Electrical Properties of In Situ Synthesized Ag-Graphene/Ni Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Conductivity and Hardness Measurement
3.2. X-ray Diffraction
3.3. Contact Angle Measurement
3.4. Welding Force
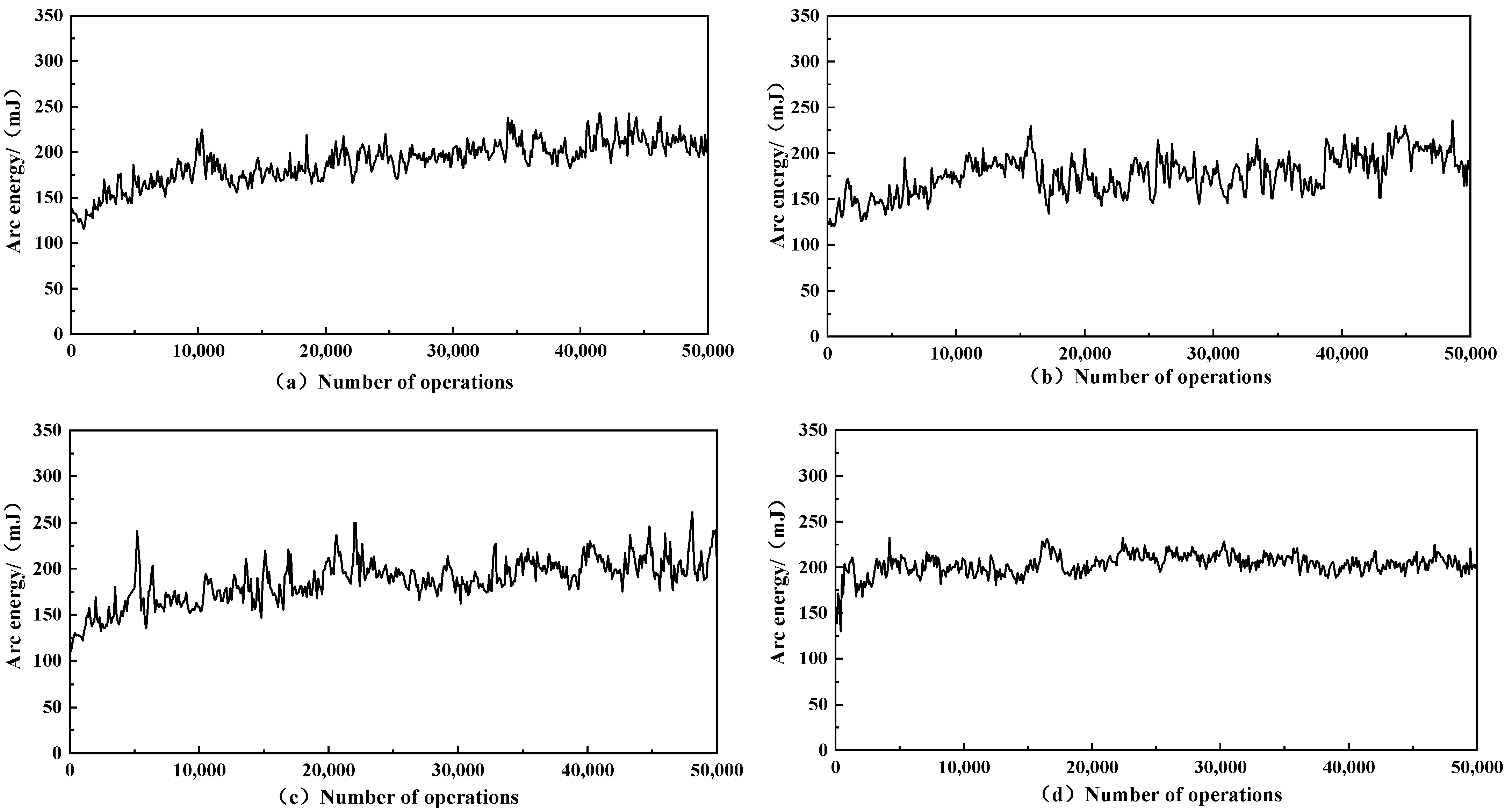
3.5. Arc Energy
4. Conclusions
- (1)
- The addition of graphene resulted in an increase in conductivity, with a 12% increase in conductivity at 0.3%wt. The hardness of the samples also tended to increase with a higher content.
- (2)
- The addition of graphene can make the contact angle of the Ag/Ni contact smaller and enhance the wettability between silver and nickel. Under the action of a high-temperature arc, the Ag/Ni contacts will reduce the spattering effect.
- (3)
- The addition of graphene reduces the fusion welding force and reduces the occurrence of fusion welding in the contacts, which can improve the life of the contacts. A graphene addition of 0.3%wt reduces the melt welding force by 8.04%, which is the best result.
- (4)
- The lowest arc energy was achieved at a graphene doping level of approximately 0.3 wt%, with an average value of approximately 176.77 mJ. Compared to the contacts without graphene doping, the average arc energy decreased by 13.06%, reducing the arc burn on the contacts.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, C.; Yi, D.; Weng, W.; Li, S.; Zhou, J.; Zheng, F. Arc erosion behavior of Ag/Ni electrical contact materials. Mater. Des. 2015, 85, 511–519. [Google Scholar] [CrossRef]
- Guo, T.F.; Chong, F.U.; Wang, J.B.; Liu, S.T.; Xue, Y.Y.; Zhao, H.G. Research and Progress of Silver-Nickel Contact Materials Containing Additives. Electr. Eng. Mater. 2015, 2, 34–38. [Google Scholar]
- Fan, Y.E.; Huang, X.W.; Wang, Y.X.; Chen, G.M.; Li, Z.P. Study on AgNi Contact Materials with MeO Additives. Electr. Eng. Mater. 2013, 2, 3–7+12. [Google Scholar]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef]
- Wen, C.; Shao, M.; Zhuo, S.; Lin, Z.; Kang, Z. Silver/graphene nanocomposite: Thermal decomposition preparation and its catalytic performance. Mater. Chem. Phys. 2012, 135, 780–785. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, J.; Cheng, X.; Zhao, N.; Sun, H.; Li, D. Transparent and conductive reduced graphene oxide/silver nanoparticles multilayer film obtained by electrical self-assembly process with graphene oxide sheets and silver colloid. RSC Adv. 2012, 3, 3391–3398. [Google Scholar] [CrossRef]
- Yu, K.; Wen, Z.; Pu, H.; Lu, G.; Bo, Z.; Kim, H.; Qian, Y.; Andrew, E.; Mao, S.; Chen, J. Hierarchical vertically oriented graphene as a catalytic counter electrode in dye-sensitized solar cells. J. Mater. Chem. A 2012, 1, 188–193. [Google Scholar] [CrossRef]
- Exarchos, D.A.; Dalla, P.T.; Tragazikis, I.K.; Dassios, K.G.; Zafeiropoulos, N.E.; Karabela, M.M.; De Crescenzo, C.; Karatza, D.; Musmarra, D.; Chianese, S.; et al. Development and Characterization of High Performance Shape Memory Alloy Coatings for Structural Aerospace Applications. Materials 2018, 11, 832. [Google Scholar] [CrossRef]
- Ouyang, J.H.; Sasaki, S.; Umeda, K. Low-pressure plasma-sprayed ZrO2–CaF2 composite coating for high temperature tribo-logical applications. Surf. Coat. Technol. 2001, 137, 21–30. [Google Scholar] [CrossRef]
- Shokri, N.; Safavi, M.S.; Etminanfar, M.; Walsh, F.C.; Khalil-Allafi, J. Enhanced corrosion protection of NiTi orthopedic implants by highly crystalline hydroxyapatite deposited by spin coating: The importance of pre-treatment. Mater. Chem. Phys. 2020, 259, 124041. [Google Scholar] [CrossRef]
- De Crescenzo, C.; Karatza, D.; Musmarra, D.; Chianese, S.; Baxevanis, T.; Dalla, P.T.; Exarchos, D.A.; Dassios, K.G.; Matikas, T.E. Ni-Ti Shape Memory Alloy Coatings for Structural Applications: Optimization of HVOF Spraying Parameters. Adv. Mater. Sci. Eng. 2018, 2018, 7867302. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Z.; Kim, D.; Zhang, B.; Tanioku, M.; Ono, T.; Matsumoto, K.; Suganuma, K. Interfacial oxidation protection and thermal-stable sinter Ag joining on bare Cu substrate by single-layer graphene coating. Appl. Surf. Sci. 2019, 497, 143797. [Google Scholar] [CrossRef]
- Zhao, L.; Guo, Q.; Shi, Y.; Liu, Y.; Osovski, S.; Li, Z.; Xiong, D.-B.; Su, Y.; Zhang, D. Interfacial Effect on the Deformation Mechanism of Bulk Nanolaminated Graphene-Al Composites. Met. Mater. Trans. A 2019, 50, 1113–1118. [Google Scholar] [CrossRef]
- Kuang, D.; Xu, L.; Liu, L.; Hu, W.; Wu, Y. Graphene–nickel composites. Appl. Surf. Sci. 2013, 273, 484–490. [Google Scholar] [CrossRef]
- Cao, M.; Xiong, D.-B.; Yang, L.; Li, S.; Xie, Y.; Guo, Q.; Li, Z.; Adams, H.; Gu, J.; Fan, T.; et al. Ultrahigh electrical conductivity of graphene embedded in metals. Adv. Funct. Mater. 2019, 29, 1806792. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef]
- Guex, L.G.; Sacchi, B.; Peuvot, K.F.; Andersson, R.L.; Pourrahimi, A.M.; Ström, V.; Farris, S.; Olsson, R.T. Experimental review: Chemical reduction of graphene oxide (GO) to reduced graphene oxide (rGO) by aqueous chemistry. Nanoscale 2017, 9, 9562–9571. [Google Scholar] [CrossRef]
- Park, S.; An, J.; Potts, J.R.; Velamakanni, A.; Murali, S.; Ruoff, R.S. Hydrazine-reduction of graphite- and graphene oxide. Carbon 2011, 49, 3019–3023. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, J.; Xiong, Z.; Yong, Y.; Zhao, X.S. Preparation, characterization and antibacterial properties of silver-modified graphene oxide. J. Mater. Chem. 2011, 21, 3350–3352. [Google Scholar] [CrossRef]
- Yuan, W.; Gu, Y.; Li, L. Green synthesis of graphene/Ag nanocomposites. Appl. Surf. Sci. 2012, 261, 753–758. [Google Scholar] [CrossRef]
- Fernández-Merino, M.J.; Guardia, L.; Paredes, J.I.; Villar-Rodil, S.; Solís-Fernández, P.; Martínez-Alonso, A.; Tascón, J.M.D. Vitamin C Is an Ideal Substitute for Hydrazine in the Reduction of Graphene Oxide Suspensions. J. Phys. Chem. C 2010, 114, 6426–6432. [Google Scholar] [CrossRef]
- Gao, R.; Hu, N.; Yang, Z.; Zhu, Q.; Chai, J.; Su, Y.; Zhang, L.; Zhang, Y. Paper-like graphene-Ag composite films with enhanced mechanical and electrical properties. Nanoscale Res. Lett. 2013, 8, 32. [Google Scholar] [CrossRef]
- Hui, K.; Dinh, D.; Tsang, C.; Cho, Y.; Zhou, W.; Hong, X.; Chun, H.-H. Green synthesis of dimension-controlled silver nanoparticle–graphene oxide with in situ ultrasonication. Acta Mater. 2014, 64, 326–332. [Google Scholar] [CrossRef]
- Yang, Y.; Ping, Y.; Gong, Y.; Wang, Z.; Fu, Q.; Pan, C. Ag/graphene composite based on high-quality graphene with high electrical and mechanical properties. Prog. Nat. Sci. 2019, 29, 384–389. [Google Scholar] [CrossRef]
- Hao, X.; Wang, X.; Zhou, S.; Zhang, H.; Liu, M. Microstructure and properties of silver matrix composites reinforced with Ag-doped graphene. Mater. Chem. Phys. 2018, 215, 327–331. [Google Scholar] [CrossRef]
- Ruud, J.A.; Witvrouw, A.; Spaepen, F. Bulk and interface stresses in silver-nickel multilayered thin films. J. Appl. Phys. 1993, 74, 2517–2523. [Google Scholar] [CrossRef]
- Tianfu, G. Preparation and Properties of the Silver-Nickel Contact Material Addition with Metal Oxides. Master’s Thesis, Xi’an Polytechnic University, Xi’an, China, 2016. [Google Scholar]
- Wang, H.; Yuan, H. Investigation on the electrical properties of AgNi contact materials with various Ni content. In Proceedings of the 63rd IEEE Holm Conference on Electrical Contacts, Denver, CO, USA, 10–13 September 2017; pp. 221–224. [Google Scholar] [CrossRef]
- Dai, C.; Yang, X.; Xie, H. One-step synthesis of reduced graphite oxide–silver nanocomposite. Mater. Res. Bull. 2011, 46, 2004–2008. [Google Scholar] [CrossRef]
- Li, C.; Ming, X.; Dekui, N.; Hongzhong, C. Anti-welding Characteristic of AgRENi Electrical Contact Material on DC Condition. Precious Mater. 2008, 3, 6–10. [Google Scholar]
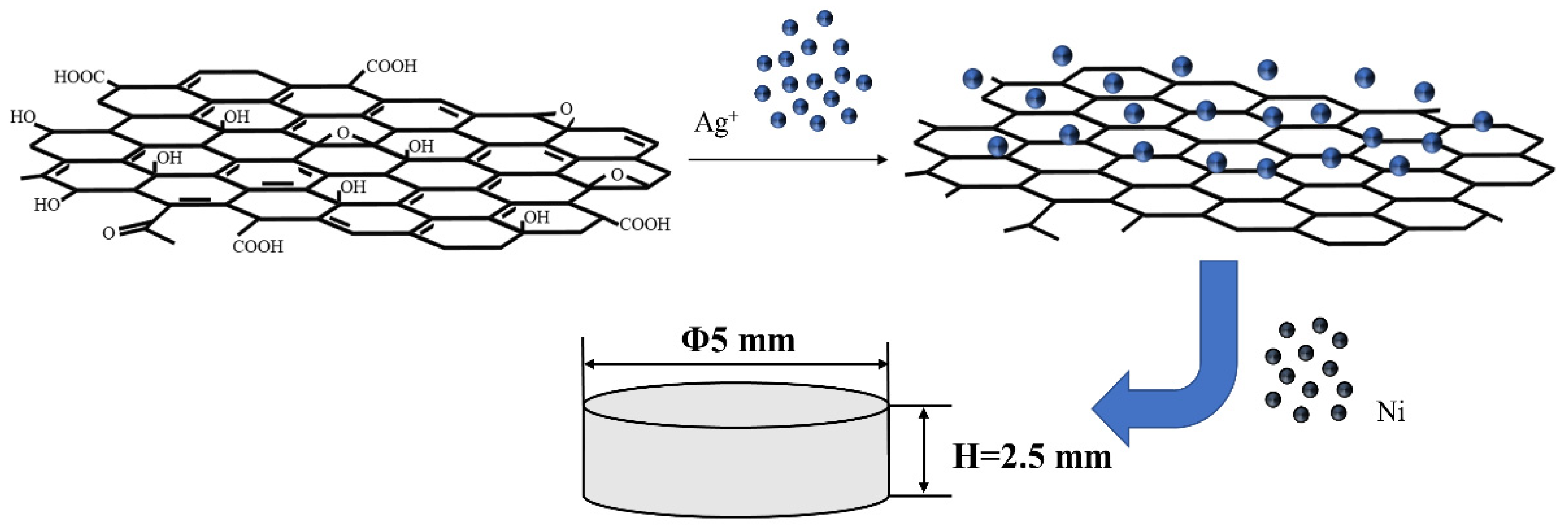
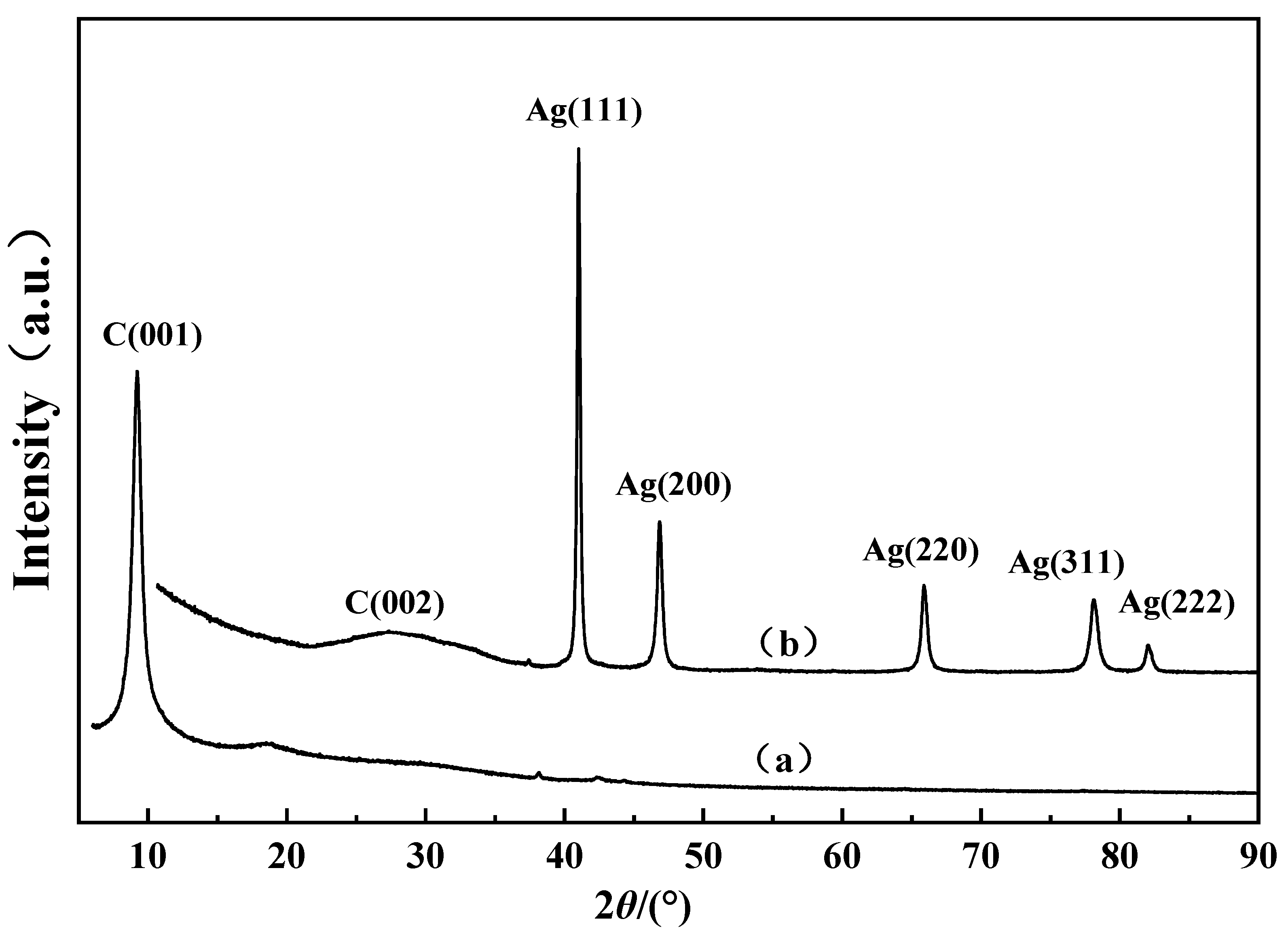
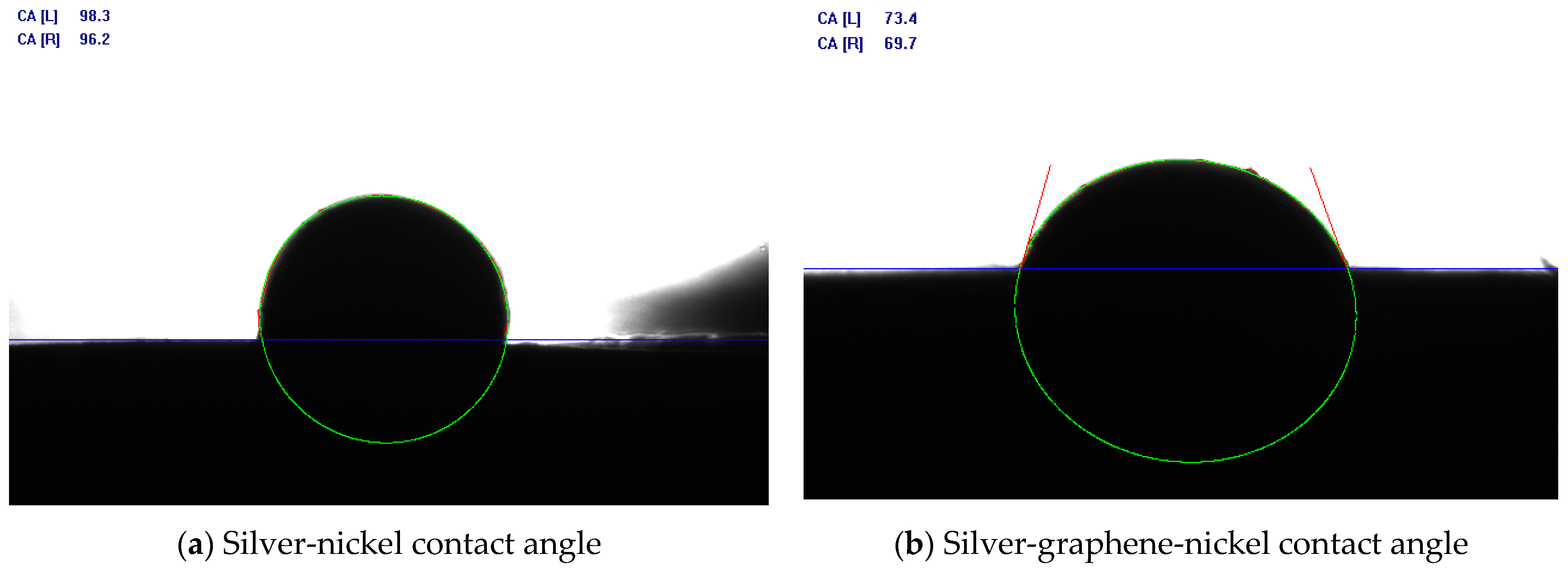
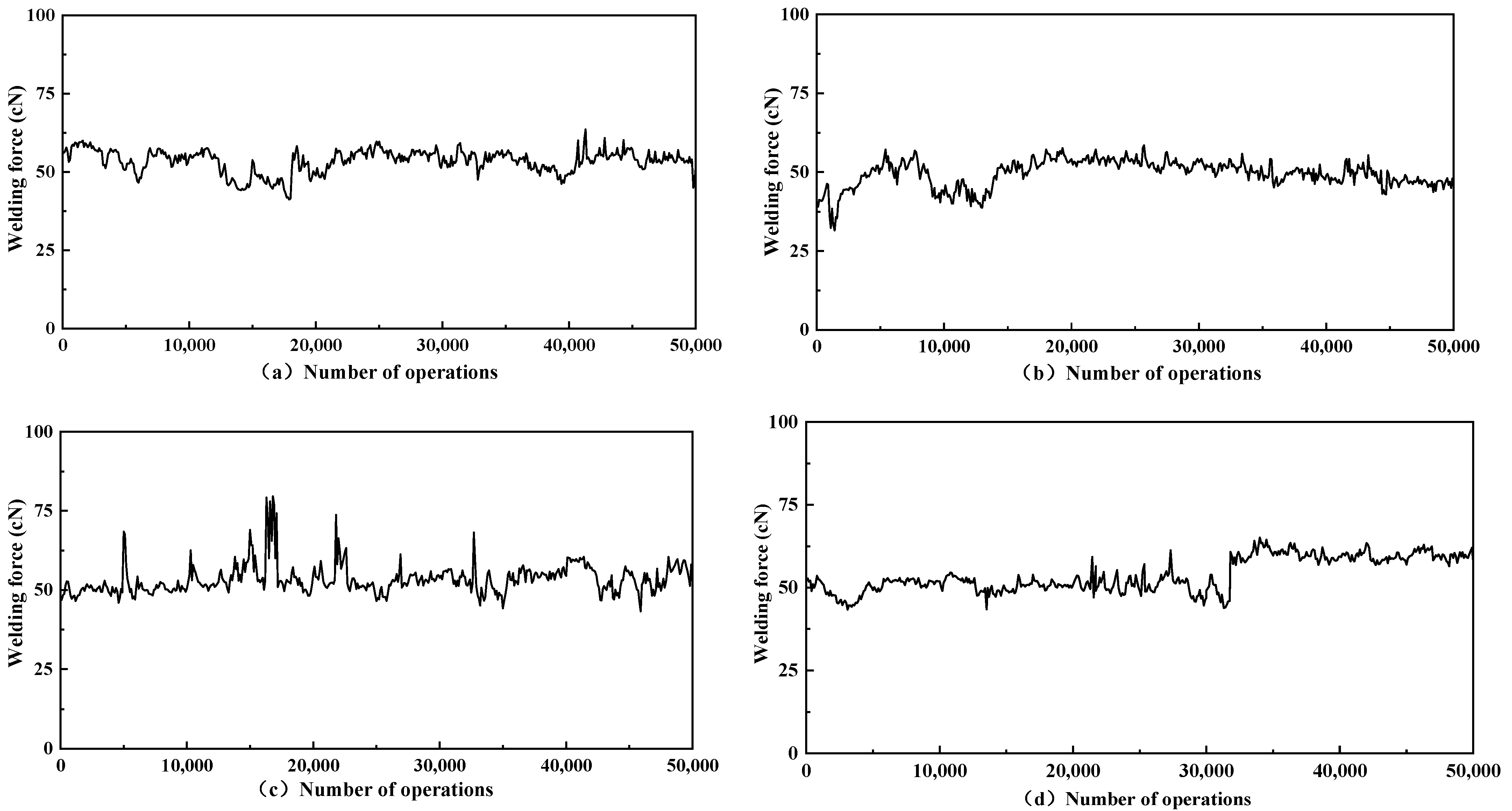
| Samples | Sample Name | Conductivity/(IACS%) | Hardness/HV |
|---|---|---|---|
| a | AgNi15-5 mg GO | 31.3 | 112.70 |
| b | AgNi15-10 mg GO | 33.8 | 114.27 |
| c | AgNi15-20 mg GO | 27.4 | 118.48 |
| d | AgNi15 | 30.0 | 114.05 |
| Samples | Sample Name | Average Welding Force/(cN) | Variance |
|---|---|---|---|
| a | AgNi15-5 mg GO | 53.46 | 13.7 |
| b | AgNi15-10 mg GO | 49.49 | 18.7 |
| c | AgNi15-20 mg GO | 53.37 | 22.0 |
| d | AgNi15 | 53.82 | 26.5 |
| Samples | Sample Name | Average Arc Energy/(mJ) | Variance |
|---|---|---|---|
| a | AgNi15-5 mg GO | 189.10 | 544.5 |
| b | AgNi15-10 mg GO | 176.77 | 486.4 |
| c | AgNi15-20 mg GO | 186.96 | 575.6 |
| d | AgNi15 | 203.33 | 128.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Hu, D.; Zhu, Y.; Guo, P. Electrical Properties of In Situ Synthesized Ag-Graphene/Ni Composites. Materials 2022, 15, 6423. https://doi.org/10.3390/ma15186423
Wang J, Hu D, Zhu Y, Guo P. Electrical Properties of In Situ Synthesized Ag-Graphene/Ni Composites. Materials. 2022; 15(18):6423. https://doi.org/10.3390/ma15186423
Chicago/Turabian StyleWang, Jingqin, Dekao Hu, Yancai Zhu, and Peijian Guo. 2022. "Electrical Properties of In Situ Synthesized Ag-Graphene/Ni Composites" Materials 15, no. 18: 6423. https://doi.org/10.3390/ma15186423
APA StyleWang, J., Hu, D., Zhu, Y., & Guo, P. (2022). Electrical Properties of In Situ Synthesized Ag-Graphene/Ni Composites. Materials, 15(18), 6423. https://doi.org/10.3390/ma15186423






