Biocompatibility of Zinc Matrix Biodegradable Composites Reinforced by Graphene Nanosheets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Extraction Preparation
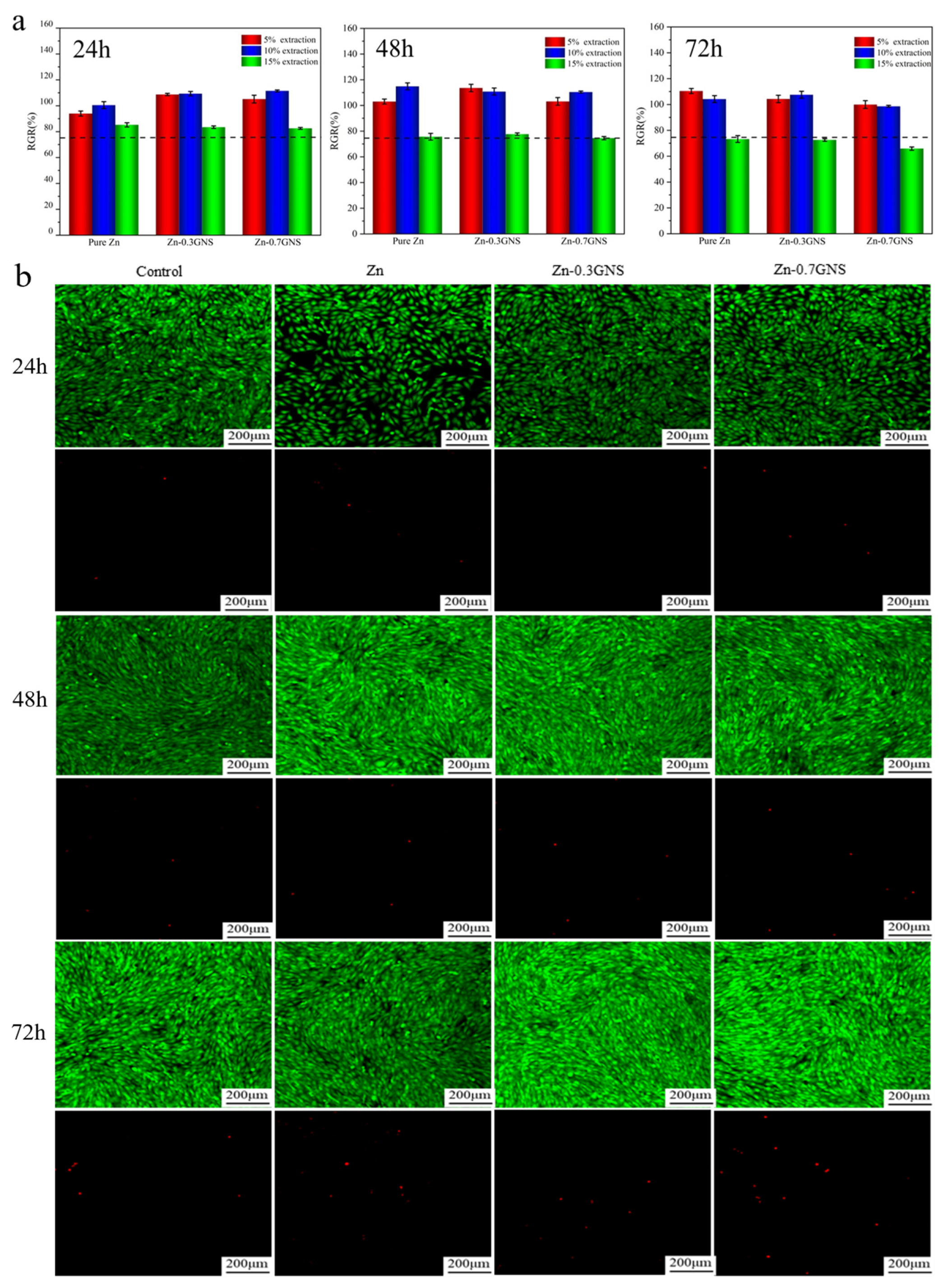
2.3. Cell Viability Test
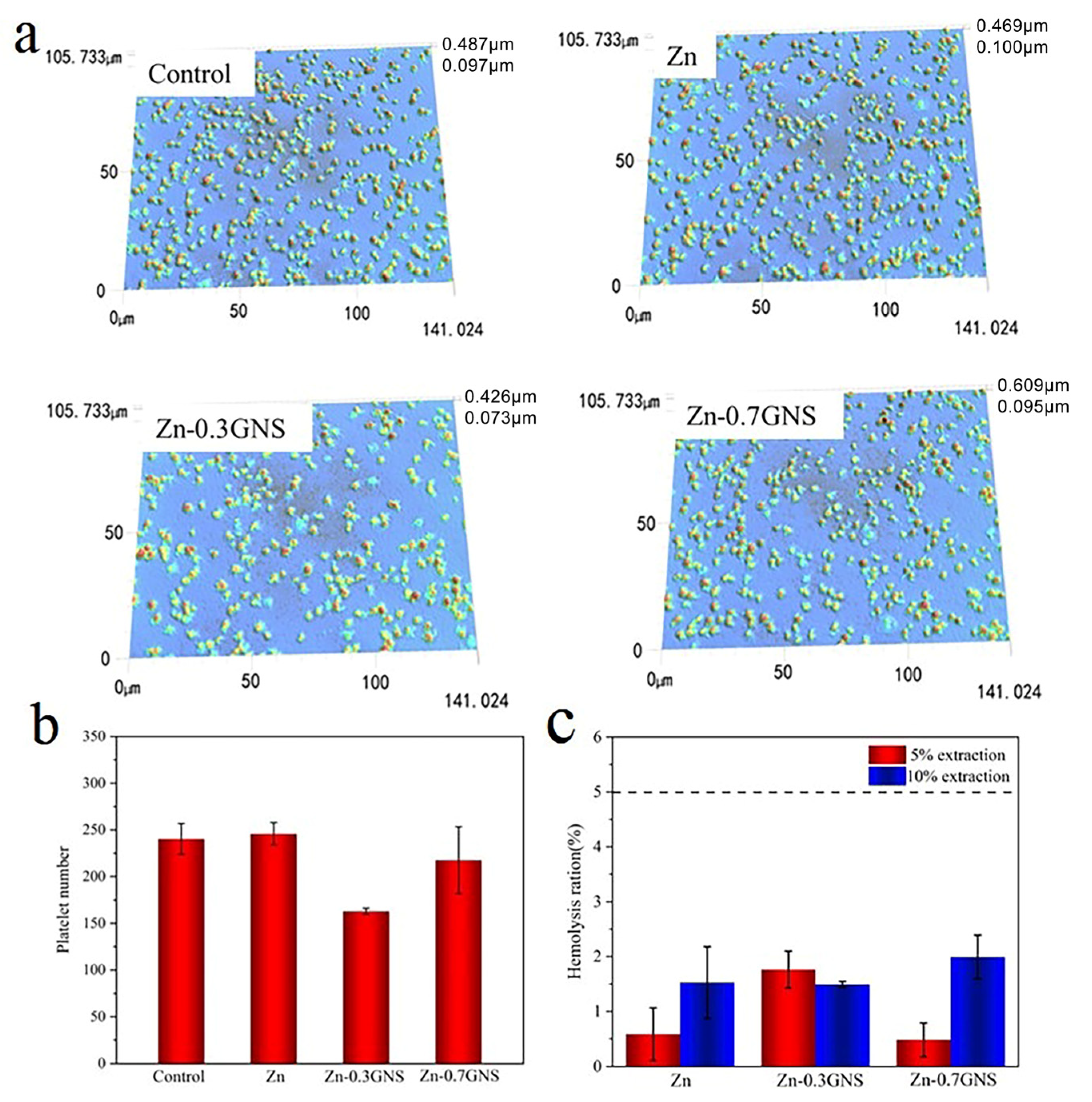
2.4. Hemolysis Test
2.5. Platelet Adhesion Test
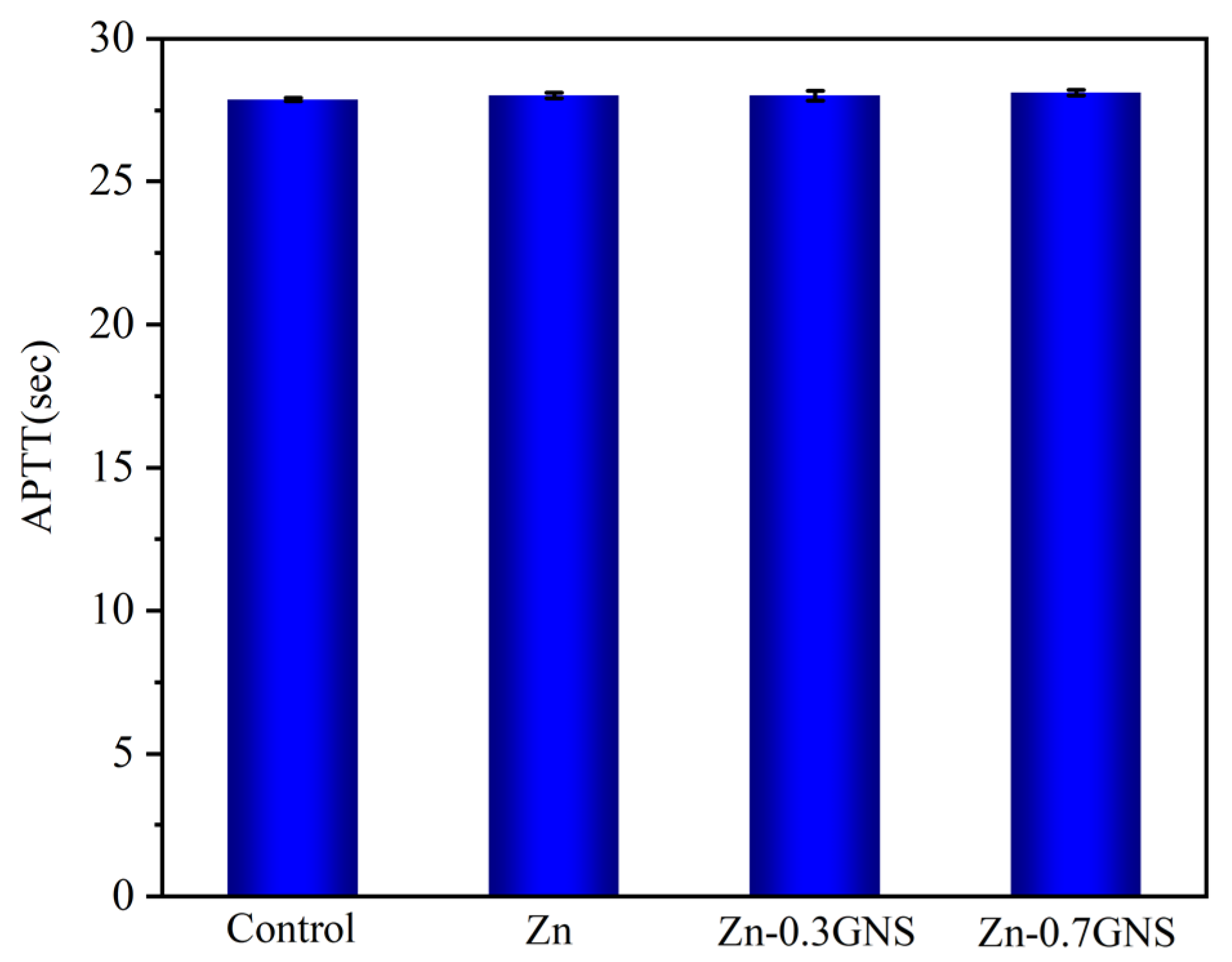
2.6. Coagulation Time
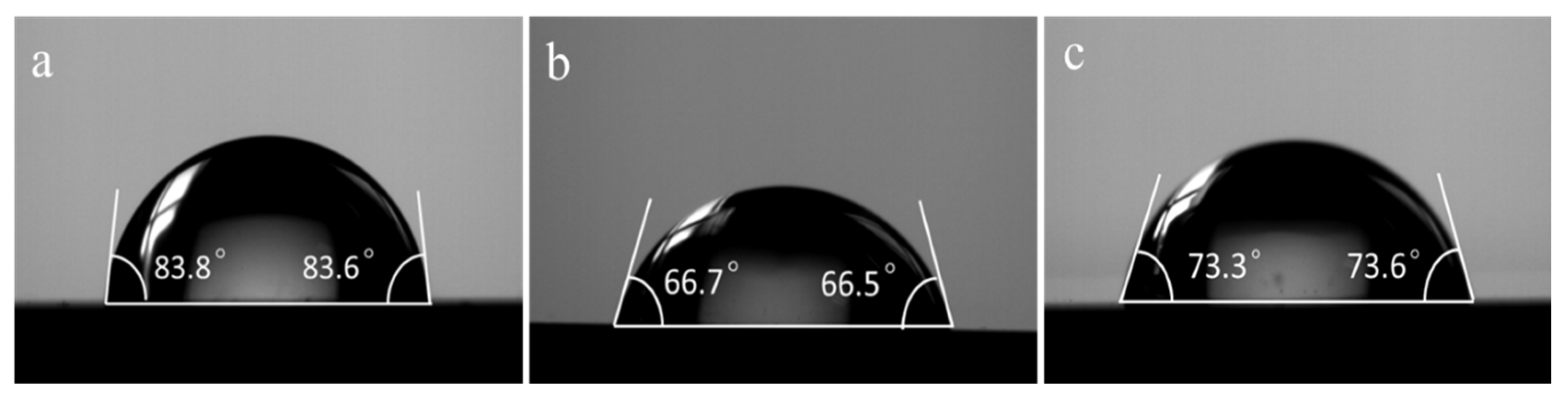
2.7. Contact Angle Measurement
3. Results and Discussion
3.1. Cytotoxicity of Zinc-Based Composites
3.2. Blood Compatibility of Zinc Matrix Composites
3.2.1. Hemolysis Rate
3.2.2. Coagulation Time
3.3. Contact Angle
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Levy, G.K.; Goldman, J.; Aghion, E. The prospects of zinc as a structural material for biodegradable implants—A review paper. Metals 2017, 7, 402. [Google Scholar] [CrossRef]
- Lin, J.; Tong, X.; Shi, Z.; Zhang, D.; Zhang, L.; Wang, K.; Wei, A.; Jin, L.; Lin, J.; Li, Y.; et al. A biodegradable Zn-1Cu-0.1Ti alloy with antibacterial properties for orthopedic applications. Acta Biomater. 2020, 106, 410–427. [Google Scholar] [CrossRef]
- Bommala, V.K.; Krishna, M.G.; Rao, C.T. Magnesium matrix composites for biomedical applications: A review. J. Magnes. Alloy. 2019, 7, 72–79. [Google Scholar] [CrossRef]
- Pierson, D.; Edick, J.; Tauscher, A.; Pokorney, E.; Bowen, P.; Gelbaugh, J.; Stinson, J.; Getty, H.; Lee, C.H.; Drelich, J.; et al. A simplified in vivo approach for evaluating the bioabsorbable behavior of candidate stent materials. J. Biomed. Mater. Res. B. 2012, 100, 58–67. [Google Scholar] [CrossRef]
- Yang, H.; Wang, C.; Liu, C.; Chen, H.; Wu, Y.; Han, J.; Jia, Z.; Lin, W.; Zhang, D.; Li, W.; et al. Evolution of the degradation mechanism of pure zinc stent in the one-year study of rabbit abdominal aorta model. Biomaterials 2017, 145, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Bowen, P.K.; Drelich, J.; Goldman, J. Zinc Exhibits Ideal Physiological Corrosion Behavior for Bioabsorbable Stents. Adv Mater. 2013, 25, 2577–2582. [Google Scholar] [CrossRef] [PubMed]
- Bowen, P.K.; Guillory, R.J., II; Shearier, E.R.; Seitz, J.M.; Drelich, J.; Bocks, M.; Zhao, F.; Goldman, J. Metallic zinc exhibits optimal biocompatibility for bioabsorbable endovascular stents. Mat. Sci. Eng. C-Mater. 2015, 56, 467–472. [Google Scholar] [CrossRef]
- Meng, Y.; Liu, L.; Zhang, D.; Dong, C.; Yan, Y.; Volinsky, A.A.; Wang, L.-N. Initial formation of corrosion products on pure zinc in saline solution. Bioact. Mater. 2019, 4, 87–96. [Google Scholar] [CrossRef]
- Weiss, J.H.; Sensi, S.L.; Koh, J.Y. Zn2+: A novel ionic mediator of neural injury in brain disease. Trends Pharmacol Sci. 2000, 21, 395–401. [Google Scholar] [CrossRef]
- Yang, H.; Qu, X.; Lin, W.; Wang, C.; Zhu, D.; Dai, K.; Zheng, Y. In vitro and in vivo studies on zinc-hydroxyapatite composites as novel biodegradable metal matrix composite for orthopedic applications. Acta Biomater. 2018, 71, 200–214. [Google Scholar] [CrossRef]
- Suzuki, T.; Kajita, Y.; Katsumata, S.-I.; Matsuzaki, H.; Suzuki, K. Zinc Deficiency Increases Serum Concentrations of Parathyroid Hormone through a Decrease in Serum Calcium and Induces Bone Fragility in Rats. J. Nutr. Sci. Vitaminol. 2015, 61, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, W.; Jia, S.; Li, B. Puerarin and zinc additively prevent mandibular bone loss through inhibiting osteoclastogenesis in ovariectomized rats. Histol. Histopathol. 2017, 32, 851–860. [Google Scholar] [PubMed]
- Kubásek, J.; Vojtěch, D.; Pospíšilová, I.; Michalcová, A.; Maixner, J. Microstructure and mechanical properties of the micrograined hypoeutectic Zn-Mg alloy. Int. J. Min. Met. Mater. 2016, 23, 1167–1176. [Google Scholar] [CrossRef]
- Erinc, M.; Sillekens, W.H.; Mannens, R.G.T.M.; Werkhoven, R.J. Applicability of existing magnesium alloys as biomedical implant materials. Magnes. Technol. 2009, 73, 209–214. [Google Scholar]
- Tang, Z.; Huang, H.; Niu, J.; Zhang, L.; Zhang, H.; Pei, J.; Tan, J.; Yuan, G. Design and characterizations of novel biodegradable Zn-Cu-Mg alloys for potential biodegradable implants. Mater. Design. 2016, 117, 84–94. [Google Scholar] [CrossRef]
- Li, H.; Yang, H.; Zheng, Y.; Zhou, F.; Qiu, K.; Wang, X. Design and characterizations of novel biodegradable ternary Zn-based alloys with IIA nutrient alloying elements Mg, Ca and Sr. Mater. Design. 2015, 83, 95–102. [Google Scholar] [CrossRef]
- Paul, W.; Sharma, C. Blood compatibility and biomedical applications of grapheme. Trends Biomater. Artif. Organs. 2011, 25, 91–94. [Google Scholar]
- Du, X.; Du, W.; Wang, Z.; Liu, K.; Li, S. Defects in graphene nanoplatelets and their interface behavior to reinforce magnesium alloys. Appl. Surf. Sci. 2019, 484, 414–423. [Google Scholar] [CrossRef]
- Zhou, K.; Yu, P.; Shi, X.; Ling, T.; Zeng, W.; Chen, A.; Yang, W.; Zhou, Z. Hierarchically Porous Hydroxyapatite Hybrid Scaffold Incorporated with Reduced Graphene Oxide for Rapid Bone Ingrowth and Repair. ACS Nano 2019, 13, 9595–9606. [Google Scholar] [CrossRef]
- Yan, J.; Chen, L.; Huang, C.-C.; Lung, S.-C.C.; Yang, L.; Wang, W.-C.; Lin, P.-H.; Suo, G.; Lin, C.-H. Consecutive evaluation of graphene oxide and reduced graphene oxide nanoplatelets immunotoxicity on monocytes. Colloid Surface B 2017, 153, 300. [Google Scholar] [CrossRef]
- Kumar, S.; Chatterjee, K. Strontium eluting graphene hybrid nanoparticles augment osteogenesis in a 3D tissue scaffold. Nanoscale 2015, 7, 2023–2033. [Google Scholar] [CrossRef] [PubMed]
- Shahin, M.; Munir, K.; Wen, C.; Li, Y. Magnesium-based composites reinforced with grapheme nanoplatelets as biodegradable implant materials. J. Alloys Compd. 2020, 828, 154461. [Google Scholar] [CrossRef]
- Shahin, M.; Munir, K.; Wen, C.; Li, Y. Magnesium matrix nanocomposites for orthopedic applications: A review from mechanical, corrosion, and biological perspectives. Acta Biomater. 2019, 96, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Newman, L.; Lozano, N.; Zhang, M.; Iijima, S.; Yudasaka, M.; Bussy, C.; Kostarelos, K. Hypochlorite degrades 2D graphene oxide sheets faster than 1D oxidised carbon nanotubes and nanohorns. NPJ 2D Mater. Appl. 2017, 1, 39. [Google Scholar] [CrossRef]
- Munir, K.; Wen, C.; Li, Y. Graphene nanoplatelets-reinforced magnesium metal matrix nanocomposites with superior mechanical and corrosion performance for biomedical applications. J. Magnes. Alloy. 2020, 8, 269–290. [Google Scholar] [CrossRef]
- Saberi, A.; Bakhsheshi-Rad, H.; Karamian, E.; Kasiri-Asgarani, M.; Ghomi, H. Magnesium-graphene nano-platelet composites: Corrosion behavior, mechanical and biological properties. J. Alloys Compd. 2022, 821, 153379. [Google Scholar] [CrossRef]
- Dai, Q.; Peng, S.; Zhang, Z.; Liu, Y.; Fan, M.; Zhao, F. Microstructure and Mechanical Properties of Zinc Matrix Biodegradable Composites Reinforced by Graphene. Front. Bioeng. Biotech. 2021, 9, 635338. [Google Scholar] [CrossRef]
- ISO 10993–5:2009; Biological Evaluation of Medical Devices–Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- Tong, X.; Zhu, L.; Wang, K.; Shi, Z.; Huang, S.; Li, Y.; Ma, J.; Wen, C.; Lin, J. Impact of gadolinium on mechanical properties, corrosion resistance, and biocompatibility of Zn-1Mg-xGd alloys for biodegradable bone-implant applications. Acta Biomater. 2022, 142, 361–373. [Google Scholar] [CrossRef]
- Tong, X.; Zhang, D.; Lin, J.; Dai, Y.; Luan, Y.; Sun, Q.; Shi, Z.; Wang, K.; Gao, Y.; Lin, J.; et al. Development of biodegradable Zn–1Mg–0.1RE (RE=Er, Dy, and Ho) alloys for biomedical applications. Acta Biomater. 2020, 117, 384–399. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Tong, X.; Sun, Q.; Luan, Y.; Zhang, D.; Shi, Z.; Wang, K.; Lin, J.; Li, Y.; Dargusch, M.; et al. Biodegradable ternary Zn–3Ge–0.5X (X=Cu, Mg, and Fe) alloys for orthopedic applications. Acta Biomater. 2020, 115, 432–446. [Google Scholar] [CrossRef]
- Murni, N.S.; Dambatta, M.S.; Yeap, S.K.; Froemming, G.R.A.; Hermawan, H. Cytotoxicity evaluation of biodegradable Zn–3Mg alloy toward normal human osteoblast cells. Mat. Sci. Eng. C-Mater. 2015, 49, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Witte, F.; Xi, T.; Zheng, Y.; Yang, K.; Yang, Y.; Zhao, D.; Meng, J.; Li, Y.; Li, W.; et al. Recommendation for modifying current cytotoxicity testing standards for biodegradable magnesium-based materials. Acta Biomater. 2015, 21, 237–249. [Google Scholar] [CrossRef]
- Oba, Y.; Yasue, A.; Kaneko, K.; Uchida, R.; Shioyasono, A.; Moriyama, K. Comparison of stability of mandibular segments following the sagittal split ramus osteotomy with poly-l-lactic acid (PLLA) screws and titanium screws fixation. Orthod. Waves 2008, 67, 1–8. [Google Scholar] [CrossRef]
- Lee, S.R. Critical role of zinc as either an antioxidant or a prooxidant in cellular systems. Oxid. Med. Cell Longevity. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Wang, J.; He, Y.; Maitz, M.F.; Collins, B.; Xiong, K.; Guo, L.; Yun, Y.; Wan, G.; Huang, N. A surface-eroding poly (1,3-trimethylene carbonate) coating for fully biodegradable magnesium-based stent applications: Toward better biofunction, biodegradation and biocompatibility. Acta Biomater. 2013, 9, 8678–8689. [Google Scholar] [CrossRef]
- Mochizuki, A.; Kaneda, H. Study on the blood compatibility and biodegradation properties of magnesium alloys. Mat. Sci. Eng. C-Mater. 2015, 47, 204–210. [Google Scholar] [CrossRef]
- Králová, Z.O.; Gorejová, R.; Oriňaková, R.; Petráková, M.; Oriňak, A.; Kupková, M.; Hrubovčáková, M.; Sopčák, T.; Baláž, M.; Maskaľová, I.; et al. Biodegradable zinc-iron alloys: Complex study of corrosion behavior mechanical properties and hemocompatibility. Prog. Nat. Sci.-Mater. 2021, 31, 279–287. [Google Scholar] [CrossRef]
- Pathak, D.K.; Pandey, P.M. Evaluation of in vitro corrosion behavior of zinc–hydroxyapatite and zinc–hydroxyapatite–iron as biodegradable composites. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Abazari, S.; Shamsipur, A.; Bakhsheshi-Rad, H.R.; Berto, F. Functionalized carbon nanotube-encapsulated magnesium-based nanocomposites with outstanding mechanical and biological properties as load-bearing bone implants. Mater. Design. 2022, 213, 110354. [Google Scholar] [CrossRef]
- Solař, P.; Kylián, O.; Marek, A.; Vandrovcová, M.; Bačáková, L.; Hanuš, J.; Vyskočil, J.; Slavínská, D.; Biederman, H. Particles induced surface nanoroughness of titanium surface and its influence on adhesion of osteoblast-like MG-63 cells. Appl. Surf. Sci. 2015, 324, 99–105. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z.; Zhang, S.; Zheng, W.; Zhao, Q.; Zhang, J.; Wang, L.; Wang, S.; Kong, D. Functionalization of the surface of electrospun poly(epsilon-caprolactone) mats using zwitterionic poly (carboxybetaine methacrylate) and cell-specific peptide for endothelial progenitor cells capture. Mat. Sci. Eng. C-Mater. 2013, 33, 1646–1653. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Zhang, D.; Zhang, X.; Su, Y.; Shi, Z.; Wang, K.; Lin, J.; Li, Y.; Lin, J.; Wen, C. Microstructure, mechanical properties, biocompatibility, and in vitro corrosion and degradation behavior of a new Zn–5Ge alloy for biodegradable implant materials. Acta Biomater. 2018, 82, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Yue, R.; Niu, J.; Li, Y.; Ke, G.; Huang, H.; Pei, J.; Ding, W.; Yuan, G. In vitro cytocompatibility, hemocompatibility and antibacterial properties of biodegradable Zn-Cu-Fe alloys for cardiovascular stents applications. Mat. Sci. Eng. C-Mater. 2020, 113, 111007. [Google Scholar] [CrossRef] [PubMed]
| Materials | Contact Angle (°) | Hemolysis Rate (%) | Applications | Ref. |
|---|---|---|---|---|
| Zn (SPS + HR) | 82.8 ± 2.4 | 1.527 ± 1.130 | Orthopedic implants | this study |
| Zn-0.3GNS(SPS + HR) | 66.4 ± 1.2 | 1.493 ± 0.089 | ||
| Zn-0.7GNS(SPS + HR) | 73 ± 2.9 | 1.994 ± 0.691 | ||
| Zn (Cast) | - | 2.083 ± 0.090 | Orthopedic implants | [38] |
| Zn-1Fe (Cast) | - | 3.750 ± 0.158 | ||
| Zn-2Fe (Cast) | - | 4.583 ± 0.170 | ||
| Zn-5Fe (Cast) | - | 17.50 ± 0.613 | ||
| Zn-10Fe (Cast) | - | 20.83 ± 0.833 | ||
| Zn-1Mg (HR) | - | 4.01 ± 0.03 | Orthopedic implants | [30] |
| Zn-1Mg-0.1Er (HR) | - | 4.49 ± 0.09 | ||
| Zn-1Mg-0.1Dy (HR) | - | 4.21 ± 0.02 | ||
| Zn-1Mg-0.1Ho (HR) | - | 3.28 ± 0.09 | ||
| Zn (MSP) | 87.3 | - | Biodegradable implants | [39] |
| Zn-3HA(MSP) | 65.5 | - | ||
| Zn-3HA-2Fe (MSP) | 77.3 | - | ||
| Zn-5HA-2Fe (MSP) | 72.7 | - | ||
| Zn (AC) | 62.4 ± 0.9 | 2.44 ± 0.08 | Orthopedic implants | [2] |
| Zn-1Cu-0.1Ti (AC) | 63.7 ± 2.6 | 3.62 ± 0.14 | ||
| Zn(Cast) | - | 0.88 ± 0.07 | Biodegradable implants | [43] |
| Zn-5Ge(Cast) | - | 0.24 ± 0.1 | ||
| Zn(SPS) | - | 1.07 ± 0.46 | Orthopedic implants | [10] |
| Zn-1HA(SPS) | - | 1.24 ± 0.78 | ||
| Zn-5HA(SPS) | - | 0.63 ± 0.55 | ||
| Zn-10HA(SPS) | - | 0.69 ± 0.44 | ||
| Zn(HE) | - | 1.16 ± 0.39 | Cardiovascular stents | [44] |
| Zn-3Cu(HE) | - | 0.96 ± 0.48 | ||
| Zn-3Cu-0.2Fe(HE) | - | 1.10 ± 0.25 | ||
| Zn-3Cu-0.5Fe (HE) | - | 1.21 ± 0.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, M.; Zhao, F.; Peng, S.; Dai, Q.; Liu, Y.; Yin, S.; Zhang, Z. Biocompatibility of Zinc Matrix Biodegradable Composites Reinforced by Graphene Nanosheets. Materials 2022, 15, 6481. https://doi.org/10.3390/ma15186481
Fan M, Zhao F, Peng S, Dai Q, Liu Y, Yin S, Zhang Z. Biocompatibility of Zinc Matrix Biodegradable Composites Reinforced by Graphene Nanosheets. Materials. 2022; 15(18):6481. https://doi.org/10.3390/ma15186481
Chicago/Turabian StyleFan, Mei, Fei Zhao, Shanshan Peng, Qianfei Dai, Yuan Liu, Sheng Yin, and Zongkui Zhang. 2022. "Biocompatibility of Zinc Matrix Biodegradable Composites Reinforced by Graphene Nanosheets" Materials 15, no. 18: 6481. https://doi.org/10.3390/ma15186481
APA StyleFan, M., Zhao, F., Peng, S., Dai, Q., Liu, Y., Yin, S., & Zhang, Z. (2022). Biocompatibility of Zinc Matrix Biodegradable Composites Reinforced by Graphene Nanosheets. Materials, 15(18), 6481. https://doi.org/10.3390/ma15186481





