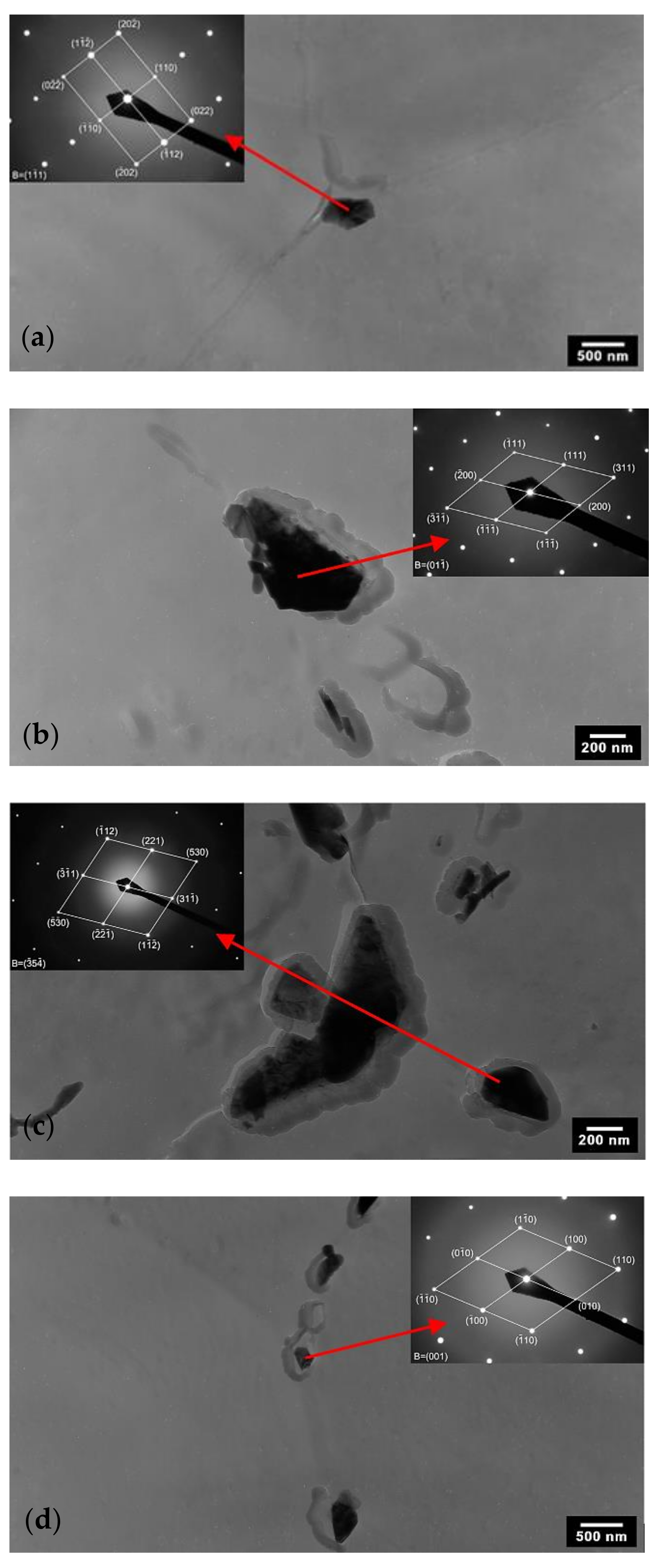

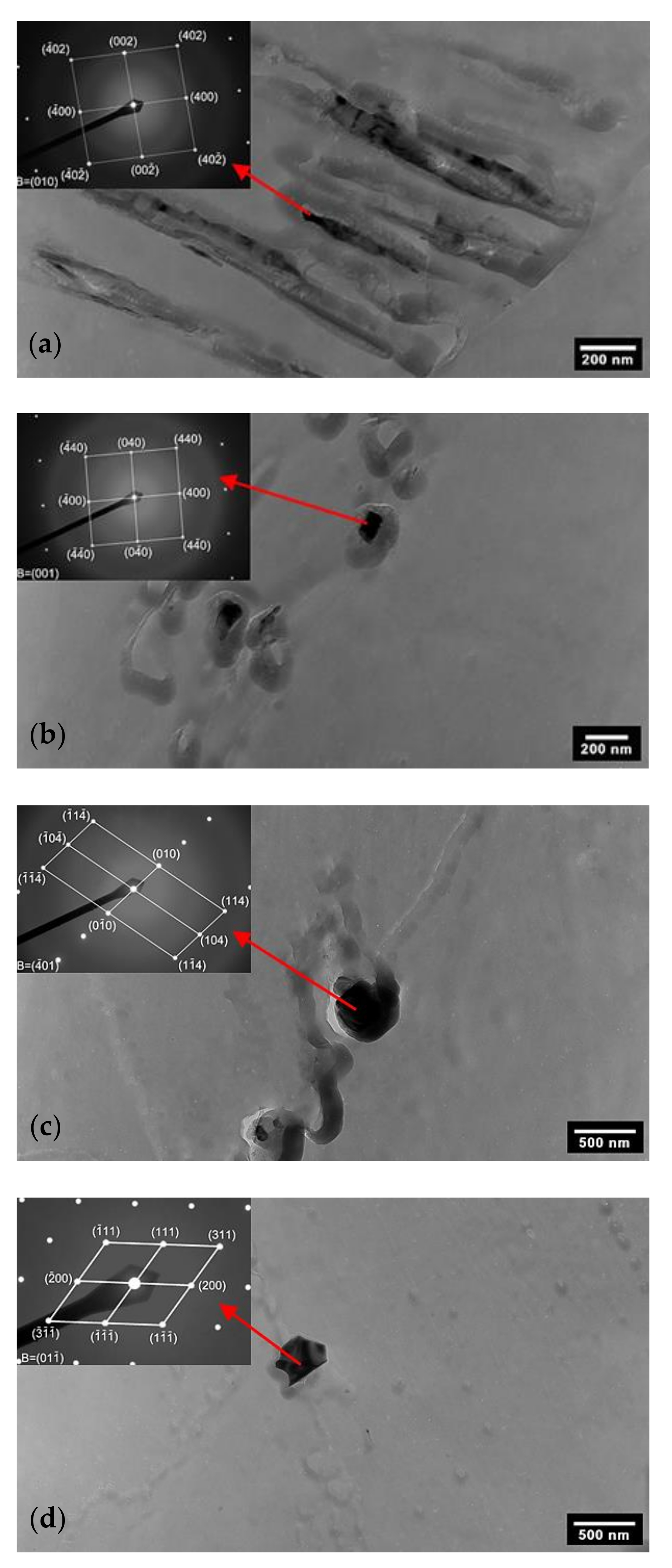
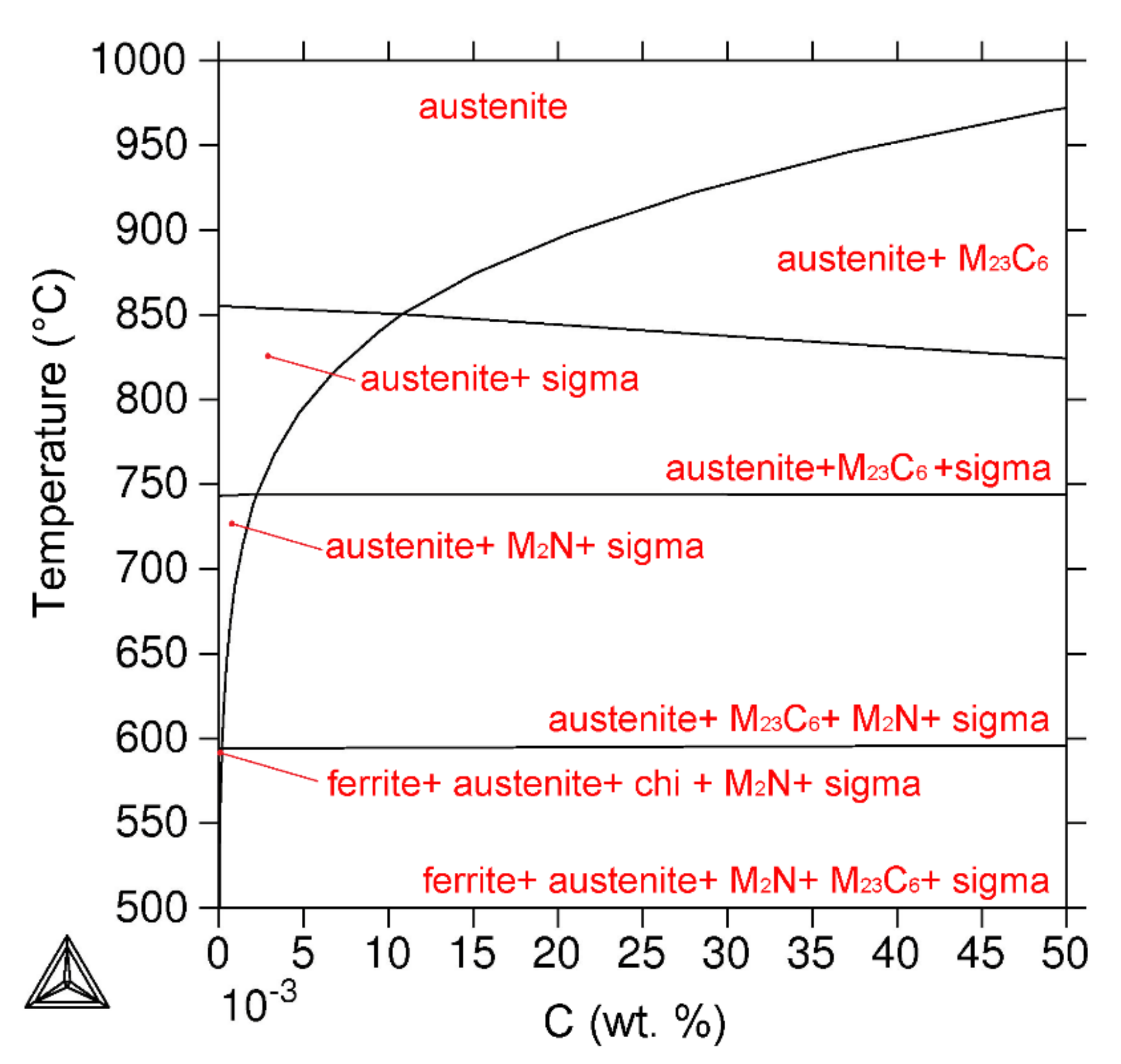
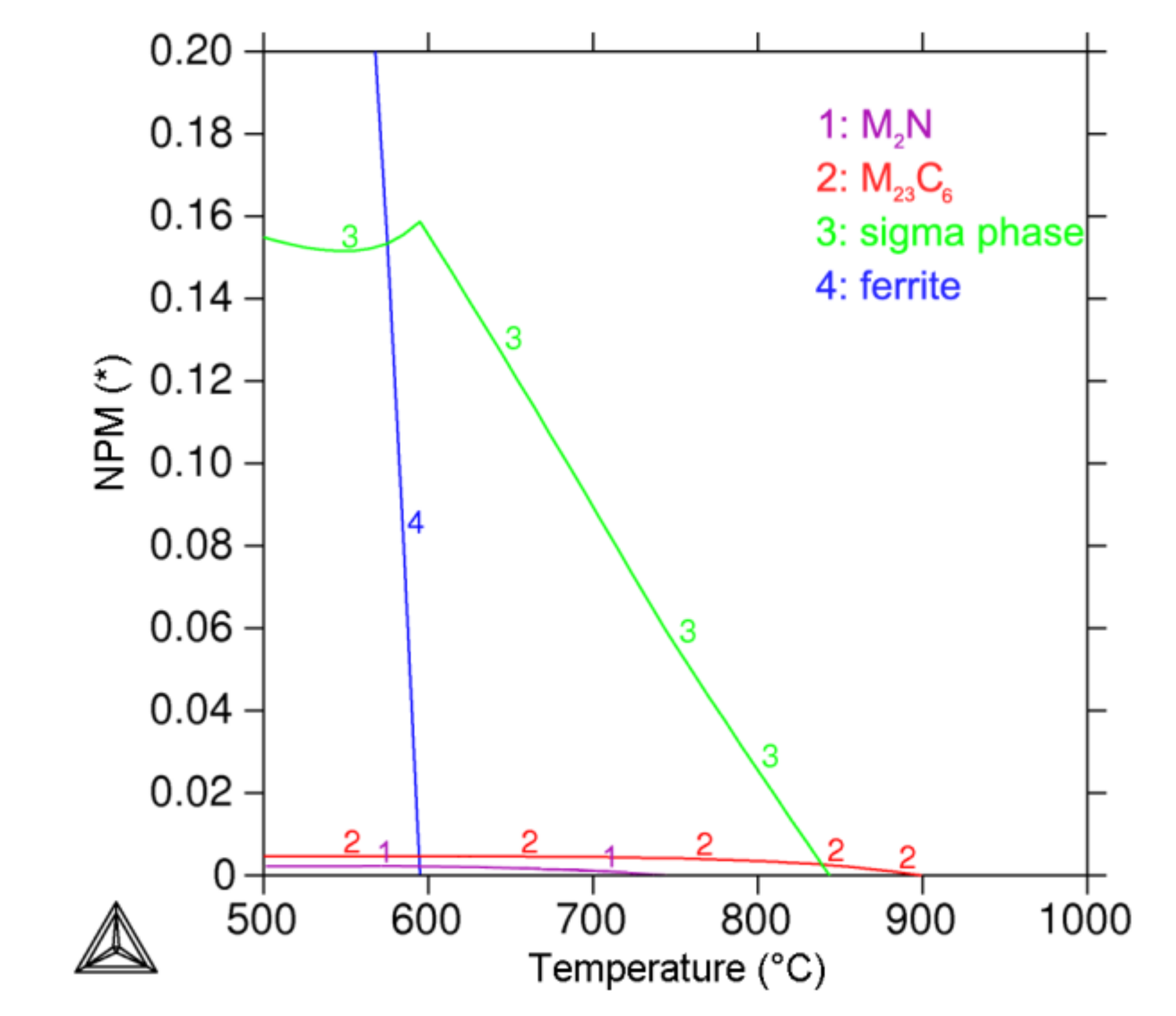

| C | N | Si | Mn | P | S | Cr | Ni | Mo | Ti | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.021 | 0.019 | 0.62 | 1.10 | 0.0027 | 0.004 | 17.47 | 12.20 | 2.10 | - | bal. |
| Sample Nr. | Annealing Time (h) | Sigma Phase | Chi Phase | M23C6 Carbide | Laves Phase |
|---|---|---|---|---|---|
| L1 | 5 | - | - | - | - |
| L2 | 10 | - | × | × | - |
| L3 | 30 | - | × | × | - |
| L4 | 100 | × | × | × | ⊗ |
| L5 | 300 | × | - | × | × |
| L6 | 1000 | × | - | × | × |
| Secondary Phases | Sample Nr. | Cr | Fe | Mo | Ni | Si |
|---|---|---|---|---|---|---|
| M23C6 carbide | L2 | 67.0 ± 1.5 | 17.7 ± 1.6 | 12.4 ± 1.8 | 2.2 ± 0.5 | 0.6 ± 0.3 |
| L3 | 67.2 ± 1.3 | 17.1 ± 1.3 | 13.2 ± 1.1 | 2.0 ± 0.7 | 0.5 ± 0.2 | |
| L4 | 68.8 ± 2.0 | 15.0 ± 1.3 | 12.8 ± 2.1 | 2.8 ± 0.4 | 0.6 ± 0.2 | |
| L5 | 68.2 ± 1.5 | 15.4 ± 2.0 | 13.8 ± 1.7 | 2.1 ± 0.6 | 0.5 ± 0.3 | |
| L6 | 67.3 ± 1.9 | 15.9 ± 2.5 | 14.5 ± 1.7 | 2.0 ± 0.5 | 0.4 ± 0.2 | |
| Average Chemical Composition of M23C6 | 67.7 | 16.2 | 13.3 | 2.2 | 0.5 | |
| Chi Phase | L2 | 21.8 ± 1.4 | 38.1 ± 1.3 | 34.7 ± 0.8 | 2.9 ± 0.5 | 2.5 ± 0.4 |
| L3 | 19.8 ± 1.5 | 39.9 ± 1.5 | 35.1 ± 1.1 | 2.5 ± 0.7 | 2.7 ± 0.4 | |
| L4 | 17.5 ± 1.8 | 39.1 ± 1.3 | 37.9 ± 1.9 | 2.3 ± 0.4 | 3.2 ± 0.3 | |
| Average Chemical Composition of Chi Phase | 19.7 | 39.0 | 35.9 | 2.6 | 2.8 | |
| Laves Phase | L4 | 11.6 ± 1.7 | 30.3 ± 2.1 | 50.5 ± 1.8 | 3.0 ± 1.1 | 4.6 ± 0.6 |
| L5 | 10.1 ± 1.2 | 29.3 ± 1.3 | 52.1 ± 1.9 | 3.9 ± 0.8 | 4.6 ± 0.7 | |
| L6 | 11.7 ± 1.3 | 28.8 ± 2.3 | 50.7 ± 2.5 | 3.6 ± 0.6 | 5.2 ± 1.4 | |
| Average Chemical Composition of Laves Phase | 11.1 | 29.5 | 51.1 | 3.5 | 4.8 | |
| Sigma Phase | L4 | 32.5 ± 0.9 | 53.1 ± 0.2 | 9.6 ± 1.6 | 2.7 ± 0.3 | 2.1 ± 0.6 |
| L5 | 30.4 ± 0.7 | 52.0 ± 0.5 | 12.6 ± 0.9 | 3.0 ± 0.4 | 2.0 ± 0.5 | |
| L6 | 32.3 ± 0.5 | 51.8 ± 0.9 | 10.5 ± 0.6 | 3.2 ± 0.4 | 2.4 ± 0.6 | |
| Average Chemical Composition of Sigma Phase | 31.7 | 52.3 | 10.9 | 3.0 | 2.2 | |
| Sample Nr. | Annealing Time (h) | Sigma Phase | Chi Phase | M23C6 Carbide | Laves Phase |
|---|---|---|---|---|---|
| DL1 | 1 | × | - | - | - |
| DL2 | 1.5 | × | - | - | - |
| DL3 | 2 | × | × | - | - |
| DL4 | 5 | × | × | × | - |
| DL5 | 10 | × | × | × | × |
| Secondary Phases | Sample Nr. | Cr | Fe | Mo | Ni | Si |
|---|---|---|---|---|---|---|
| Sigma Phase | DL51 | 23.9 ± 2.0 | 60.2 ± 3.2 | 5.7 ± 0.7 | 8.1 ± 0.5 | 2.1 ± 0.7 |
| DL52 | 26.3 ± 1.6 | 60.3 ± 2.9 | 8.7 ± 2.1 | 2.9 ± 1.2 | 1.8 ± 0.4 | |
| DL53 | 26.3 ± 1.7 | 58.5 ± 3.1 | 6.8 ± 1.7 | 2.7 ± 1.0 | 5.7 ± 2.1 | |
| DL54 | 26.4 ± 1.5 | 56.2 ± 2.0 | 9.8 ± 1.9 | 4.9 ± 1.6 | 2.8 ± 0.8 | |
| DL55 | 28.7 ± 1.9 | 56.3 ± 2.4 | 10.0 ± 2.4 | 3.6 ± 1.0 | 1.3 ± 0.6 | |
| Average Chemical Composition of Sigma Phase | 26.3 ± 1.5 | 58.3 ± 1.8 | 8.2 ± 1.7 | 4.4 ± 2.0 | 2.7 ± 1.6 | |
| Chi Phase | DL53 | 23.2 ± 0.8 | 34.9 ± 1.2 | 32.9 ± 1.7 | 3.9 ± 0.9 | 5.0 ± 1.4 |
| DL54 | 24.3 ± 1.0 | 38.9 ± 2.2 | 29.0 ± 1.2 | 4.4 ± 2.4 | 3.5 ± 2.3 | |
| DL55 | 24.0 ± 1.8 | 40.5 ± 2.3 | 28.5 ± 2.3 | 4.0 ± 1.7 | 3.1 ± 0.9 | |
| Average Chemical Composition of Chi Phase | 23.8 ± 0.5 | 38.1 ± 2.4 | 30.1 ± 2.0 | 4.1 ± 0.2 | 3.9 ± 0.8 | |
| M23C6 carbide | DL54 | 62.0 ± 3.0 | 18.3 ± 1.8 | 12.4 ± 2.7 | 4.9 ± 1.4 | 2.4 ± 0.6 |
| DL55 | 64.5 ± 2.4 | 16.2 ± 1.9 | 15.6 ± 1.9 | 2.8 ± 2.0 | 0.9 ± 0.3 | |
| Average Chemical Composition of M23C6 | 63.3 ± 1.3 | 17.3 ± 1.1 | 14.0 ± 1.6 | 3.9 ± 1.1 | 1.7 ± 0.8 | |
| Laves Phase | DL55 | 11.3 ± 1.1 | 17.0 ± 0.6 | 54.5 ± 2.6 | 11.1 ± 1.9 | 6.2 ± 1.2 |
| ASS | tmin (h) | Tcrit (°C) |
|---|---|---|
| AISI 316L (0% CW) | 10 | 750 |
| AISI 316L (40% CW) | 2 | 750 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bártová, K.; Dománková, M.; Bárta, J.; Pastier, P. Influence of 40% Cold Working and Annealing on Precipitation in AISI 316L Austenitic Stainless Steel. Materials 2022, 15, 6484. https://doi.org/10.3390/ma15186484
Bártová K, Dománková M, Bárta J, Pastier P. Influence of 40% Cold Working and Annealing on Precipitation in AISI 316L Austenitic Stainless Steel. Materials. 2022; 15(18):6484. https://doi.org/10.3390/ma15186484
Chicago/Turabian StyleBártová, Katarína, Mária Dománková, Jozef Bárta, and Peter Pastier. 2022. "Influence of 40% Cold Working and Annealing on Precipitation in AISI 316L Austenitic Stainless Steel" Materials 15, no. 18: 6484. https://doi.org/10.3390/ma15186484
APA StyleBártová, K., Dománková, M., Bárta, J., & Pastier, P. (2022). Influence of 40% Cold Working and Annealing on Precipitation in AISI 316L Austenitic Stainless Steel. Materials, 15(18), 6484. https://doi.org/10.3390/ma15186484






