Synthesis and Properties of Optically Transparent Fluoro-Containing Polyimide Films with Reduced Linear Coefficients of Thermal Expansion from Organo-Soluble Resins Derived from Aromatic Diamine with Benzanilide Units
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
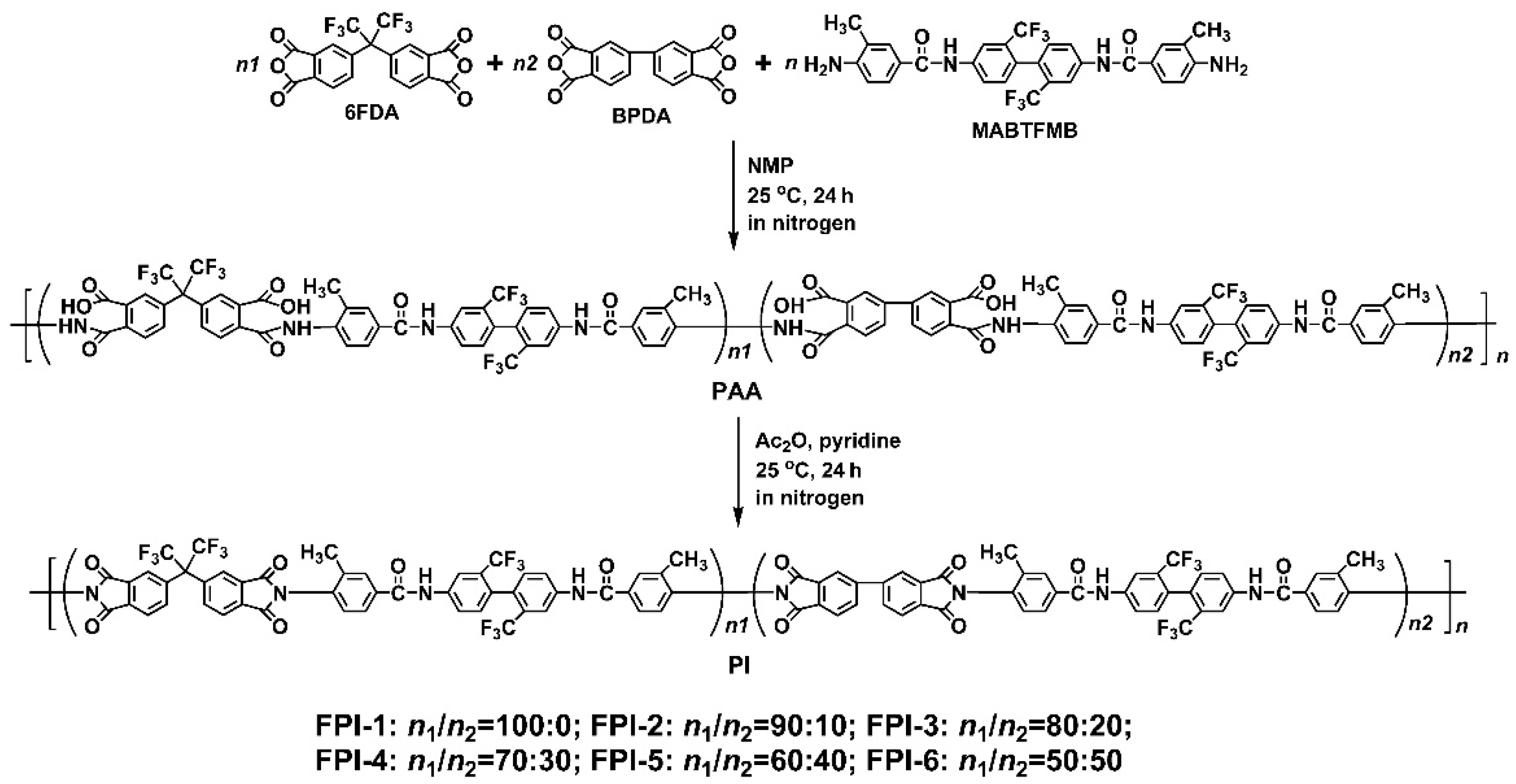
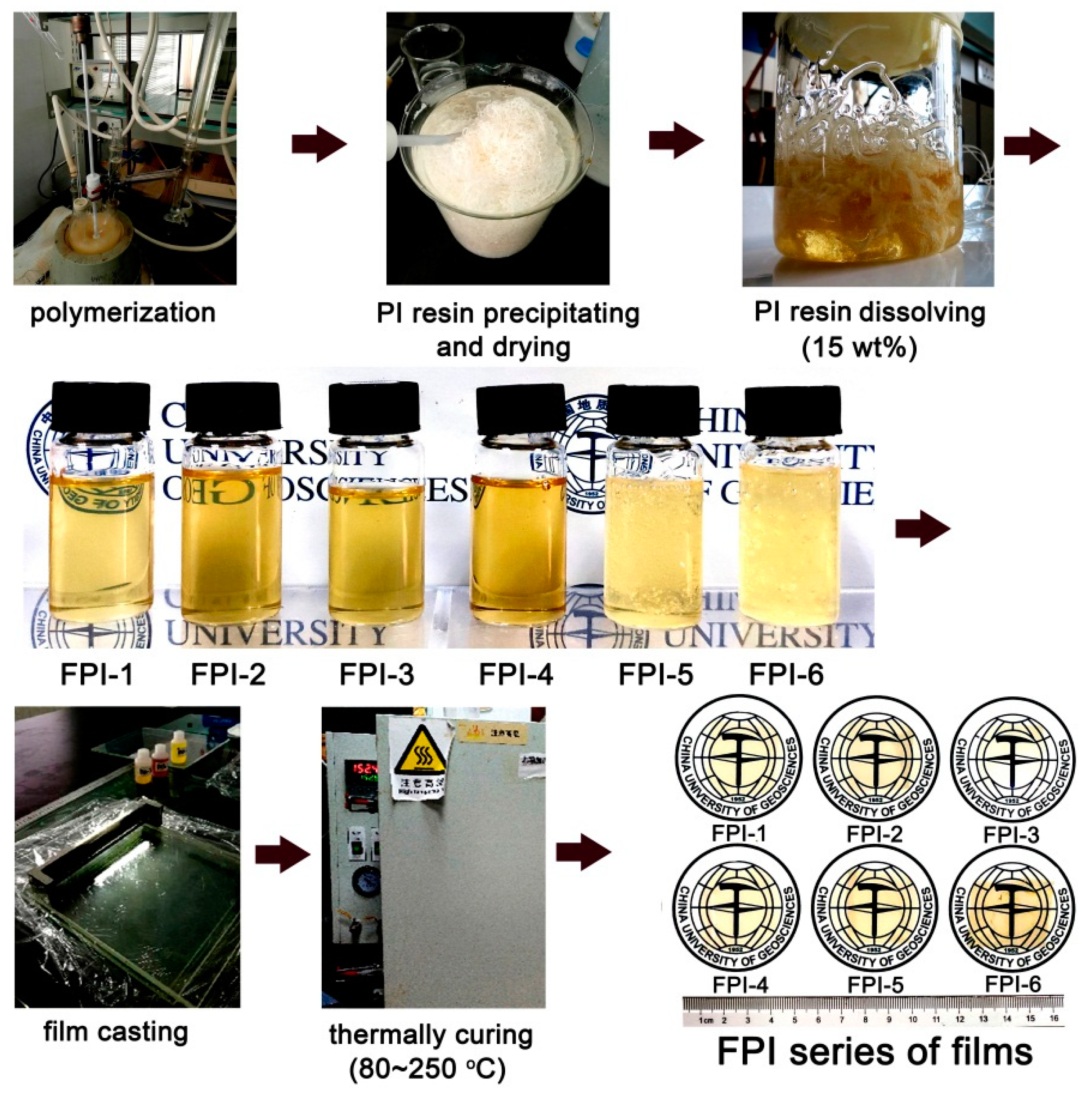
2.2. PI Synthesis
2.3. Characterization
3. Results
3.1. PI Synthesis and Characterization
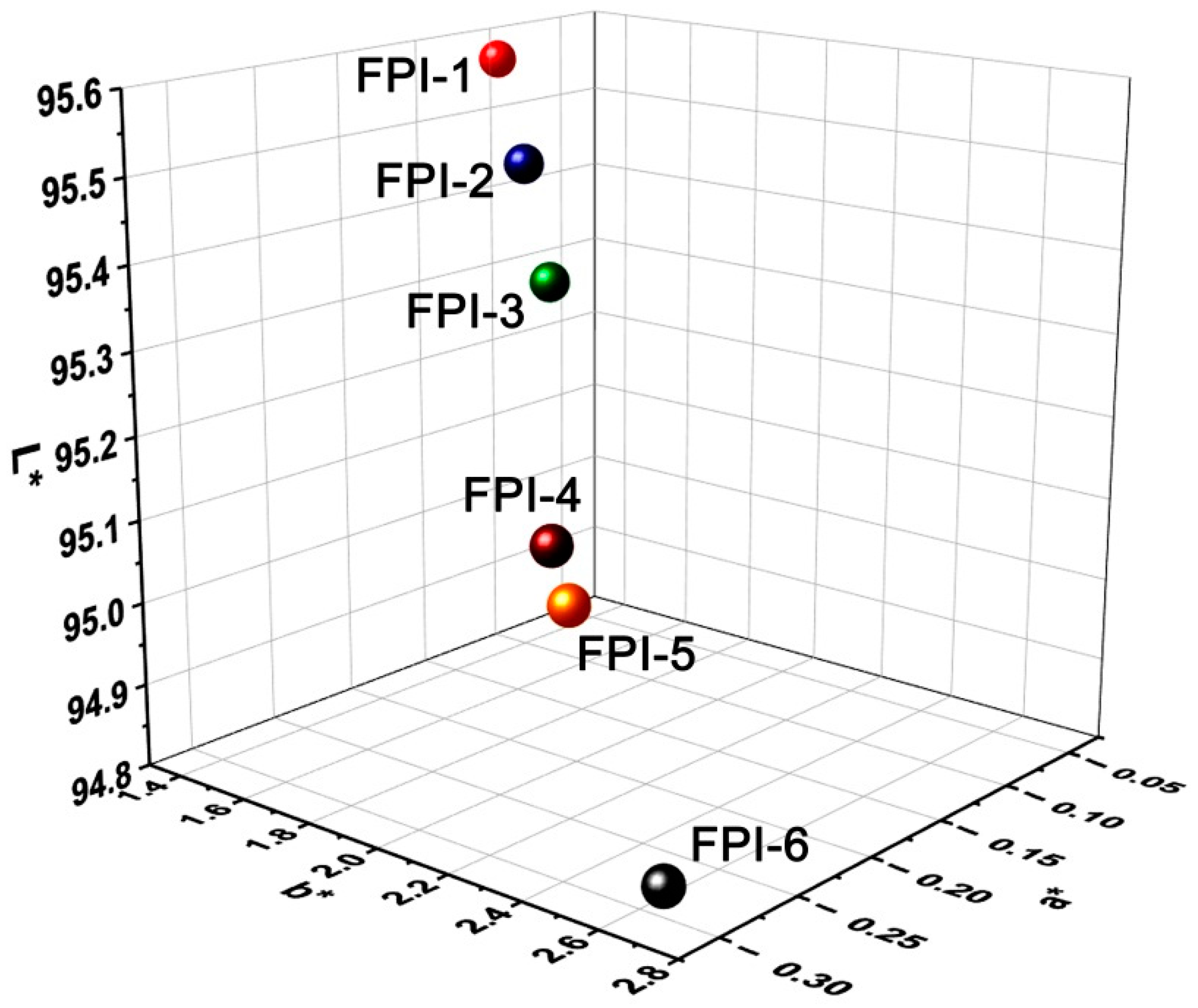
3.2. Optical Properties
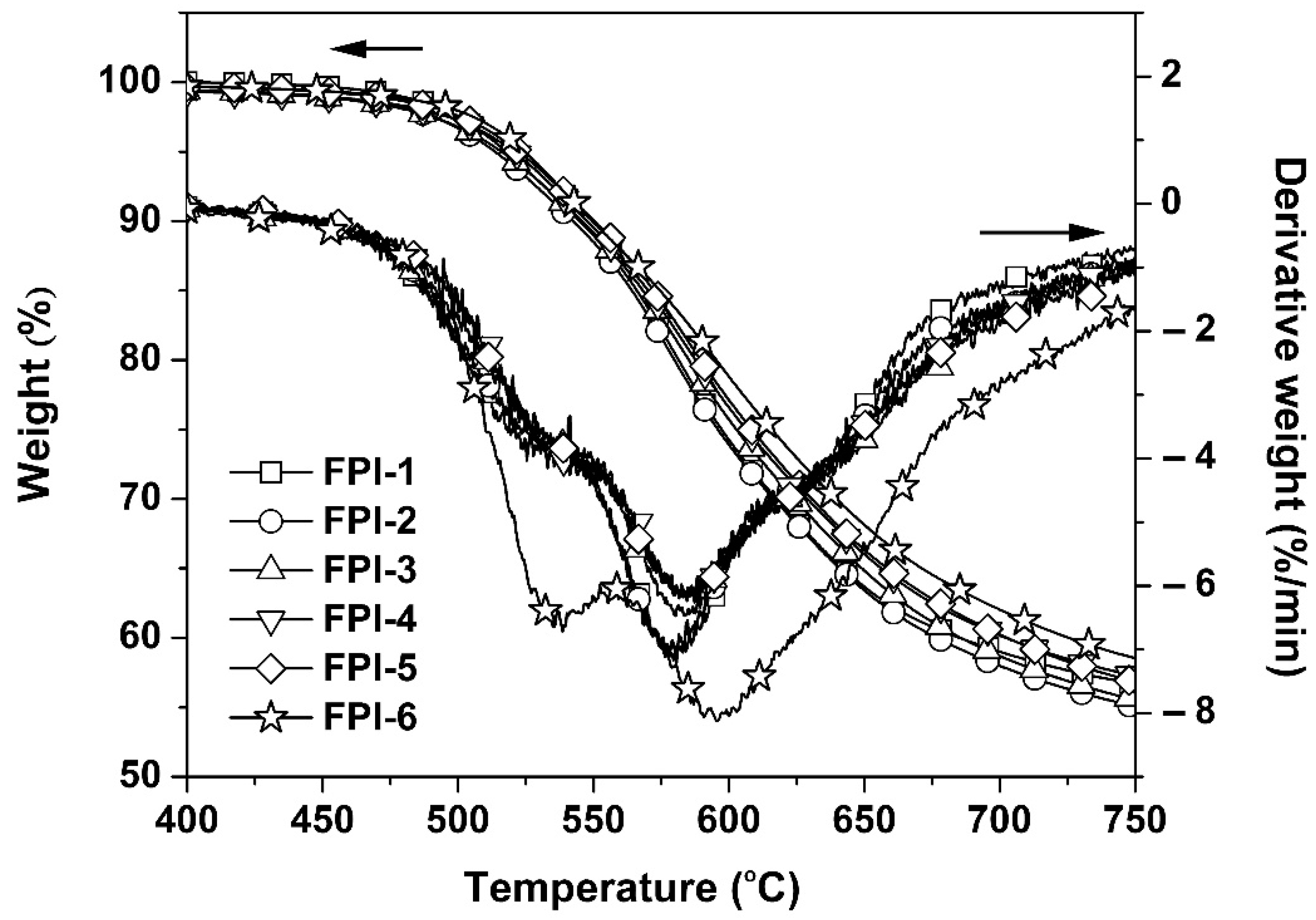
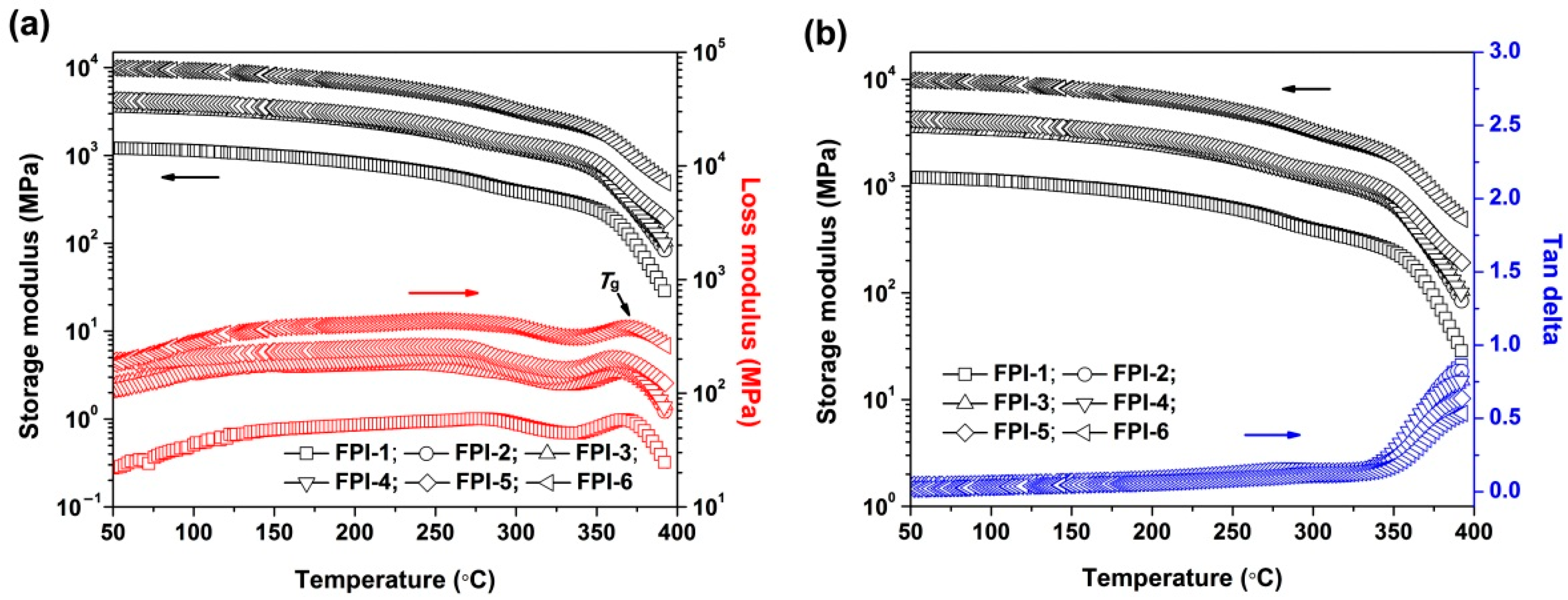
3.3. Thermal Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ni, H.; Liu, J.; Wang, Z.; Yang, S. A review on colorless and optically transparent polyimide films: Chemistry, process and engineering applications. J. Ind. Eng. Chem. 2015, 28, 16–27. [Google Scholar] [CrossRef]
- Zuo, H.T.; Gan, F.; Dong, J.; Zhang, P.; Zhao, X.; Zhang, Q.H. Highly transparent and colorless polyimide film with low dielectric constant by introducing meta-substituted structure and trifluoromethyl groups. Chin. J. Polym. Sci. 2021, 39, 455–464. [Google Scholar] [CrossRef]
- Yan, X.; Dai, F.; Ke, Z.; Yan, K.; Chen, C.; Qian, G.; Li, H. Synthesis of colorless polyimides with high Tg from asymmetric twisted benzimidazole diamines. Eur. Polym. J. 2022, 164, 110975. [Google Scholar] [CrossRef]
- Yi, C.; Li, W.; Shi, S.; He, K.; Ma, P.; Chen, M.; Yang, C. High-temperature-resistant and colorless polyimide: Preparations, properties, and applications. Sol. Energy 2020, 195, 340–354. [Google Scholar] [CrossRef]
- Zhi, X.X.; Zhang, Y.; Zhang, X.M.; Wang, H.L.; Wu, L.; An, Y.C.; Wei, X.Y.; Liu, J.G. Preparation and properties of semi-alicyclic colorless polyimide films and light-colored sheets with low dielectric features for potential applications in optoelectronic integrated circuits. Express Polym. Lett. 2021, 15, 1051–1062. [Google Scholar] [CrossRef]
- Min, H.; Kang, B.; Shin, Y.S.; Kim, B.S.; Lee, S.W.; Cho, J.H. Transparent and colorless polyimides containing multiple trifluoromethyl groups as gate insulators for flexible organic transistors with superior electrical stability. ACS Appl. Mater. Interf. 2020, 12, 18739–18747. [Google Scholar] [CrossRef]
- Kim, Y.N.; Lee, J.; Kim, Y.O.; Kim, J.; Han, H.; Jung, Y.C. Colorless polyimides with excellent optical transparency and self-healing properties based on multi-exchange dynamic network. Appl. Mater. Today 2021, 25, 101226. [Google Scholar] [CrossRef]
- Tapaswi, P.K.; Ha, C.S. Recent trends on transparent colorless polyimides with balanced thermal and optical properties: Design and synthesis. Macromol. Chem. Phys. 2019, 220, 1800313. [Google Scholar] [CrossRef]
- Yu, X.H.; Liu, J.N.; Wu, D.Y. Colorless PI structure design and evaluation for achieving low CTE target. Mater. Today Commun 2019, 21, 100562. [Google Scholar] [CrossRef]
- Zhang, Y.; Qu, L.; Liu, J.; Wu, X.; Zhang, Y.; Zhang, R.; Qi, H.; Zhang, X. Synthesis and characterization of high temperature resistant and optically transparent polyimide coatings for potential applications in quartz optical fibers protection. J. Coat. Technol. Res. 2019, 16, 511–520. [Google Scholar] [CrossRef]
- Hasegawa, M. Development of solution-processable, optically transparent polyimides with ultra-low linear coefficients of thermal expansion. Polymers 2017, 9, 520. [Google Scholar] [CrossRef] [PubMed]
- Bae, W.J.; Kovalev, M.K.; Kalinina, F.; Kim, M.; Cho, C. Towards colorless polyimide/silica hybrids for flexible substrates. Polymer 2016, 105, 124–132. [Google Scholar] [CrossRef]
- Fukukawa, K.; Okazaki, M.; Sakata, Y.; Urakami, T.; Yamashita, W.; Tamai, S. Synthesis and properties of multi-block semi-alicyclic polyimides for thermally stable transparent and low CTE film. Polymer 2013, 54, 1053–1063. [Google Scholar] [CrossRef]
- Lao, H.; Mushtaq, N.; Chen, G.; Wang, B.; Ba, Y.; Fang, X. Synthesis and properties of transparent random and multi-block polyamide-imide films with high modulus and low CTE. Eur. Polym. J. 2021, 153, 110512. [Google Scholar] [CrossRef]
- Nam, K.H.; Jin, J.; Lee, D.H.; Han, H.; Goh, M.; Yu, J.; Ku, B.C.; You, N.H. Towards solution-processable, thermally robust, transparent polyimide-chain-end tethered organosilicate nanohybrids. Compos. Part B Eng. 2019, 163, 290–296. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, H.; Kang, C.; Gao, L. Synthesis and characterization of amide-bridged colorless polyimide films with low CTE and high optical performance for flexible OLED displays. Polym. Chem. 2021, 12, 5364–5376. [Google Scholar] [CrossRef]
- Ozawa, H.; Ishiguro, E.; Kyoya, Y.; Kikuchi, Y.; Matsumoto, T. Colorless polyimides derived from an alicyclic dianhydride, CpODA. Polymers 2021, 13, 2824. [Google Scholar] [CrossRef]
- Ahn, C.; Kim, T.Y.; Hong, P.H.; Choi, S.; Lee, Y.J.; Kwon, H.; Jeon, H.; Ko, D.W.; Park, I.; Han, H.; et al. Highly transparent, colorless optical film with outstanding mechanical strength and folding reliability using mismatched charge-transfer complex intensification. Adv. Func. Mater. 2022, 32, 2111040. [Google Scholar] [CrossRef]
- Hasegawa, M.; Watanabe, Y.; Tsukuda, S.; Ishii, J. Solution-processable colorless polyimides with ultralow coefficients of thermal expansion for optoelectronic applications. Polym. Int. 2016, 65, 1063–1073. [Google Scholar] [CrossRef]
- Jiang, G.L.; Wang, D.Y.; Du, H.P.; Wu, X.; Zhang, Y.; Tan, Y.Y.; Wu, L.; Liu, J.G.; Zhang, X.M. Reduced coefficients of linear thermal expansion of colorless and transparent semi-alicyclic polyimide films via incorporation of rigid-rod amide moiety: Preparation and properties. Polymers 2020, 12, 413. [Google Scholar] [CrossRef]
- Zhi, X.X.; Jiang, G.L.; Zhang, Y.; Jia, Y.; Wu, L.; An, Y.C.; Liu, J.G.; Liu, Y.G. Preparation and properties of colorless and transparent semi-alicyclic polyimide films with enhanced high-temperature dimensional stability via incorporation of alkyl-substituted benzanilide units. J. Appl. Polym. Sci. 2022, 139, 51544. [Google Scholar] [CrossRef]
- Huang, H.W.; Horie, K.; Yokota, R. Differences in thermo-mechanical properties and intermolecular charge-transfer characteristics of the aromatic polyimide PI(BPDA/PDA) from various precursors. Macromol. Chem. Phys. 1999, 200, 791–798. [Google Scholar] [CrossRef]
- Miake, S.; Kato, J.; Muroya, Y.; Katsumura, Y.; Yamashita, T. Synthesis of polyimides with aliphatic moieties and their charge transfer structure and photophysical process. J. Photopolym. Sci. Technol. 2003, 16, 255–260. [Google Scholar] [CrossRef][Green Version]
- Hasegawa, M.; Shindo, Y.; Sugimura, T.; Ohshima, S.; Horie, K.; Kochi, M.; Yokota, R.; Mita, I. Photophysical processes in aromatic polyimides. Studies with model compounds. J. Polym. Sci. Part B Polym. Phys. 1993, 31, 1617–1625. [Google Scholar] [CrossRef]
- Jiang, P.; Shen, J.; Wang, Y.; Zhang, J.; Liu, X.; Tu, G. The influences of sulfoxide electron traps in transparent polyimides with low retardation, yellow index, and CTE. Macromol. Mater. Eng. 2021, 306, 2000606. [Google Scholar] [CrossRef]
- Lian, R.; Lei, X.; Xiao, Y.; Xue, S.; Xiong, G.; Zhang, Z.; Yan, D.; Zhang, Q. Synthesis and properties of colorless copolyimides derived from 4,4′-diaminodiphenyl ether-based diamines with different substituents. Polym. Chem. 2021, 12, 4803–4811. [Google Scholar] [CrossRef]
- Li, X.; Lei, H.; Guo, J.; Wang, J.; Qi, S.; Tian, G.; Wu, D. Composition design and properties investigation of BPDA/PDA/TFDB co-polyimide films with low dielectric permittivity. J. Appl. Polym. Sci. 2019, 136, 47989. [Google Scholar] [CrossRef]
- Jang, W.; Seo, J.; Lee, C.; Paek, S.H.; Han, H. Residual stress and mechanical properties of polyimide thin films. J. Appl. Polym. Sci. 2009, 113, 976–983. [Google Scholar] [CrossRef]
- Tian, Y.; Luo, L.; Yang, Q.; Zhang, L.; Wang, M.; Wu, D.; Wang, X.; Liu, X. Construction of stable hydrogen bonds at high temperature for preparation of polyimide films with ultralow coefficient of thermal expansion and high Tg. Polymer 2020, 188, 122100. [Google Scholar] [CrossRef]
- Kang, S.J.; Hong, S.I.; Park, C.R. New film-forming aromatic poly(amide-imide)s containing isoindoloquinazolinedione unit in the backbone. 2. Physical properties of the film cast from poly(biphenylphthalicdianhydride-oxydianiline-4,4′-diamino-3′- carbamoyl-benzanilide) [poly(BPDA-ODA-DACB)]. J. Appl. Polym. Sci. 2000, 78, 118–123. [Google Scholar] [CrossRef]
- Dezern, J.F. Synthesis and characterization of BTDA-based polyamide-imides. J. Polym. Sci. Part A Polym. Chem. 1988, 26, 2157–2169. [Google Scholar] [CrossRef]
- Luo, L.; Pang, Y.; Jiang, X.; Wang, X.; Zhang, P.; Chen, Y.; Peng, C.; Liu, X. Preparation and characterization of novel polyimide films containing amide groups. J. Polym. Res. 2012, 19, 9783. [Google Scholar] [CrossRef]
- Chang, C.L.; Zhu, W.G.; Lin, S.H. Flexible cover window with colorless polyimide hard coating thin film for foldable displays. SID Inter. Symp. Dig. Technol. Pap. 2021, 52, 961–964. [Google Scholar] [CrossRef]
- Wu, Q.; Ma, X.; Zheng, F.; Lu, X.; Lu, Q. High performance transparent polyimides by controlling steric hindrance of methyl side groups. Eur. Polym. J. 2019, 120, 109235. [Google Scholar] [CrossRef]
- Choi, M.C.; Kim, Y.; Ha, C.S. Polymers for flexible displays: From material selection to device applications. Prog. Polym. Sci. 2008, 33, 581–630. [Google Scholar] [CrossRef]
- Chang, J.H. Equibiaxially stretchable colorless and transparent polyimides for flexible display substrates. Rev. Adv. Mater. Sci. 2020, 59, 1–9. [Google Scholar] [CrossRef]




| PI | 6FDA (g, mmol) | BPDA (g, mmol) | MABTFMB (g, mmol) | NMP (g) | Ac2O (g, mmol) | Pyridine (g, mmol) |
|---|---|---|---|---|---|---|
| FPI-1 | 8.8848, 20 | 0 | 11.7306, 20 | 61.8 | 10.2, 100 | 6.3, 80 |
| FPI-2 | 7.9963, 18 | 0.5884, 2 | 11.7306, 20 | 60.9 | 10.2, 100 | 6.3, 80 |
| FPI-3 | 7.1078, 16 | 1.1769, 4 | 11.7306, 20 | 60.0 | 10.2, 100 | 6.3, 80 |
| FPI-4 | 6.2194, 14 | 1.7653, 6 | 11.7306, 20 | 59.1 | 10.2, 100 | 6.3, 80 |
| FPI-5 | 5.3309, 12 | 2.3538, 8 | 11.7306, 20 | 58.2 | 10.2, 100 | 6.3, 80 |
| FPI-6 | 4.4424, 10 | 2.9422, 10 | 11.7306, 20 | 57.4 | 10.2, 100 | 6.3, 80 |
| PI | Mn a (g/mol, 104) | Mw a (g/mol, 104) | PDI a | Solubility b | |||||
|---|---|---|---|---|---|---|---|---|---|
| NMP | DMAc | DMF | CHCl3 | THF | GBL | ||||
| FPI-1 | 4.87 | 9.66 | 1.98 | ++ | ++ | ++ | +− | +− | ++ |
| FPI-2 | 4.05 | 7.81 | 1.93 | ++ | ++ | ++ | +− | +− | +− |
| FPI-3 | 3.92 | 7.38 | 1.88 | ++ | ++ | ++ | − | − | +− |
| FPI-4 | 4.70 | 9.48 | 2.02 | ++ | ++ | ++ | − | − | +− |
| FPI-5 | 4.57 | 9.01 | 1.97 | ++ | ++ | +− | − | − | − |
| FPI-6 | 4.82 | 9.65 | 2.00 | ++ | ++ | +− | − | − | − |
| Samples | λcut a (nm) | T400 b (%) | T450 b (%) | L* c | a* c | b* c | Haze (%) |
|---|---|---|---|---|---|---|---|
| FPI-1 | 340 | 82.1 | 86.4 | 95.54 | −0.06 | 1.14 | 0.38 |
| FPI-2 | 352 | 79.8 | 85.4 | 95.47 | −0.15 | 1.63 | 0.58 |
| FPI-3 | 358 | 78.4 | 84.7 | 95.34 | −0.16 | 1.75 | 0.61 |
| FPI-4 | 363 | 73.3 | 81.8 | 95.14 | −0.27 | 2.24 | 1.20 |
| FPI-5 | 366 | 72.0 | 82.3 | 95.09 | −0.28 | 2.33 | 1.21 |
| FPI-6 | 367 | 63.0 | 74.0 | 94.82 | −0.29 | 2.62 | 2.29 |
| PI | T5% a (°C) | Tmax1 a (°C) | Tmax2 a (°C) | Rw750 a (%) | Tg a (°C) | E′50 b (GPa) | E′300 b (GPa) | CTE c (×10−6/K) |
|---|---|---|---|---|---|---|---|---|
| FPI-1 | 513.7 | 525.3 | 579.4 | 56.6 | 367 | 1.21 | 0.39 | 30.6 |
| FPI-2 | 515.8 | 529.9 | 580.5 | 55.0 | 360 | 3.56 | 1.19 | 29.9 |
| FPI-3 | 517.7 | 532.0 | 584.1 | 55.5 | 365 | 3.91 | 1.19 | 27.1 |
| FPI-4 | 520.3 | 533.1 | 584.1 | 57.2 | 357 | 4.25 | 1.29 | 25.1 |
| FPI-5 | 522.3 | 532.3 | 584.4 | 56.8 | 361 | 4.53 | 1.38 | 24.2 |
| FPI-6 | 524.4 | 535.4 | 595.4 | 58.5 | 367 | 9.87 | 3.20 | 23.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Wang, H.; Du, X.; Qi, H.; Pan, Z.; Wang, X.; Dai, S.; Yang, C.; Liu, J. Synthesis and Properties of Optically Transparent Fluoro-Containing Polyimide Films with Reduced Linear Coefficients of Thermal Expansion from Organo-Soluble Resins Derived from Aromatic Diamine with Benzanilide Units. Materials 2022, 15, 6346. https://doi.org/10.3390/ma15186346
Ren X, Wang H, Du X, Qi H, Pan Z, Wang X, Dai S, Yang C, Liu J. Synthesis and Properties of Optically Transparent Fluoro-Containing Polyimide Films with Reduced Linear Coefficients of Thermal Expansion from Organo-Soluble Resins Derived from Aromatic Diamine with Benzanilide Units. Materials. 2022; 15(18):6346. https://doi.org/10.3390/ma15186346
Chicago/Turabian StyleRen, Xi, Hanli Wang, Xuanzhe Du, Haoran Qi, Zhen Pan, Xiaolei Wang, Shengwei Dai, Changxu Yang, and Jingang Liu. 2022. "Synthesis and Properties of Optically Transparent Fluoro-Containing Polyimide Films with Reduced Linear Coefficients of Thermal Expansion from Organo-Soluble Resins Derived from Aromatic Diamine with Benzanilide Units" Materials 15, no. 18: 6346. https://doi.org/10.3390/ma15186346
APA StyleRen, X., Wang, H., Du, X., Qi, H., Pan, Z., Wang, X., Dai, S., Yang, C., & Liu, J. (2022). Synthesis and Properties of Optically Transparent Fluoro-Containing Polyimide Films with Reduced Linear Coefficients of Thermal Expansion from Organo-Soluble Resins Derived from Aromatic Diamine with Benzanilide Units. Materials, 15(18), 6346. https://doi.org/10.3390/ma15186346







