A Fukui Analysis of an Arginine-Modified Carbon Surface for the Electrochemical Sensing of Dopamine
Abstract
:1. Introduction
2. Experimentation
2.1. Computational Methods
2.2. Reagents and Chemicals
2.3. Instrumentation
2.4. Fabrication of AMPE
3. Results and Discussion
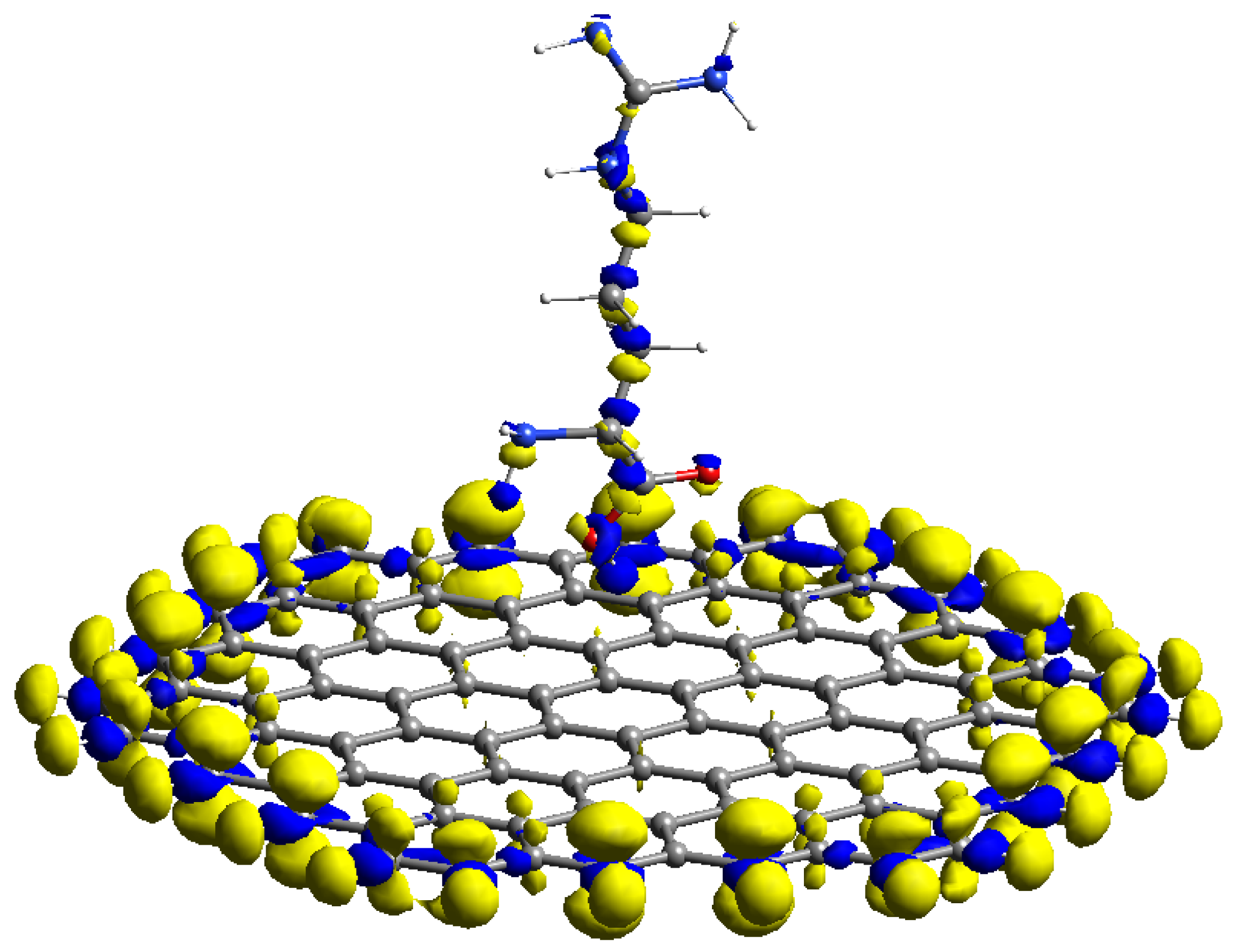
3.1. Computational Modeling of AGR Complexes
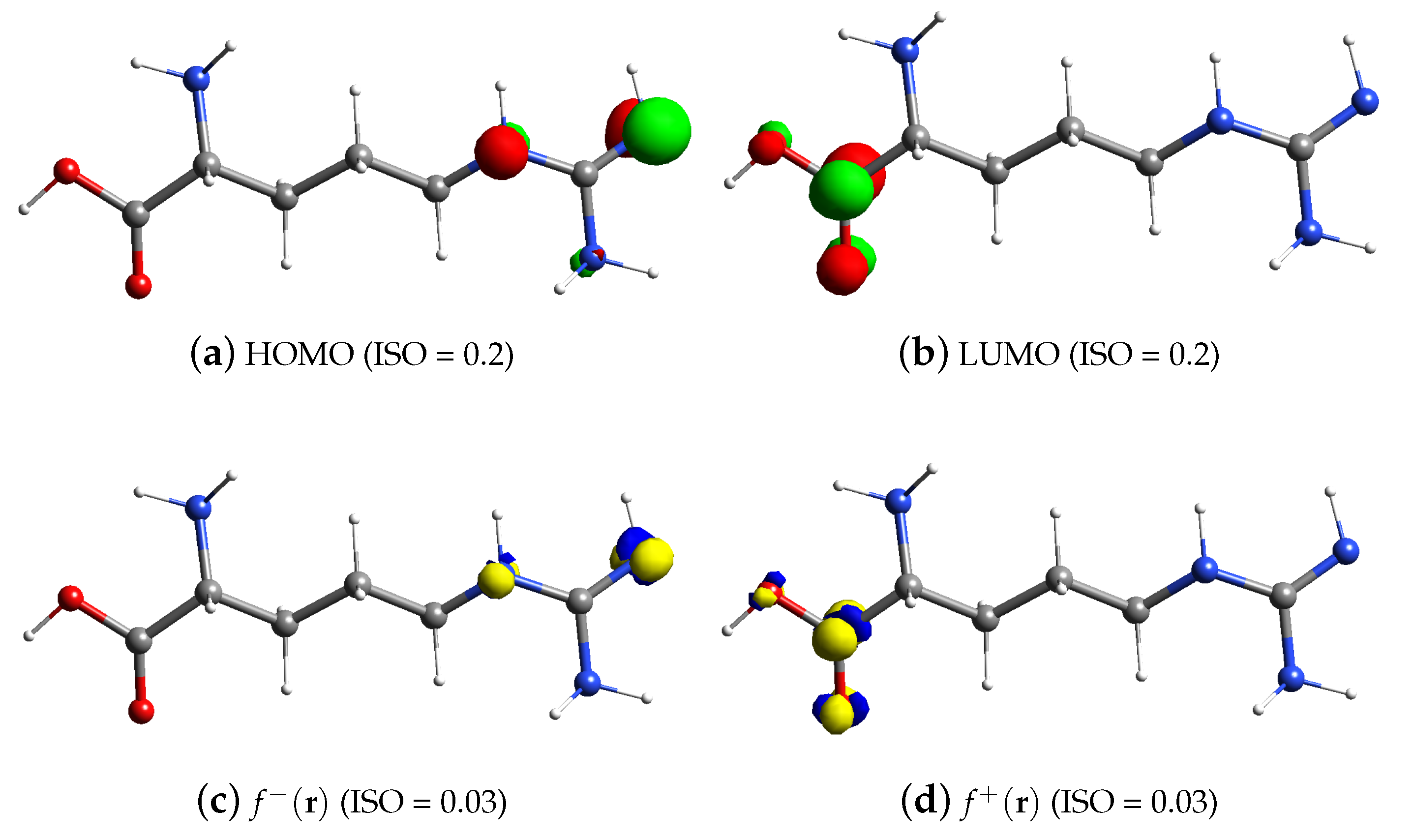
3.2. FMO and Fukui Analysis of Arginine
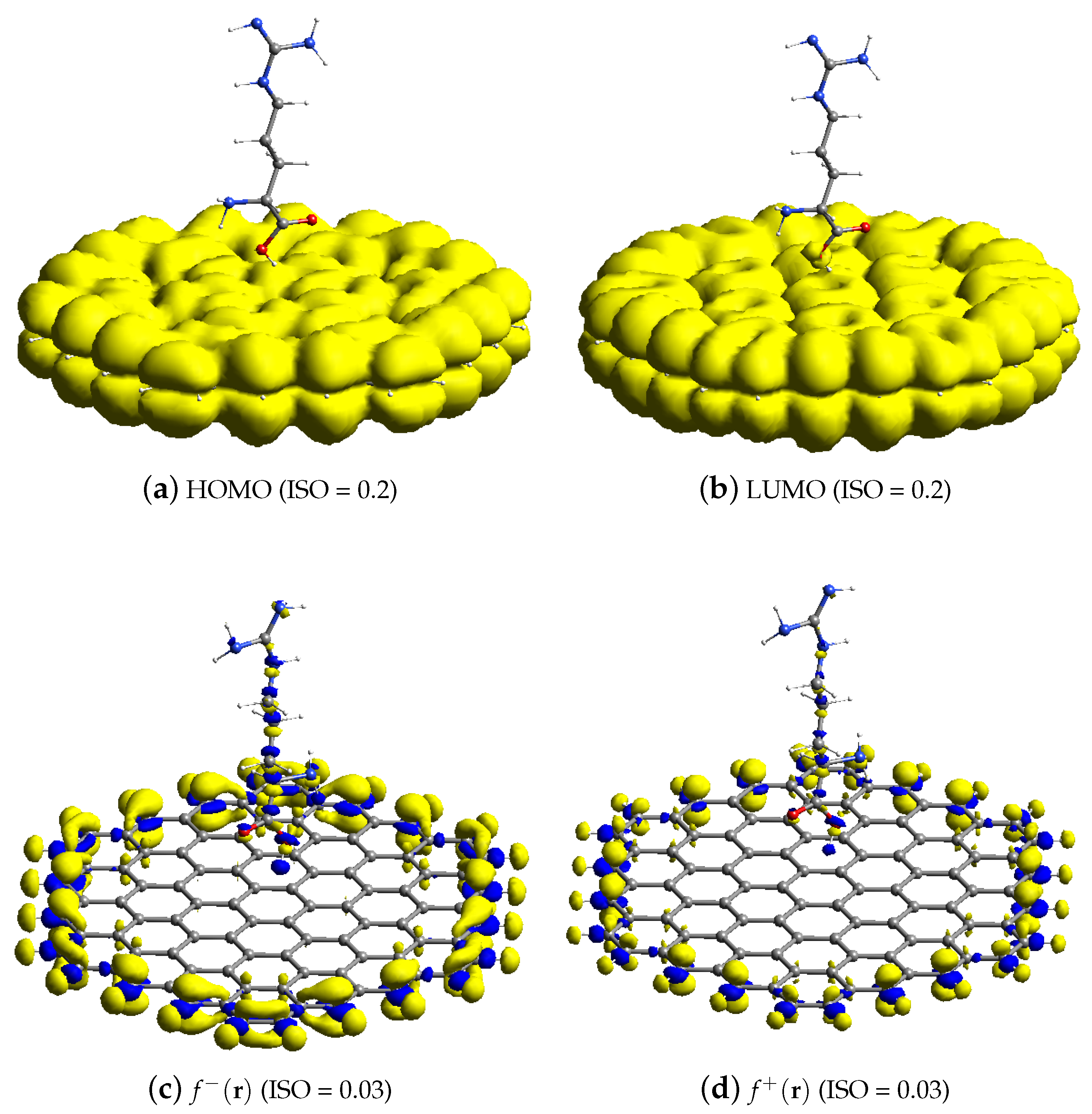
3.3. FMO and Analytical Fukui Analysis of Arginine–GR
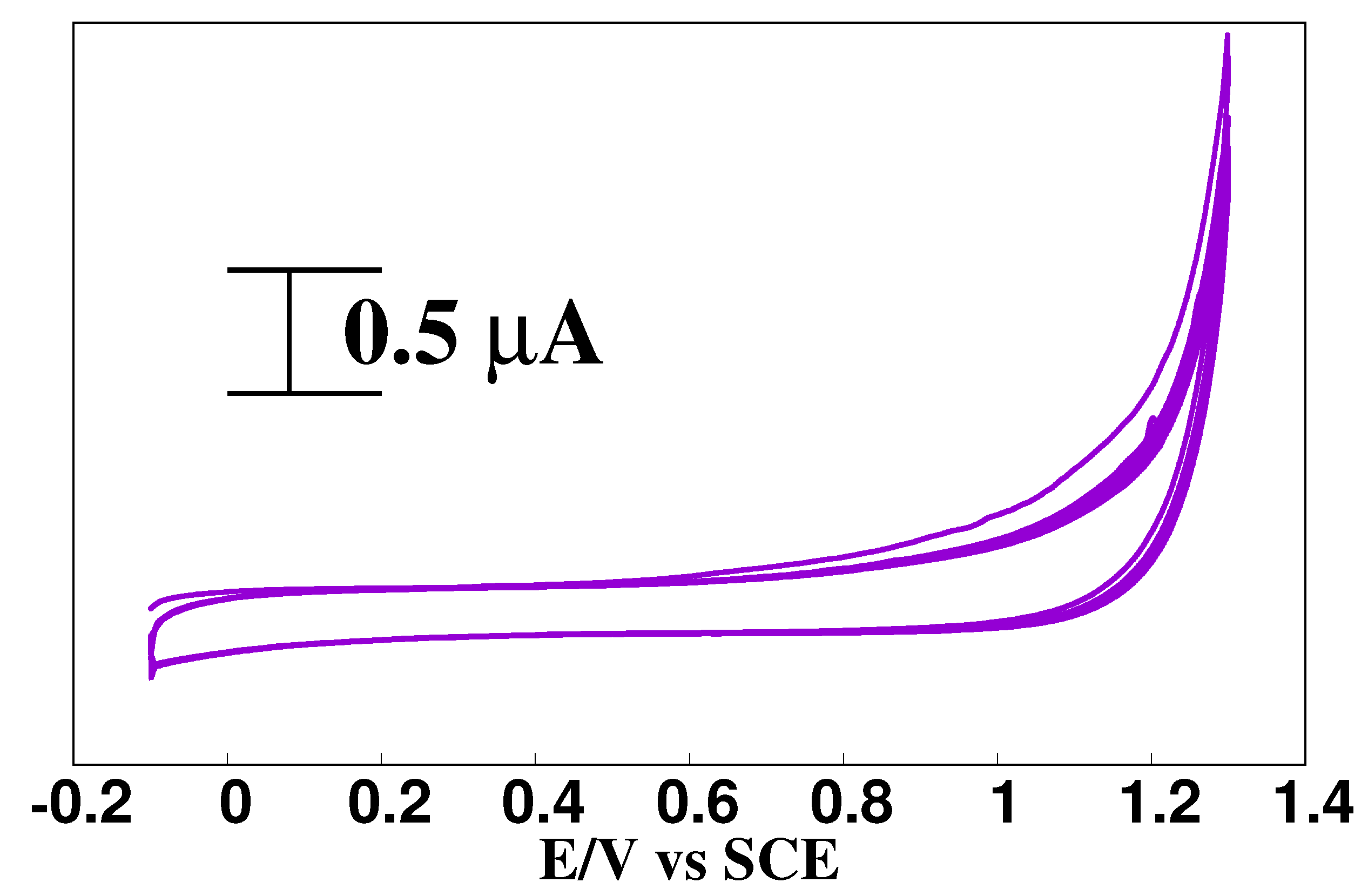
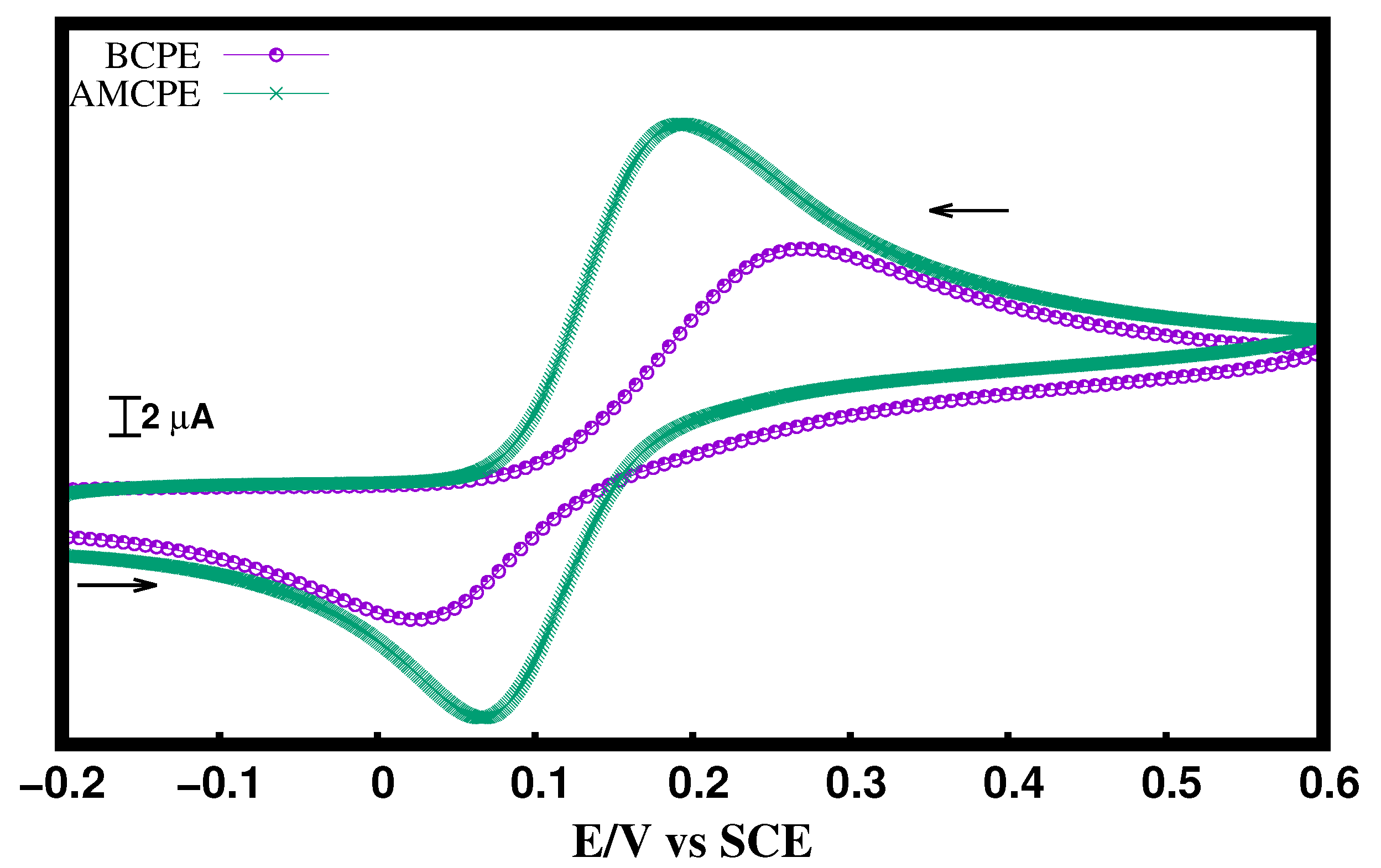
3.4. Analytical Use of the AMCPE Interface for DA Sensing
3.4.1. Analysis of DA at the AMCPE Interface
3.4.2. The Impact of Concentration of DA at AMCPE
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carlsson, A.; Lindqvist, M.; Magnusson, T. 3, 4-Dihydroxyphenylalanine and 5-hydroxytryptophan as reserpine antagonists. Nature 1957, 180, 1200. [Google Scholar] [CrossRef]
- Whitton, A.E.; Reinen, J.M.; Slifstein, M.; Ang, Y.S.; McGrath, P.J.; Iosifescu, D.V.; Abi-Dargham, A.; Pizzagalli, D.A.; Schneier, F.R. Baseline reward processing and ventrostriatal dopamine function are associated with pramipexole response in depression. Brain 2020, 143, 701–710. [Google Scholar] [CrossRef]
- Damier, P.; Hirsch, E.C.; Agid, Y.; Graybiel, A.M. The substantia nigra of the human brain: II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain 1999, 122, 1437–1448. [Google Scholar] [CrossRef]
- Jentsch, J.; Roth, R.H.; Taylor, J.R. Role for dopamine in the behavioral functions of the prefrontal corticostriatal system: Implications for mental disorders and psychotropic drug action. In Cognition, Emotion and Autonomic Responses: The Integrative Role of the Prefrontal Cortex and Limbic Structures; Elsevier: Amsterdam, The Netherlands, 2000; Volume 126, pp. 433–453. [Google Scholar]
- Kamal Eddin, F.B.; Wing Fen, Y. Recent Advances in Electrochemical and Optical Sensing of Dopamine. Sensors 2020, 20, 1039. [Google Scholar] [CrossRef]
- Kudur Jayaprakash, G.; Kumara Swamy, B.E.; Nicole González Ramírez, H.; Tumbre Ekanthappa, M.; Flores-Moreno, R. Quantum chemical and electrochemical studies of lysine modified carbon paste electrode surfaces for sensing dopamine. New J. Chem. 2018, 42, 4501–4506. [Google Scholar] [CrossRef]
- Kamal Eddin, F.B.; Fen, Y.W.; Omar, N.A.S.; Liew, J.Y.C.; Wan Mohd Ebtisyam Mustaqim Mohd Daniyal. Femtomolar detection of dopamine using surface plasmon resonance sensor based on chitosan/graphene quantum dots thin film. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 263, 120202. [Google Scholar] [CrossRef]
- Sarre, S.; Michotte, Y.; Herregodts, P.; Deleu, D.; De Klippel, N.; Ebinger, G. High-performance liquid chromatography with electrochemical detection for the determination of levodopa, catecholamines and their metabolites in rat brain dialysates. J. Chromatogr. B Biomed. Appl. 1992, 575, 207–212. [Google Scholar] [CrossRef]
- Kim, H.R.; Kim, T.H.; Hong, S.H.; Kim, H.G. Direct detection of tetrahydrobiopterin (BH4) and dopamine in rat brain using liquid chromatography coupled electrospray tandem mass spectrometry. Biochem. Biophys. Res. Commun. 2012, 419, 632–637. [Google Scholar] [CrossRef]
- Zhang, J.; Hou, S.; Zhang, J.; Liang, N.; Zhao, L. A facile aptamer-based sensing strategy for dopamine detection through the fluorescence energy transfer between dye and single-wall carbon nanohorns. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 279, 121415. [Google Scholar] [CrossRef]
- Jayaprakash, G.K.; Swamy, B.E.K.; Chandrashekar, B.N.; Flores-Moreno, R. Theoretical and cyclic voltammetric studies on electrocatalysis of benzethonium chloride at carbon paste electrode for detection of dopamine in presence of ascorbic acid. J. Mol. Liq. 2017, 240, 395–401. [Google Scholar] [CrossRef]
- Zima, J.; Švancara, I.; Barek, J.; Vytřas, K. Recent Advances in Electroanalysis of Organic Compounds at Carbon Paste Electrodes. Crit. Rev. Anal. Chem. 2009, 39, 204–227. [Google Scholar] [CrossRef]
- Švancara, I.; Vytřas, K.; Kalcher, K.; Walcarius, A.; Wang, J. Carbon Paste Electrodes in Facts, Numbers, and Notes: A Review on the Occasion of the 50-Years Jubilee of Carbon Paste in Electrochemistry and Electroanalysis. Electroanalysis 2009, 21, 7–28. [Google Scholar] [CrossRef]
- Jayaprakash, G.K.; Kumara Swamy, B.; Rajendrachari, S.; Sharma, S.; Flores-Moreno, R. Dual descriptor analysis of cetylpyridinium modified carbon paste electrodes for ascorbic acid sensing applications. J. Mol. Liq. 2021, 334, 116348. [Google Scholar] [CrossRef]
- Kudur Jayaprakash, G.; Swamy, B.K.; Casillas, N.; Flores-Moreno, R. Analytical Fukui and cyclic voltammetric studies on ferrocene modified carbon electrodes and effect of Triton X-100 by immobilization method. Electrochim. Acta 2017, 258, 1025–1034. [Google Scholar] [CrossRef]
- Zhang, F.; Gu, S.; Ding, Y.; Zhou, L.; Zhang, Z.; Li, L. Electrooxidation and determination of cefotaxime on Au nanoparticles/poly (L-arginine) modified carbon paste electrode. J. Electroanal. Chem. 2013, 698, 25–30. [Google Scholar] [CrossRef]
- Raghu, P.; Madhusudana Reddy, T.; Reddaiah, K.; Jaidev, L.; Narasimha, G. A novel electrochemical biosensor based on horseradish peroxidase immobilized on Ag-nanoparticles/poly(l-arginine) modified carbon paste electrode toward the determination of pyrogallol/hydroquinone. Enzyme Microb. Technol. 2013, 52, 377–385. [Google Scholar] [CrossRef]
- Zhang, F.; Gu, S.; Ding, Y.; Zhang, Z.; Li, L. A novel sensor based on electropolymerization of β-cyclodextrin and l-arginine on carbon paste electrode for determination of fluoroquinolones. Anal. Chim. Acta 2013, 770, 53–61. [Google Scholar] [CrossRef]
- Tigari, G.; Manjunatha, J. Electrochemical preparation of poly (arginine)-modified carbon nanotube paste electrode and its application for the determination of pyridoxine in the presence of riboflavin: An electroanalytical approach. J. Anal. Test. 2019, 3, 331–340. [Google Scholar] [CrossRef]
- Edwin, D.S.; Manjunatha, J.G.; Raril, C.; Girish, T.; Ravishankar, D.K.; Arpitha, H.J. Electrochemical analysis of indigo carmine using polyarginine modified carbon paste electrode. J. Electrochem. Sci. Eng. 2021, 11, 87–96. [Google Scholar] [CrossRef]
- Chandrashekar, B.; Kumara Swamy, B.; Pandurangachar, M.; Sathisha, T.; Sherigara, B. Electropolymerisation of l-arginine at carbon paste electrode and its application to the detection of dopamine, ascorbic and uric acid. Colloids Surf. B Biointerfaces 2011, 88, 413–418. [Google Scholar] [CrossRef]
- McCreery, R.L. Advanced Carbon Electrode Materials for Molecular Electrochemistry. Chem. Rev. 2008, 108, 2646–2687. [Google Scholar] [CrossRef] [PubMed]
- Toral-Sanchez, E.; Rangel-Mendez, J.R.; Chazaro-Ruiz, L.F. Characterization of iron-modified carbon paste electrodes and their application in As(V) detection. J. Appl. Electrochem. 2016, 46, 205–215. [Google Scholar] [CrossRef]
- Sahoo, S.; Sahoo, P.K.; Satpati, A.K. Gold Nano Particle and Reduced Graphene Oxide Composite Modified Carbon Paste Electrode for the Ultra Trace Detection of Arsenic (III). Electroanalysis 2017, 29, 1400–1409. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density Functional Approach to the Frontier–Electron Theory of Chemical Reactivity. J. Am. Chem. Soc. 1984, 106, 4049–4050. [Google Scholar] [CrossRef]
- Flores-Moreno, R. Symmetry Conservation in Fukui Functions. J. Chem. Theory Comput. 2010, 6, 48–54. [Google Scholar] [CrossRef]
- Flores-Moreno, R.; Melin, J.; Ortiz, J.V.; Merino, G. Efficient Evaluation of Analytic Fukui Functions. J. Chem. Phys. 2008, 129, 224105. [Google Scholar] [CrossRef]
- Martínez-Araya, J.I. Why is the dual descriptor a more accurate local reactivity descriptor than Fukui functions? J. Math. Chem. 2015, 53, 451–465. [Google Scholar] [CrossRef]
- Morell, C.; Grand, A.; Toro-Labbéá, A. Theoretical support for using the △f(r) descriptor. Chem. Phys. Lett. 2006, 425, 342–346. [Google Scholar] [CrossRef]
- Martínez, J. Local reactivity descriptors from degenerate frontier molecular orbitals. Chem. Phys. Lett. 2009, 478, 310–322. [Google Scholar] [CrossRef]
- Flores-Moreno, R.; Pineda-Urbina, K.; Gómez-Sandoval, Z. Sinapsis,Version XII-V. Sinapsis Developers. 2012. Available online: http://sinapsis.sourceforge.net (accessed on 20 July 2022).
- Geudtner, G.; Calaminici, P.; Carmona-Espíndola, J.; del Campo, J.M.; Domínguez-Soria, V.D.; Moreno, R.F.; Gabriel, U.; Annick, K.; Andreas, M.; Zúñinga-Gutierrez, B.; et al. deMon2k. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 548–555. [Google Scholar] [CrossRef]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate Spin-Dependent Electron Liquid Correlation Energies for Local Spin Density Calculations: A Critical Analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef]
- Godbout, N.; Salahub, D.R.; Andzelm, J.; Wimmer, E. Optimization of Gaussian-Type Basis Sets for Local Spin Density Functional Calculations. Part I. Boron through Neon, Optimization Technique and Validation. Can. J. Chem. 1992, 70, 560–571. [Google Scholar] [CrossRef]
- Andzelm, J.; Radzio, E.; Salahub, D.R. Compact basis sets for LCAO-LSD calculations. Part I: Method and bases for Sc to Zn. J. Comput. Chem. 1985, 6, 520–532. [Google Scholar] [CrossRef]
- Andzelm, J.; Russo, N.; Salahub, D.R. Ground and excited states of group IVA diatomics from local-spin-density calculations: Model potentials for Si, Ge, and Sn. J. Chem. Phys. 1987, 87, 6562–6572. [Google Scholar] [CrossRef]
- Calaminici, P.; Janetzko, F.; Köster, A.M.; Mejia-Olvera, R.; Zuniga-Gutierrez, B. Density functional theory optimized basis sets for gradient corrected functionals: 3d transition metal systems. J. Chem. Phys. 2007, 126, 044108. [Google Scholar] [CrossRef]
- Hareesha, N.; Manjunatha, J.G.; Raril, C.; Tigari, G. Sensitive and Selective Electrochemical Resolution of Tyrosine with Ascorbic Acid through the Development of Electropolymerized Alizarin Sodium Sulfonate Modified Carbon Nanotube Paste Electrodes. ChemistrySelect 2019, 4, 4559–4567. [Google Scholar] [CrossRef]
- Manjunatha, J. A surfactant enhanced graphene paste electrode as an effective electrochemical sensor for the sensitive and simultaneous determination of catechol and resorcinol. Chem. Data Collect 2020, 25, 100331. [Google Scholar] [CrossRef]
- Hareesha, N.; Manjunatha, J. A simple and low-cost poly (dl-phenylalanine) modified carbon sensor for the improved electrochemical analysis of Riboflavin. J. Sci. Adv. Mater. Devices 2020, 5, 502–511. [Google Scholar] [CrossRef]
- Hareesha, N.; Manjunatha, J.G. Surfactant and polymer layered carbon composite electrochemical sensor for the analysis of estriol with ciprofloxacin. Mater. Res. Innov. 2020, 24, 349–362. [Google Scholar] [CrossRef]
- Raril, C.; Manjunatha, J.G. Fabrication of novel polymer-modified graphene-based electrochemical sensor for the determination of mercury and lead ions in water and biological samples. J. Anal. Sci. Technol. 2020, 11, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Charithra, M.M.; Manjunatha, J.G.G.; Raril, C. Surfactant Modified Graphite Paste Electrode as an Electrochemical Sensor for the Enhanced Voltammetric Detection of Estriol with Dopamine and Uric acid. Adv. Pharm. Bull. 2020, 10, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Kudur Jayaprakash, G. Pre-post redox electron transfer regioselectivity at the alanine modified nano graphene electrode interface. Chem. Phys. Lett. 2022, 789, 139295. [Google Scholar] [CrossRef]
- Fukui, K.; Yonezawa, T.; Shingu, H. A Molecular Orbital Theory of Reactivity in Aromatic Hydrocarbons. J. Chem. Phys. 1952, 20, 722–725. [Google Scholar] [CrossRef]
- Fukui, K.; Yonezawa, T.; Nagata, C.; Shingu, H. Molecular Orbital Theory of Orientation in Aromatic, Heteroaromatic, and Other Conjugated Molecules. J. Chem. Phys. 1954, 22, 1433–1442. [Google Scholar] [CrossRef]
- Fukui, K. Role of Frontier Orbitals in Chemical Reactions. Science 1982, 218, 747–754. [Google Scholar] [CrossRef] [Green Version]
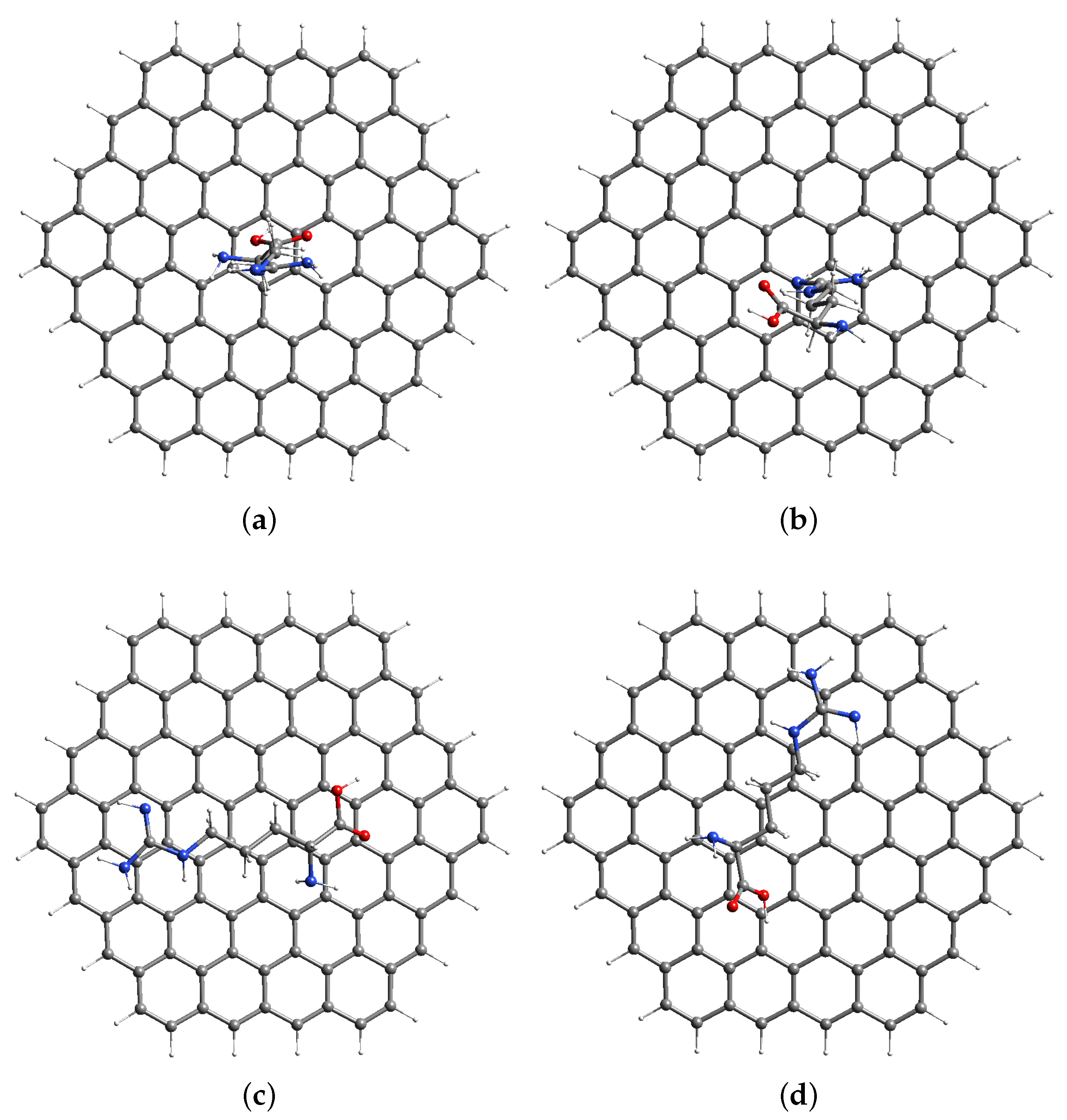

| Model | PBE/TZVP (kJ/mol) |
|---|---|
| Arginine–GR a | 0.000 |
| Arginine–GR b | 9.466 |
| Arginine–GR c | 20.574 |
| Arginine–GR d | 8.135 |
| SI/No. | DA Spiked | DA Sensed | Deviation | Recovery |
|---|---|---|---|---|
| (L) | (L) | (L) | (%) | |
| 1 | 5 | 4.75 | −0.35 | 95.0 |
| 2 | 10 | 9.60 | −0.40 | 96.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Revanappa, S.K.; Soni, I.; Siddalinganahalli, M.; Jayaprakash, G.K.; Flores-Moreno, R.; Bananakere Nanjegowda, C. A Fukui Analysis of an Arginine-Modified Carbon Surface for the Electrochemical Sensing of Dopamine. Materials 2022, 15, 6337. https://doi.org/10.3390/ma15186337
Revanappa SK, Soni I, Siddalinganahalli M, Jayaprakash GK, Flores-Moreno R, Bananakere Nanjegowda C. A Fukui Analysis of an Arginine-Modified Carbon Surface for the Electrochemical Sensing of Dopamine. Materials. 2022; 15(18):6337. https://doi.org/10.3390/ma15186337
Chicago/Turabian StyleRevanappa, Santhosh Kumar, Isha Soni, Manjappa Siddalinganahalli, Gururaj Kudur Jayaprakash, Roberto Flores-Moreno, and Chandrashekar Bananakere Nanjegowda. 2022. "A Fukui Analysis of an Arginine-Modified Carbon Surface for the Electrochemical Sensing of Dopamine" Materials 15, no. 18: 6337. https://doi.org/10.3390/ma15186337
APA StyleRevanappa, S. K., Soni, I., Siddalinganahalli, M., Jayaprakash, G. K., Flores-Moreno, R., & Bananakere Nanjegowda, C. (2022). A Fukui Analysis of an Arginine-Modified Carbon Surface for the Electrochemical Sensing of Dopamine. Materials, 15(18), 6337. https://doi.org/10.3390/ma15186337









