Interactions between O2 Nanobubbles and the Pulmonary Surfactant in the Presence of Inhalation Medicines
Abstract
1. Introduction
2. Materials and Methods
2.1. Generation of O2-NBs Dispersions in Water (ADON—Aquous Disspersion of Oxygen Nanobubbles) and Saline (SDON—Saline Disspersion of Oxygen Nanobubbles)
2.2. Experimental Evaluation of Interactions between Oxygen Nanobubbles and Pulmonary Surfactant Models
2.2.1. Interactions with the Lipid Model (LM) of the Pulmonary Surfactant
2.2.2. Interactions with the Multicomponent Pulmonary Surfactant Model MPS (Lipids + Proteins)
3. Results and Discussion
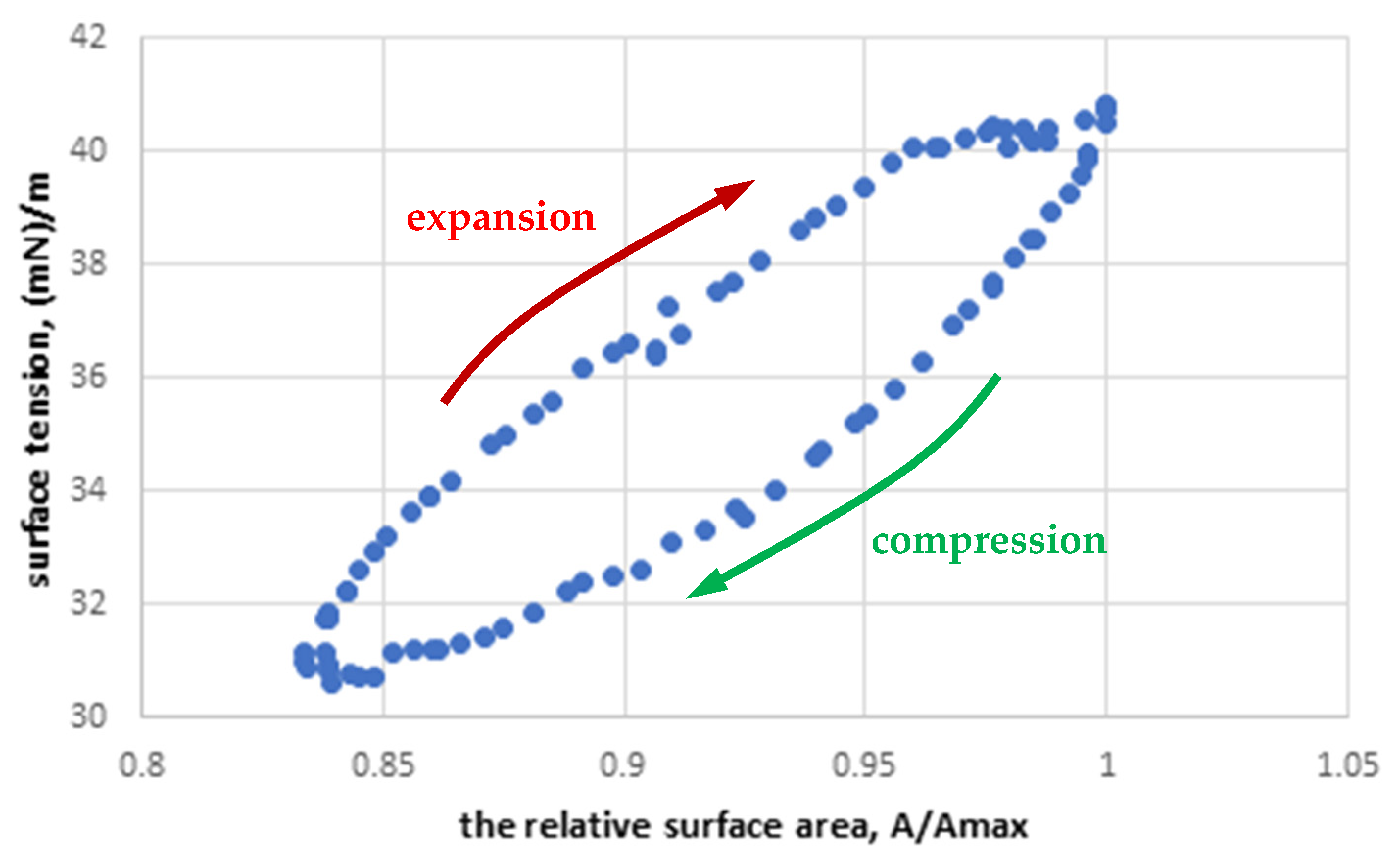
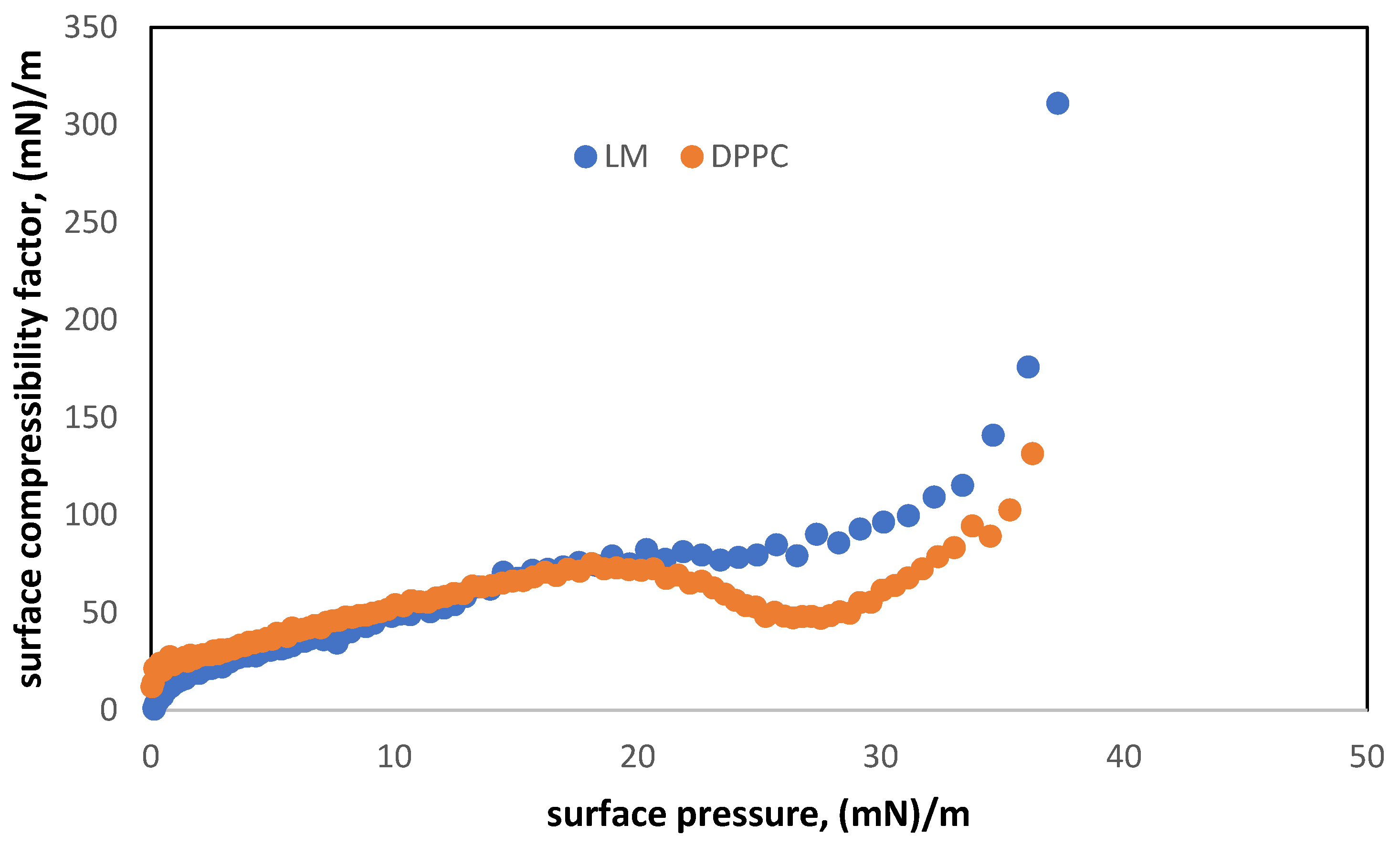
3.1. Characterization of Two-Component Monolayers (LM)
3.2. Interactions between Oxygen Nanobubbles and the Lipid Model (LM) of the Pulmonary Surfactant
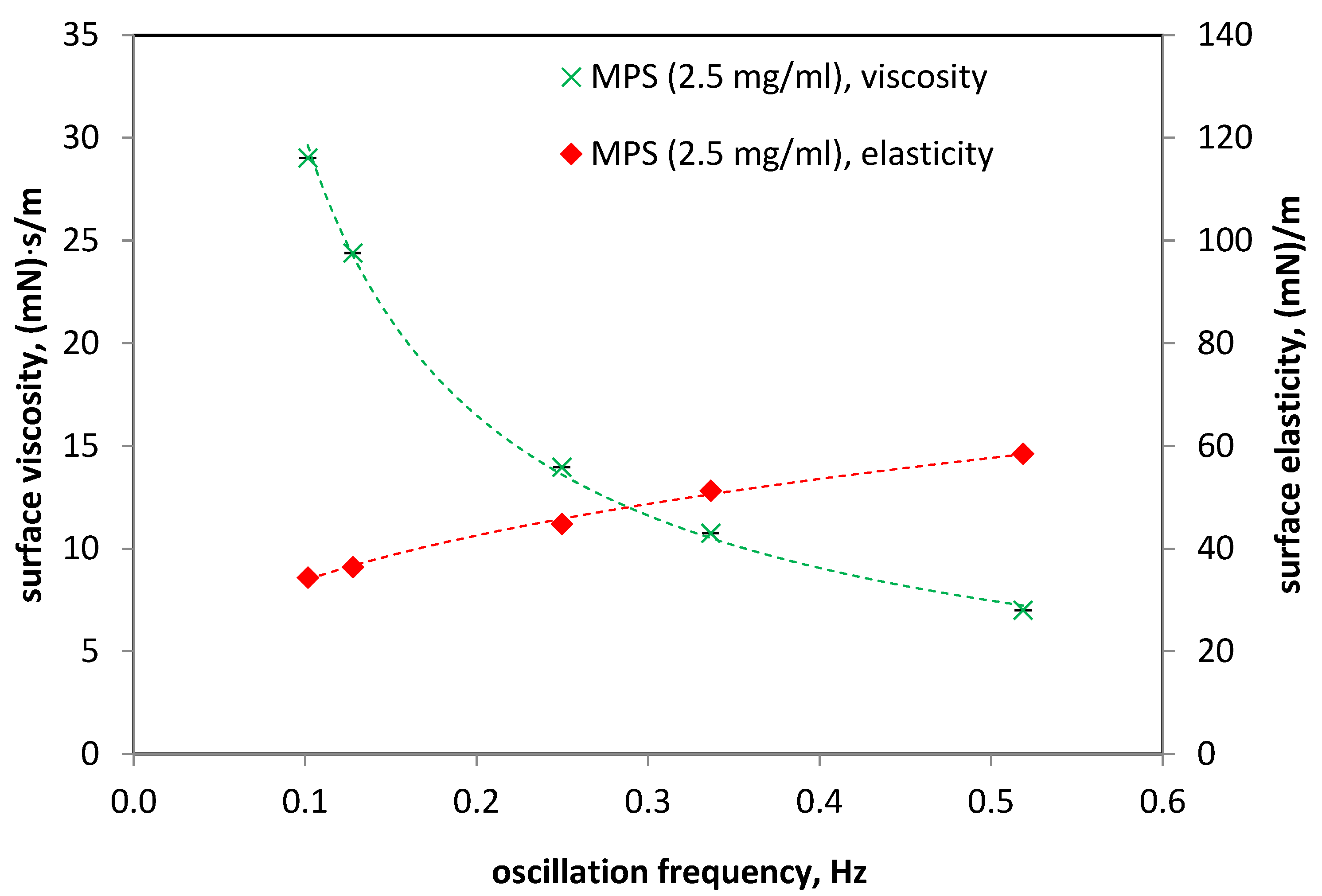
3.3. Dynamics of Air–Liquid Interface of Multicomponent Pulmonary Surfactant Model MPS (Lipids + Proteins)
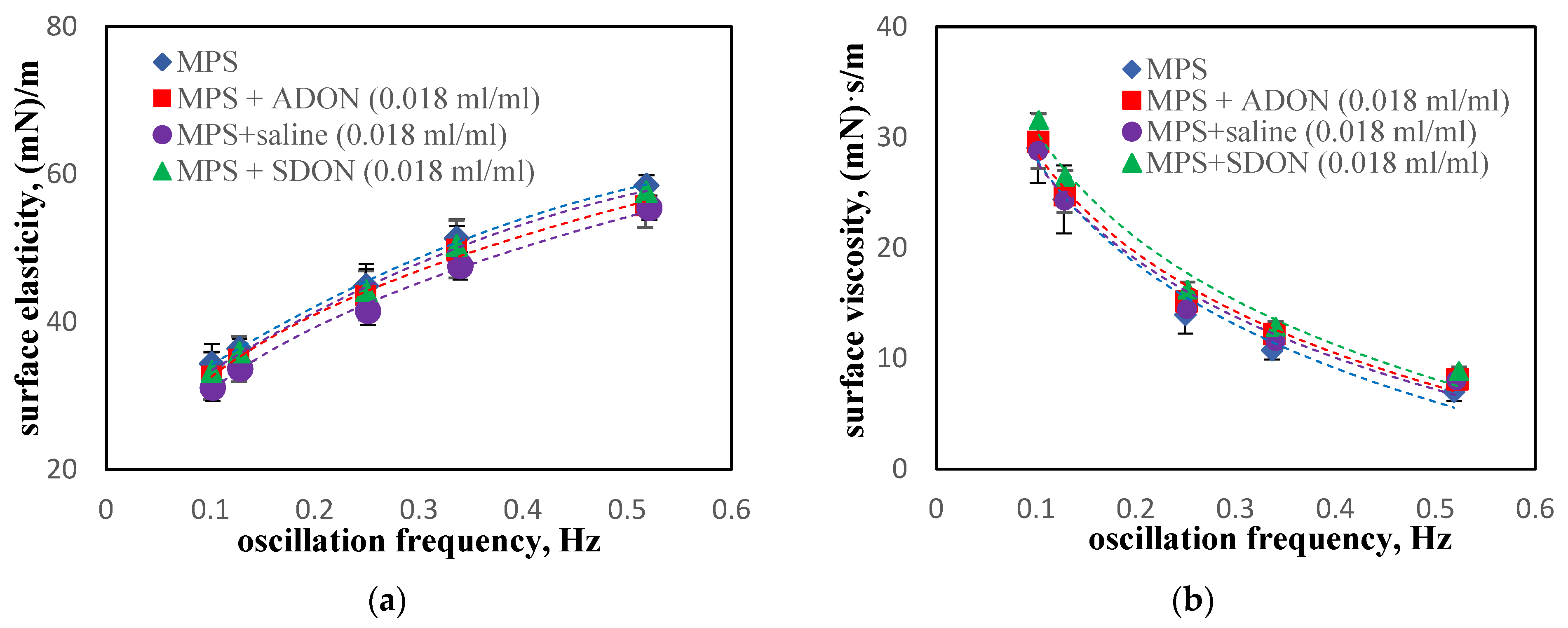
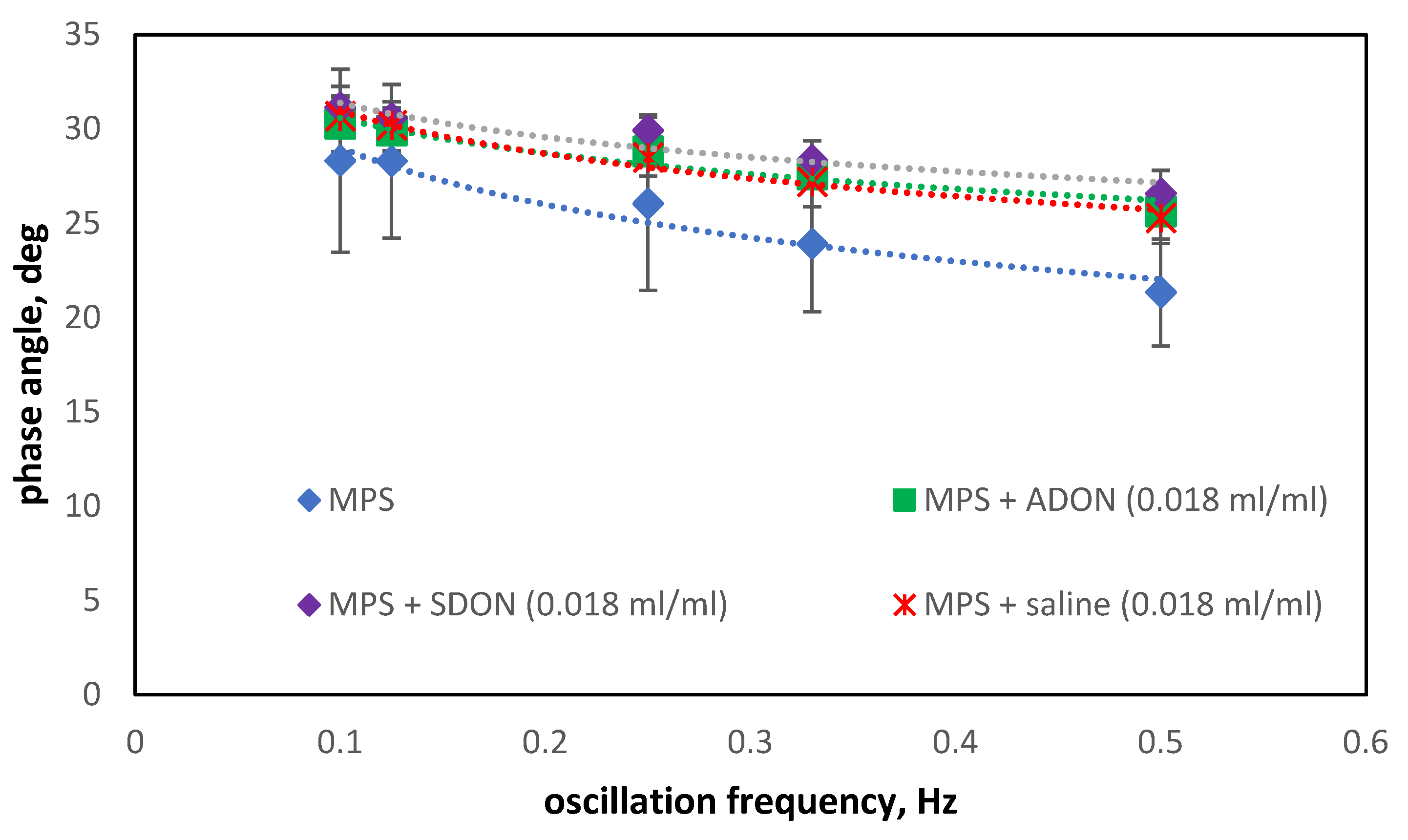
3.4. Influence of Oxygen Nanobubbles on MPS
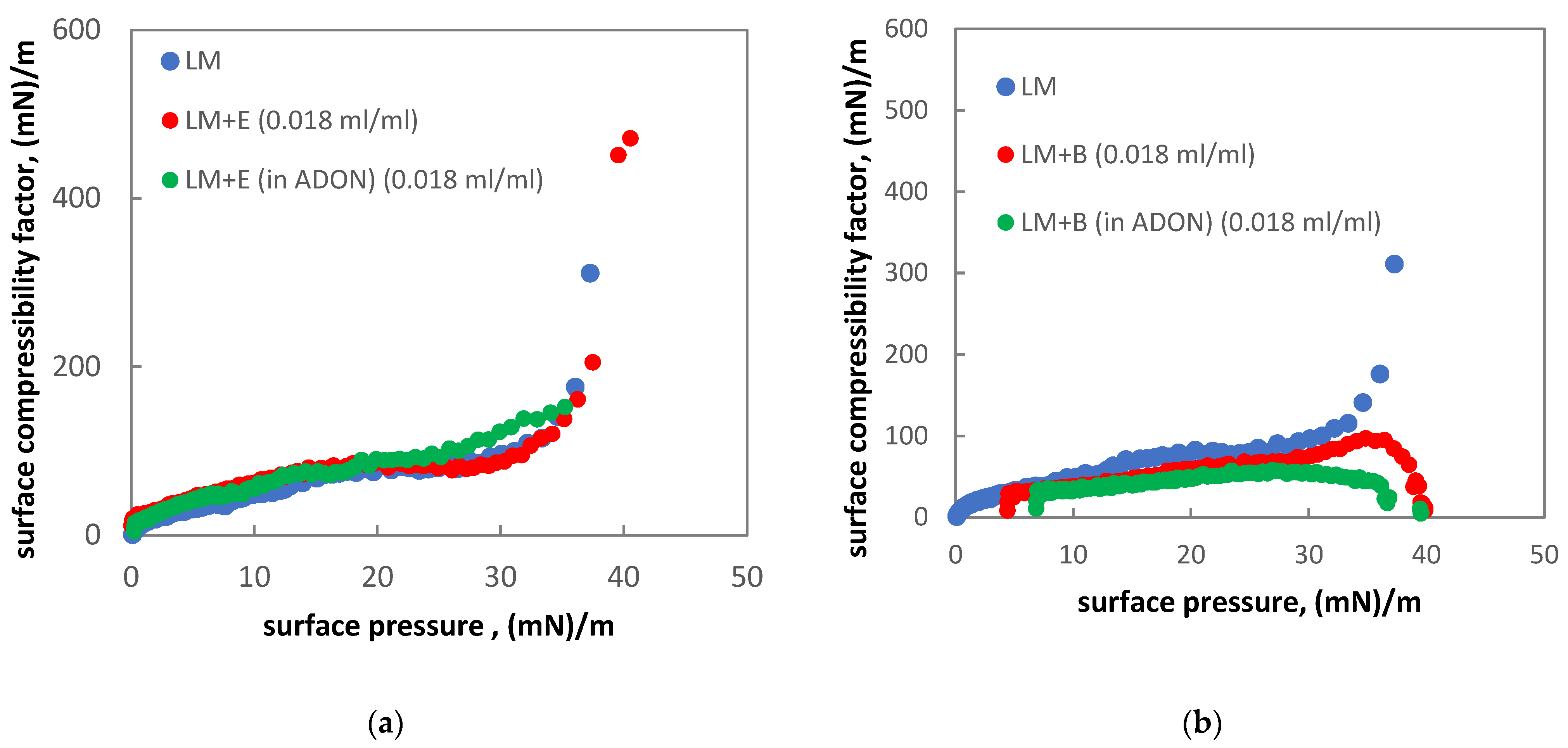
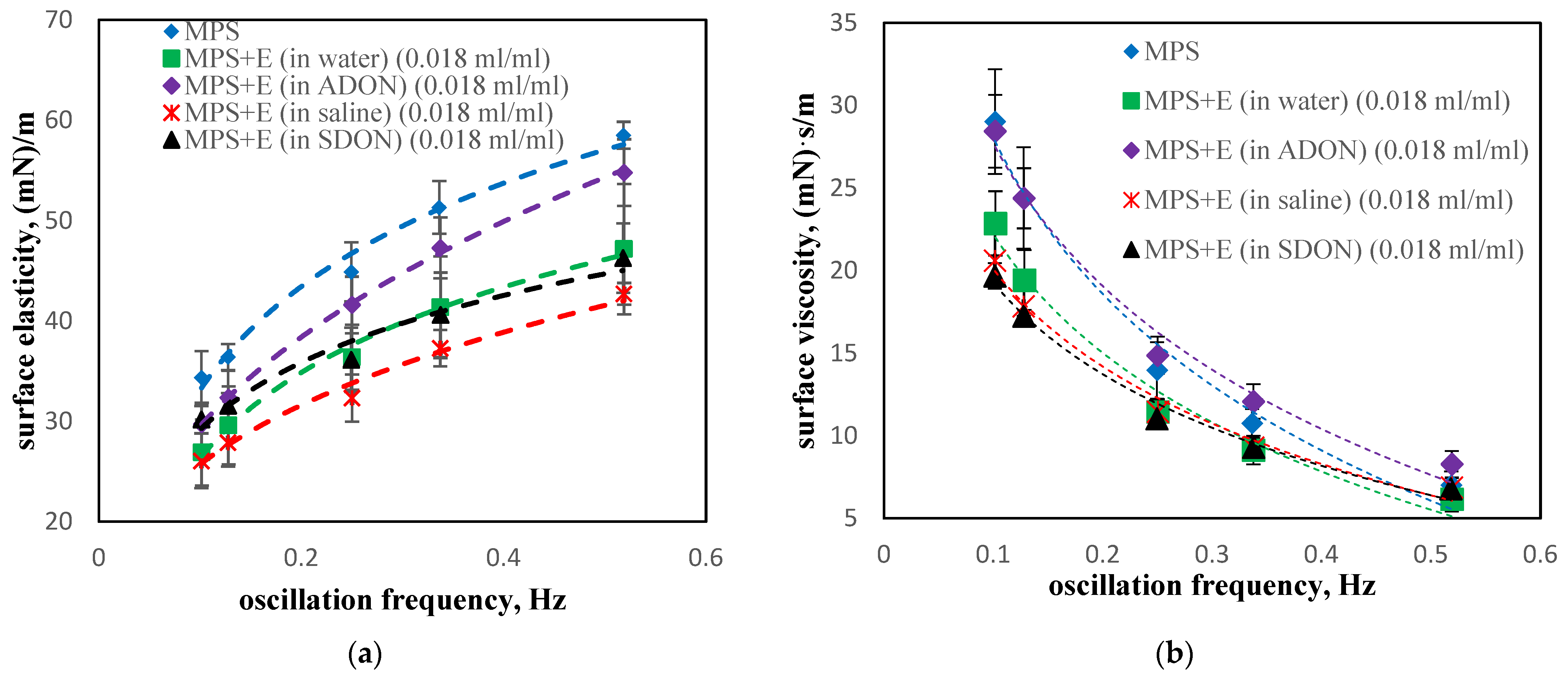
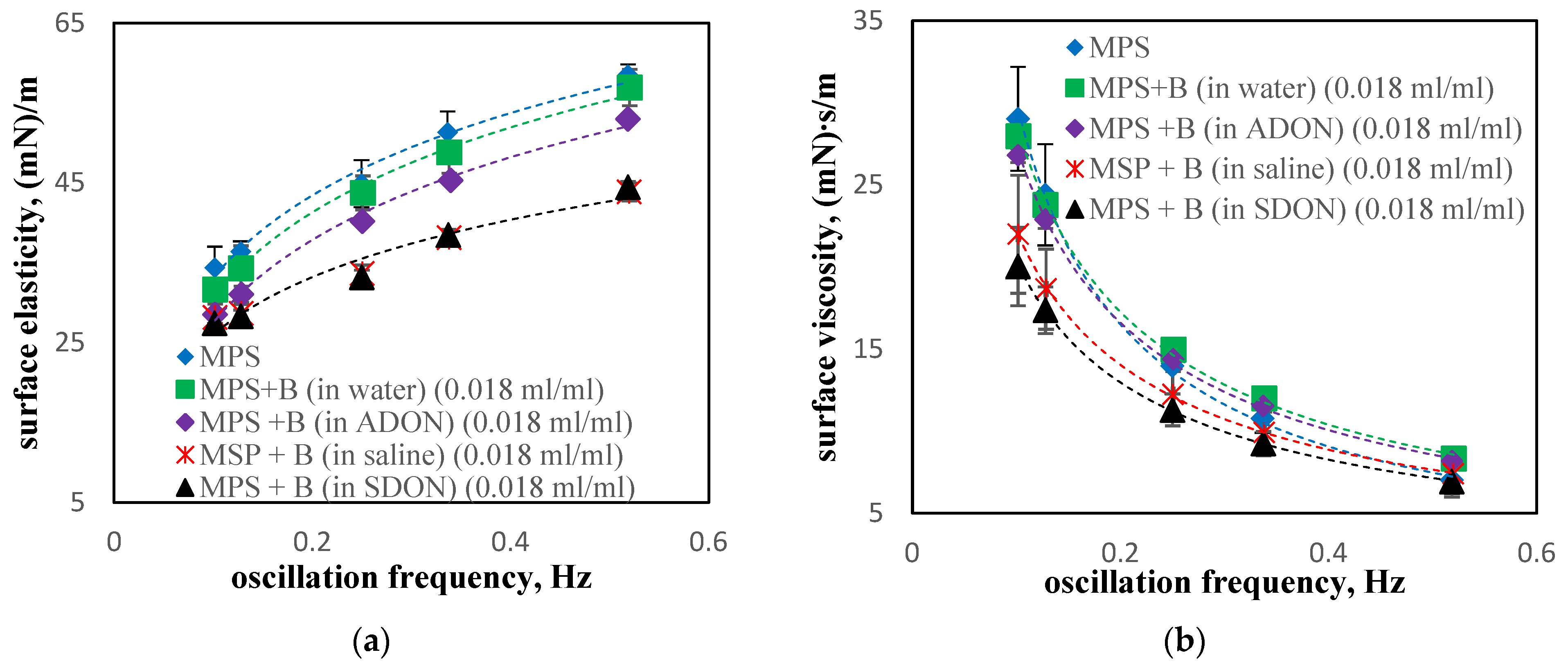
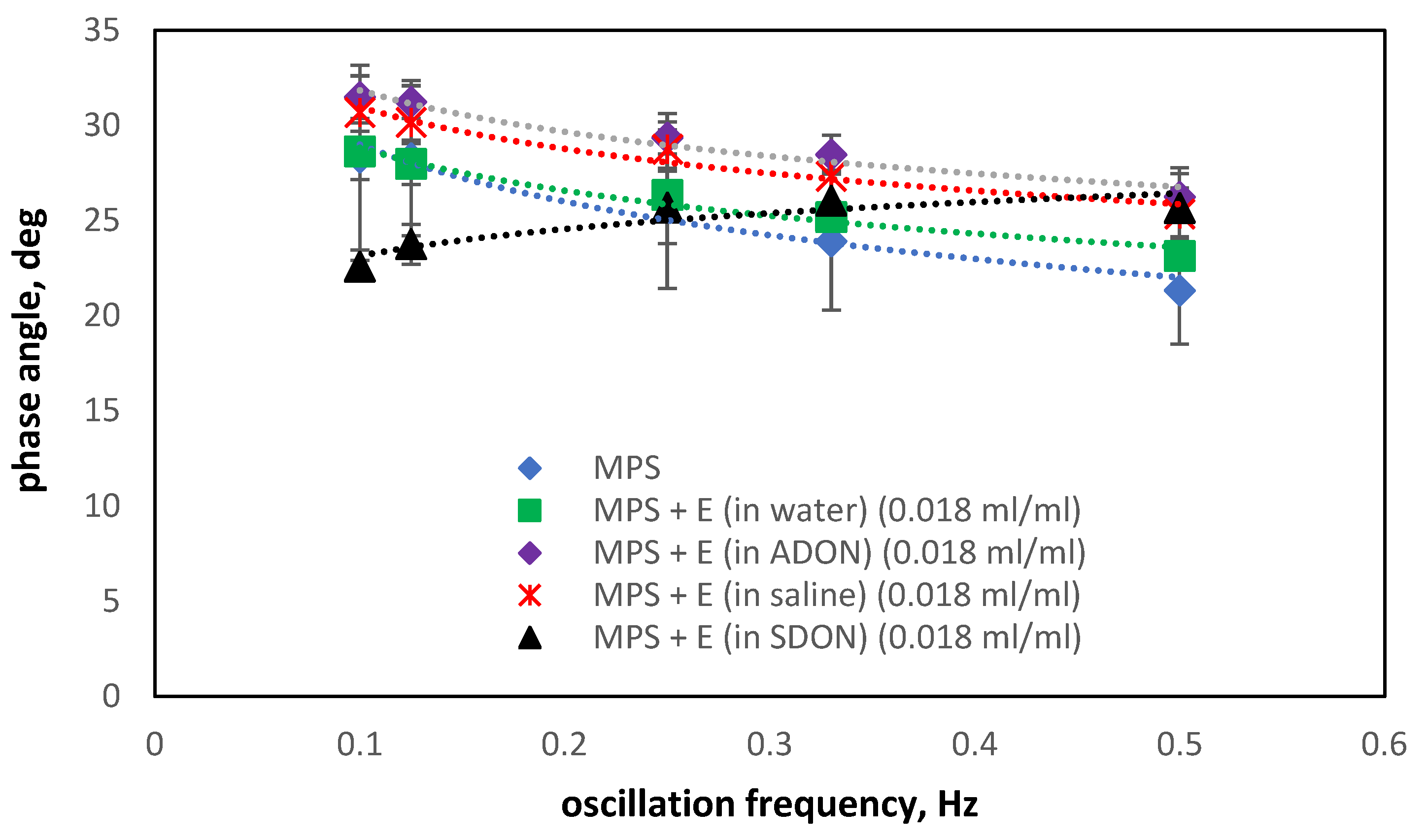
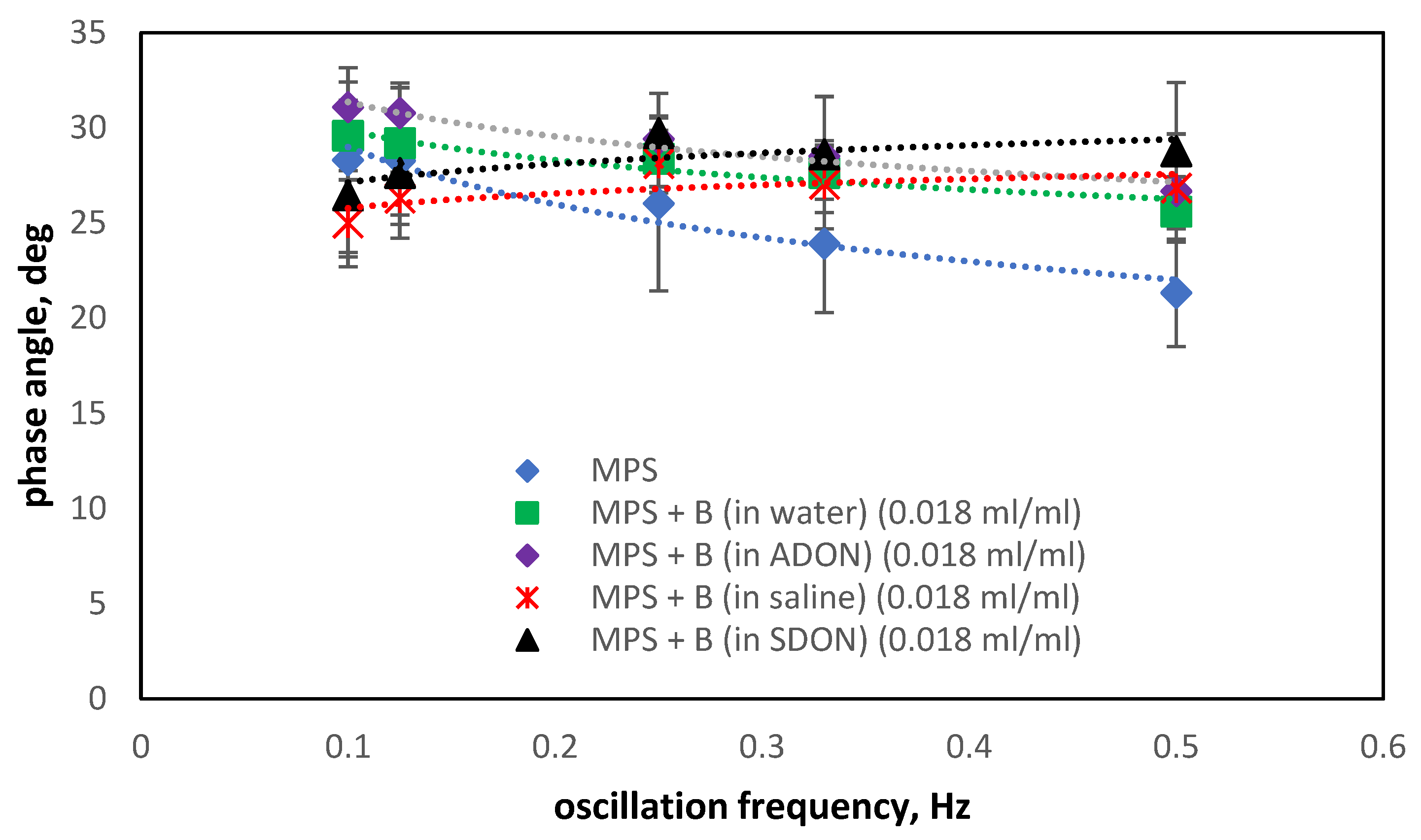
3.5. Influence of Drugs and Oxygen Nanobubbles on MPS
- The selection of the PS model for the studies is important. The LM model is simpler and allows us to evaluate the changes in the surface processes more precisely using a Langmuir trough; however, it does not inform about the full dynamics of the PS system due to poor reconstruction of the composition of the interfacial layer and low deformation (compression) rates compared to the physiological system. The MPS model and dynamic surface oscillations in the pendant drop method reflect the dynamics of the physiological system and provide quantitative results reflecting the surface response to sinusoidal deformation.
- The influence of oxygen nanobubbles on both models of PS is marginal. The PS properties are more altered by the drug product components (e.g., surfactants present in budesonide suspensions) than by O2-NBs. Since the safety of tested inhalation drugs (ectoine and budesonide) on the pulmonary system has been confirmed in clinical studies, it seems that very small effects on the PS surface activity detected here for NBs present no issue regarding the safety of their future use as drug carriers in medical inhalants.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsuge, H. Micro- and Nanobubbles. Fundamentals and Applications, 1st ed.; Pan Stanford Publishing Pte Ltd.: Singapore, 2015. [Google Scholar]
- Alheshibri, M.; Qian, J.; Jehannin, M.; Craig, V.S.J. A History of Nanobubbles. Langmuir 2016, 32, 11086–11100. [Google Scholar] [CrossRef] [PubMed]
- Ulatowski, K.; Sobieszuk, P.; Mróz, A.; Ciach, T. Stability of nanobubbles generated in water using porous membrane system. Chem. Eng. Process. Process Intensif. 2019, 136, 62–71. [Google Scholar] [CrossRef]
- Gao, Z.; Wu, W.; Sun, W.; Wang, B. Understanding the Stabilization of a Bulk Nanobubble: A Molecular Dynamics Analysis. Langmuir 2021, 37, 11281–11291. [Google Scholar] [CrossRef]
- Sakr, M.; Maraqa, M.A.; Hamouda, M.A.; Hassan, A.A.; Ali, J.; Jung, J. A critical review of the recent developments in micro–nano bubbles applications for domestic and industrial wastewater treatment. Alex. Eng. J. 2022, 61, 6591–6612. [Google Scholar] [CrossRef]
- Li, H.; Hu, L.; Xia, Z. Impact of groundwater salinity on bioremediation enhanced by micro-nano bubbles. Materials 2013, 6, 3676–3687. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Li, P.; Wang, Y.; Yang, J.; Zhang, W. Micronanobubble aeration promotes senescence of submerged macrophytes with low total antioxidant capacity in urban landscape water. Environ. Sci. Water Res. Technol. 2020, 6, 523–531. [Google Scholar] [CrossRef]
- Ikeura, H.; Kobayashi, F.; Tamaki, M. Removal of residual pesticide, fenitrothion, in vegetables by using ozone microbubbles generated by different methods. J. Food Eng. 2011, 103, 345–349. [Google Scholar] [CrossRef]
- Kutty, S.R.M.; Winarto, F.E.W.; Gilani, S.I.U.; Anizam, A.A.; Karimah, W.W.Z.; Isa, M.H. Degradation of organic matter using a submerged microbubble diffuser in a biological wastewater treatment system. Waste Manag. Environ. 2010, 1, 415–423. [Google Scholar]
- Ahmed, A.K.A.; Shi, X.; Hua, L.; Manzueta, L.; Qing, W.; Marhaba, T.; Zhang, W. Influences of Air, Oxygen, Nitrogen, and Carbon Dioxide Nanobubbles on Seed Germination and Plant Growth. J. Agric. Food Chem. 2018, 66, 5117–5124. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, X.; Wang, H.; Hu, B.; Lei, Z.; Kobayashi, M.; Adachi, Y.; Shimizu, K.; Zhang, Z. Effects of nanobubble water on the growth of: Lactobacillus acidophilus 1028 and its lactic acid production. RSC Adv. 2019, 9, 30760–30767. [Google Scholar] [CrossRef]
- Ulatowski, K.; Wierzchowski, K.; Fiuk, J.; Sobieszuk, P. Effect of Nanobubble Presence on Murine Fibroblasts and Human Leukemia Cell Cultures. Langmuir 2022, 38, 8575–8584. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Yamada, S.; Kumada, Y.; Matsuo, H.; Toriyama, T.; Kawahara, H. Immersing Feet in Carbon Dioxide-enriched Water Prevents Expansion and Formation of Ischemic Ulcers after Surgical Revascularization in Diabetic Patients with Critical Limb Ischemia. Ann. Vasc. Dis. 2008, 1, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Afshari, R.; Akhavan, O.; Hamblin, M.R.; Varma, R.S. Review of Oxygenation with Nanobubbles: Possible Treatment for Hypoxic COVID-19 Patients. ACS Appl. Nano Mater. 2021, 4, 11386–11412. [Google Scholar] [CrossRef]
- Sobieszuk, P.; Strzyżewska, A.; Ulatowski, K. Investigation of the possibility of culturing aerobic yeast with oxygen nanobubble addition and evaluation of the results of batch and semi-batch cultures of Saccharomyces cerevisiae. Chem. Eng. Process. Process Intensif. 2021, 159, 108247. [Google Scholar] [CrossRef]
- Riyad, G.; Al-omary, H. Physiological Effects of Carbon Dioxide Treatment on Diabetic Foot Ulcer Patients. IOSR J. Pharm. 2018, 13, 1–7. [Google Scholar]
- Shalan, N.; Al-Bazzaz, A.; Al-Ani, I.; Najem, F.; Al-Masri, M. Effect of Carbon Dioxide Therapy on Diabetic Foot Ulcer. J. Diabetes Mellit. 2015, 5, 284–289. [Google Scholar] [CrossRef][Green Version]
- Paknahad, A.A.; Kerr, L.; Wong, D.A.; Kolios, M.C.; Tsai, S.S.H. Biomedical nanobubbles and opportunities for microfluidics. RSC Adv. 2021, 11, 32750. [Google Scholar] [CrossRef]
- Bhandari, P.; Novikova, G.; Goergen, C.J.; Irudayaraj, J. Ultrasound beam steering of oxygen nanobubbles for enhanced bladder cancer therapy. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Ma, X.; He, W.; Liu, C.; Liu, Z. Functional micro/nanobubbles for ultrasound medicine and visualizable guidance. Sci. China Chem. 2021, 646, 891–899. [Google Scholar] [CrossRef]
- Jin, J.; Yang, L.; Chen, F.; Gu, N. Drug delivery system based on nanobubbles. Interdiscip. Mater. 2022, 1–24. [Google Scholar] [CrossRef]
- Czajkowska-Malinowska, M.; Kania, A.; Kuca, P.J.; Nasiłowski, J.; Skoczyński, S.; Sokołowski, R.; Śliwiński, P.S. Treatment of acute respiratory failure in the course of COVID-19. Practical hints from the expert panel of the Assembly of Intensive Care and Rehabilitation of the Polish Respiratory Society. Adv. Respir. Med. 2020, 88, 245–266. [Google Scholar] [CrossRef] [PubMed]
- Odziomek, M.; Ulatowski, K.; Dobrowolska, K.; Sobieszuk, P.; Sosnowski, T.R. Aqueous dispersions of oxygen nanobubbles for potential application in inhalation therapy. Sci. Rep. 2022, 12, 12455. [Google Scholar] [CrossRef] [PubMed]
- Odziomek, M.; Sosnowski, T.R.; Gradoń, L. Conception, preparation and properties of functional carrier particles for pulmonary drug delivery. Int. J. Pharm. 2012, 433, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Odziomek, M.; Sosnowski, T.R.; Gradoń, L. The Influence of Functional Carrier Particles (FCPs) on the Molecular Transport Rate Through the Reconstructed Bronchial Mucus: In Vitro Studies. Transp. Porous Med. 2015, 106, 439–454. [Google Scholar] [CrossRef][Green Version]
- Gradoń, L.; Podgórski, A. Hydrodynamical model of pulmonary clearance. Chem. Eng. Sci. 1989, 44, 741–749. [Google Scholar] [CrossRef]
- Sosnowski, T.R. Particles on the lung surface—physicochemical and hydrodynamic effects. Curr. Opin. Colloid Interface Sci. 2018, 36, 1–9. [Google Scholar] [CrossRef]
- Grippi, M.A.; Elias, J.A.; Fishman, J.A.; Kotloff, R.M.; Pack, A.I.; Senior, R.M.; Siegel, M.D. (Eds.) Fishman’s Pulmonary Diseases and Disorders, 5th ed.; McGraw Hill: New York, NY, USA, 2015. [Google Scholar]
- Taeusch, H.W.; Bernardino de la Serna, J.; Perez-Gil, J.; Alonso, C.; Zasadzinski, J.A. Inactivation of pulmonary surfactant due to serum-inhibited adsorption and reversal by hydrophilic polymers: Experimental. Biophys. J. 2005, 89, 1769–1779. [Google Scholar] [CrossRef]
- Podgórski, A.; Sosnowski, T.R.; Gradoń, L. Deactivation of the Pulmonary Surfactant Dynamics by Toxic Aerosols and Gases. J. Aerosol Med. 2001, 14, 455–466. [Google Scholar] [CrossRef]
- Ma, G.; Allen, H.C. DPPC Langmuir monolayer at the air-water interface: Probing the tail and head groups by vibrational sum frequency generation spectroscopy. Langmuir 2006, 22, 5341–5439. [Google Scholar] [CrossRef]
- Ładniak, A.; Jurak, M.; Wiącek, A.E. Langmuir monolayer study of phospholipid DPPC on the titanium dioxide–chitosan–hyaluronic acid subphases. Adsorption 2019, 25, 469–476. [Google Scholar] [CrossRef]
- Crowe, J.H.; Carpenter, J.F.; Crowe, L.M.; Anchordoguy, T.J. Are freezing and dehydration similar stress vectors? A comparison of modes of interaction of stabilizing solutes with biomolecules. Cryobiology 1990, 27, 219–231. [Google Scholar] [CrossRef]
- Drugbank Database. Available online: https://go.drugbank.com/drugs/DB01222 (accessed on 18 May 2022).
- McNaught, A.D.; Wilkinson, A. IUPAC. Compendium of Chemical Terminology, 2nd ed.; (the “Gold Book”); Blackwell Scientific Publications: Oxford, UK, 1997. [Google Scholar]
- Lyklema, J. Fundamentals of Interface and Colloid Science; Academic Press: Cambridge, MA, USA, 2000. [Google Scholar]
- Davies, J.T.; Rideal, E.K. Interfacial Phenomena; Academic Press: Cambridge, MA, USA, 1963. [Google Scholar]
- Guzmán, E.; Liggieri, L.; Santini, E.; Ferrari, M.; Ravera, F. Mixed DPPC–cholesterol Langmuir monolayers in presence of hydrophilic silica nanoparticles. Colloids Surf. B Biointerfaces 2013, 105, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.; Santini, E.; Ferrari, M.; Liggieri, L.; Ravera, F. Effect of the incorporation of nanosized titanium dioxide on the interfacial properties of 1,2-dipalmitoyl-sn-glycerol-3-phosphocholine Langmuir monolayers. Langmuir 2017, 33, 10715–10725. [Google Scholar] [CrossRef] [PubMed]
- Kondej, D.; Sosnowski, T.R. Effect of clay nanoparticles on model lung surfactant: A potential marker of hazard from nanoaerosol inhalation. Env. Sci. Pollut. Res. 2016, 23, 4660–4669. [Google Scholar] [CrossRef]
- Ruiz, G.; Pazin, W.M.; do Carmo Morato, L.F.; Oliveira, O.N.; Constantino, C.J. Correlating mono- and bilayers of lipids to investigate the pronounced effects of steroid hormone 17α-ethynylestradiol on membrane models of DPPC/cholesterol. J. Mol. Liq. 2020, 311, 113324. [Google Scholar] [CrossRef]
- Uchida, T.; Liu, S.; Enari, M.; Oshita, S.; Yamazaki, K.; Gohara, K. Effect of NaCl on the Lifetime of Micro- and Nanobubbles. Nanomaterials 2016, 6, 31. [Google Scholar] [CrossRef]
- Sosnowski, T.R.; Dobrowolska, K.E. In vitro interactions of nebulized budesonide inhalation suspension with lung surfactant: The importance of drug formulation. In Respiratory Drug Delivery; Dalby, R.N., Byron, P.R., Hindle, M., Peart, J., Traini, D., Young, P.M., Farr, S.J., Suman, J.D., Watt, A., Eds.; Virginia Commonwealth University: Richmond, VA, USA, 2020; Volume 3, pp. 737–742. [Google Scholar]
- Schürch, S.; Goerke, J.; Clements, J.A. Direct determination of volume- and time-dependence of alveolar surface tension in excised lungs. Proc. Natl. Acad. Sci. USA 1978, 75, 3417–3421. [Google Scholar] [CrossRef]
- Jabłczyńska, K.; Sosnowski, T.R. Adsorption and co-adsorption of polyaldehydedextran nanoparticles and nonionic surfactantat an air–water interface: Potential implicationsfor pulmonary drug delivery. Chem. Eng. Process. 2017, 38, 67–77. [Google Scholar] [CrossRef]
- Kondej, D.; Sosnowski, T.R. Interfacial rheology for the assessment of potential health effects of inhaled carbon nanomaterials at variable breathing conditions. Sci. Rep. 2020, 10, 14044. [Google Scholar] [CrossRef]
- Schott, H. Hydrophilic-lipophilic balance, solubility parameter, and oil-water partition coefficient as universal parameters of nonionic surfactants. J. Pharm. Sci. 1995, 84, 1215–1222. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrowolska, K.; Odziomek, M.; Ulatowski, K.; Kędziora, W.; Soszyńska, K.; Sobieszuk, P.; Sosnowski, T.R. Interactions between O2 Nanobubbles and the Pulmonary Surfactant in the Presence of Inhalation Medicines. Materials 2022, 15, 6353. https://doi.org/10.3390/ma15186353
Dobrowolska K, Odziomek M, Ulatowski K, Kędziora W, Soszyńska K, Sobieszuk P, Sosnowski TR. Interactions between O2 Nanobubbles and the Pulmonary Surfactant in the Presence of Inhalation Medicines. Materials. 2022; 15(18):6353. https://doi.org/10.3390/ma15186353
Chicago/Turabian StyleDobrowolska, Katarzyna, Marcin Odziomek, Karol Ulatowski, Weronika Kędziora, Karolina Soszyńska, Paweł Sobieszuk, and Tomasz R. Sosnowski. 2022. "Interactions between O2 Nanobubbles and the Pulmonary Surfactant in the Presence of Inhalation Medicines" Materials 15, no. 18: 6353. https://doi.org/10.3390/ma15186353
APA StyleDobrowolska, K., Odziomek, M., Ulatowski, K., Kędziora, W., Soszyńska, K., Sobieszuk, P., & Sosnowski, T. R. (2022). Interactions between O2 Nanobubbles and the Pulmonary Surfactant in the Presence of Inhalation Medicines. Materials, 15(18), 6353. https://doi.org/10.3390/ma15186353






