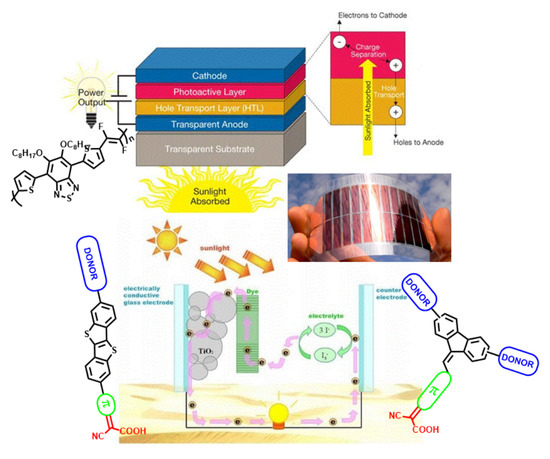
Functional Organic Materials for Photovoltaics: The Synthesis as a Tool for Managing Properties for Solid State Applications
Abstract
:1. Introduction
2. Results
2.1. Fluorine Impact on Arylenevinylene Materials: Synthesis, Chemico-Physical Properties and Application in BHJ Solar Cells
2.1.1. Synthesis of Polyarylenevinylenes for BHJ Solar Cells
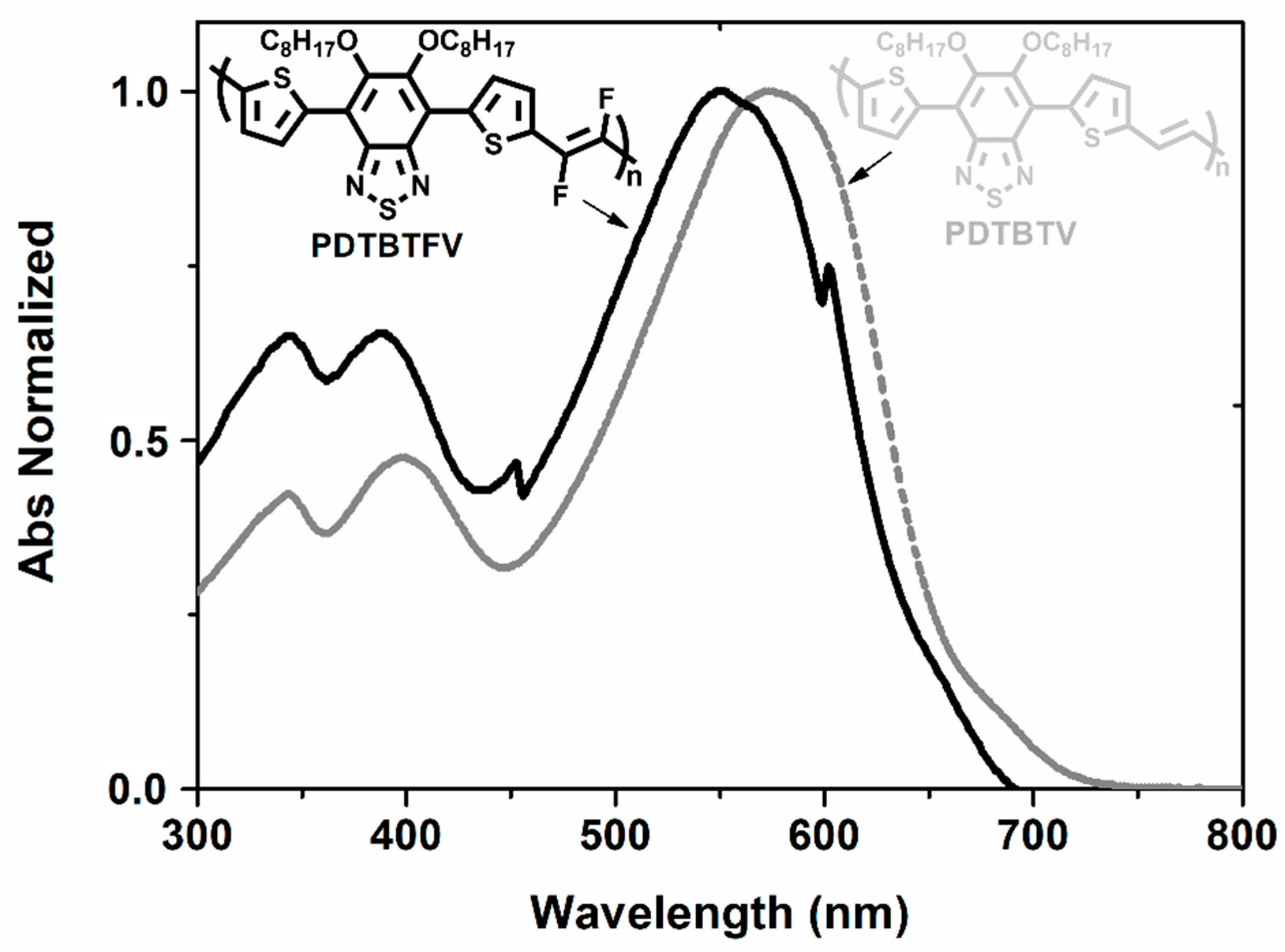
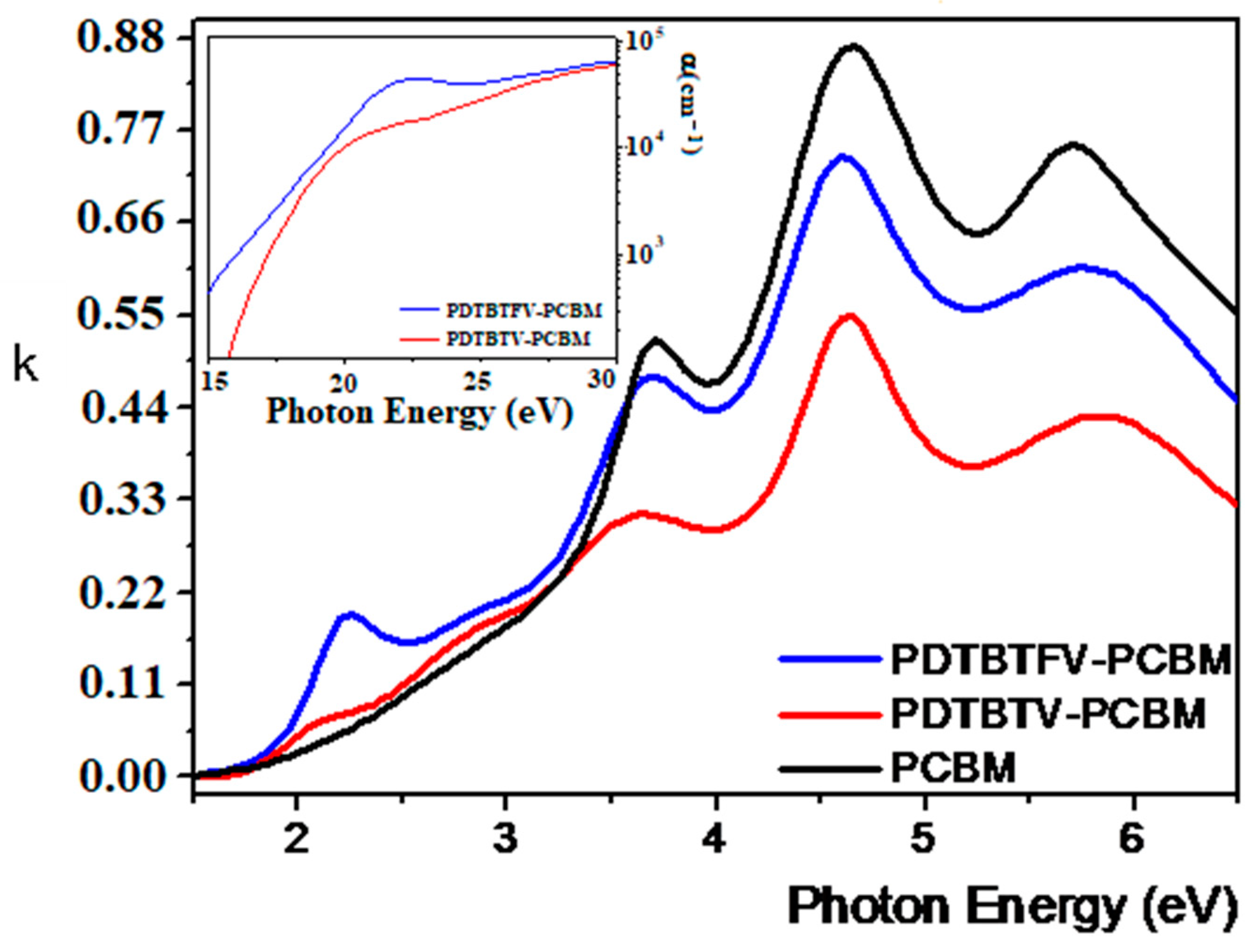
2.1.2. Chemico-Physical Properties and Photovoltaic Performance in BHJ Solar Cells of PDTBTFV and PDTBTV: Impact of Fluoro-Functionalization of Vinylene Units
2.2. Small Molecules for Dye-Sensitized Solar Cells DSSCs: From Synthesis to Photovoltaic Performance in Device
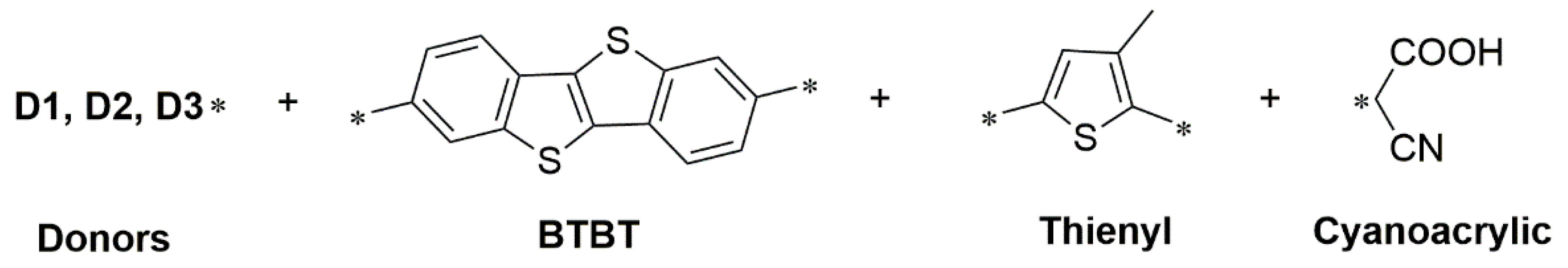
2.2.1. Benzothienobenzothiophene (BTBT)-Based Organic Dyes
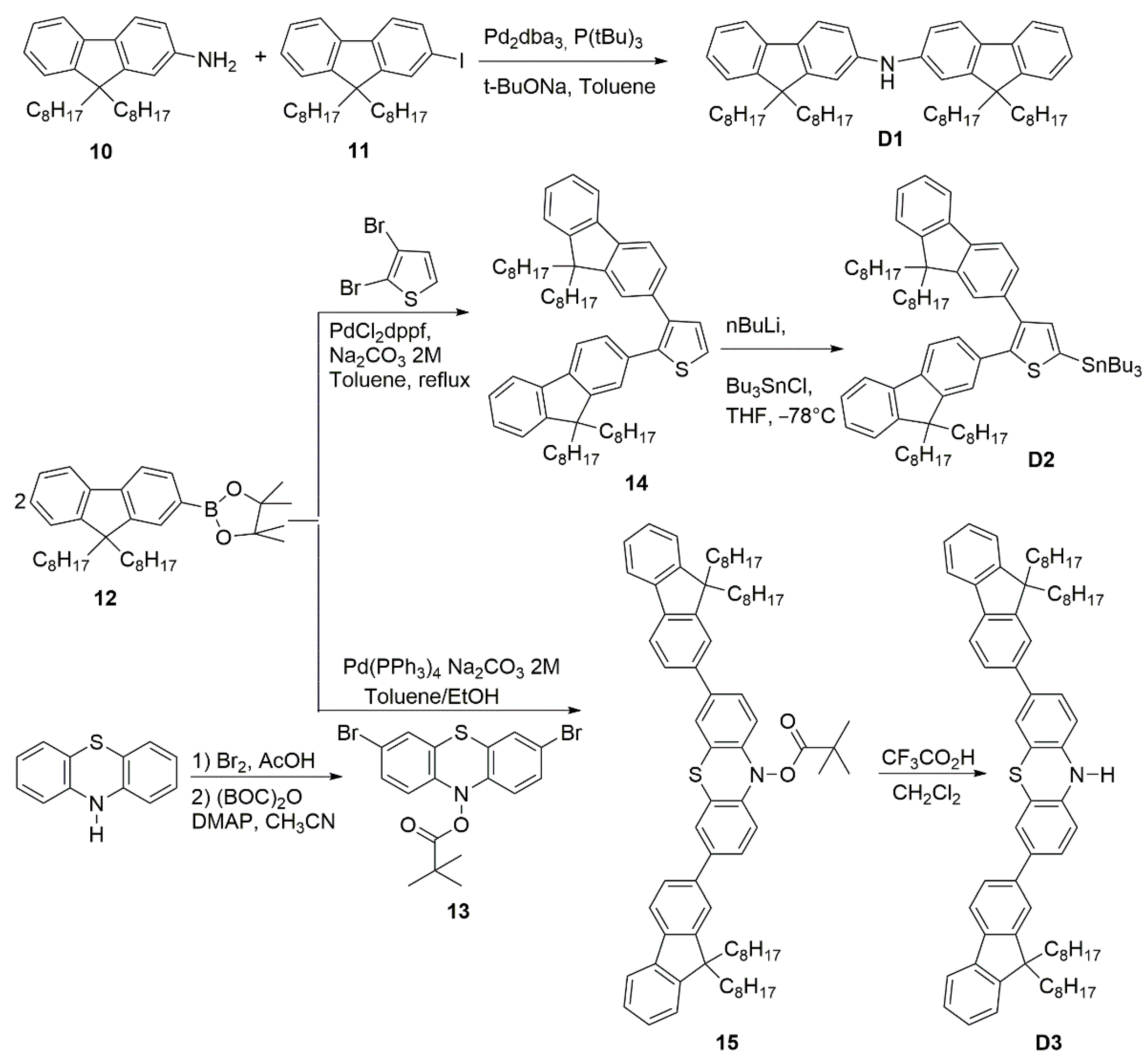
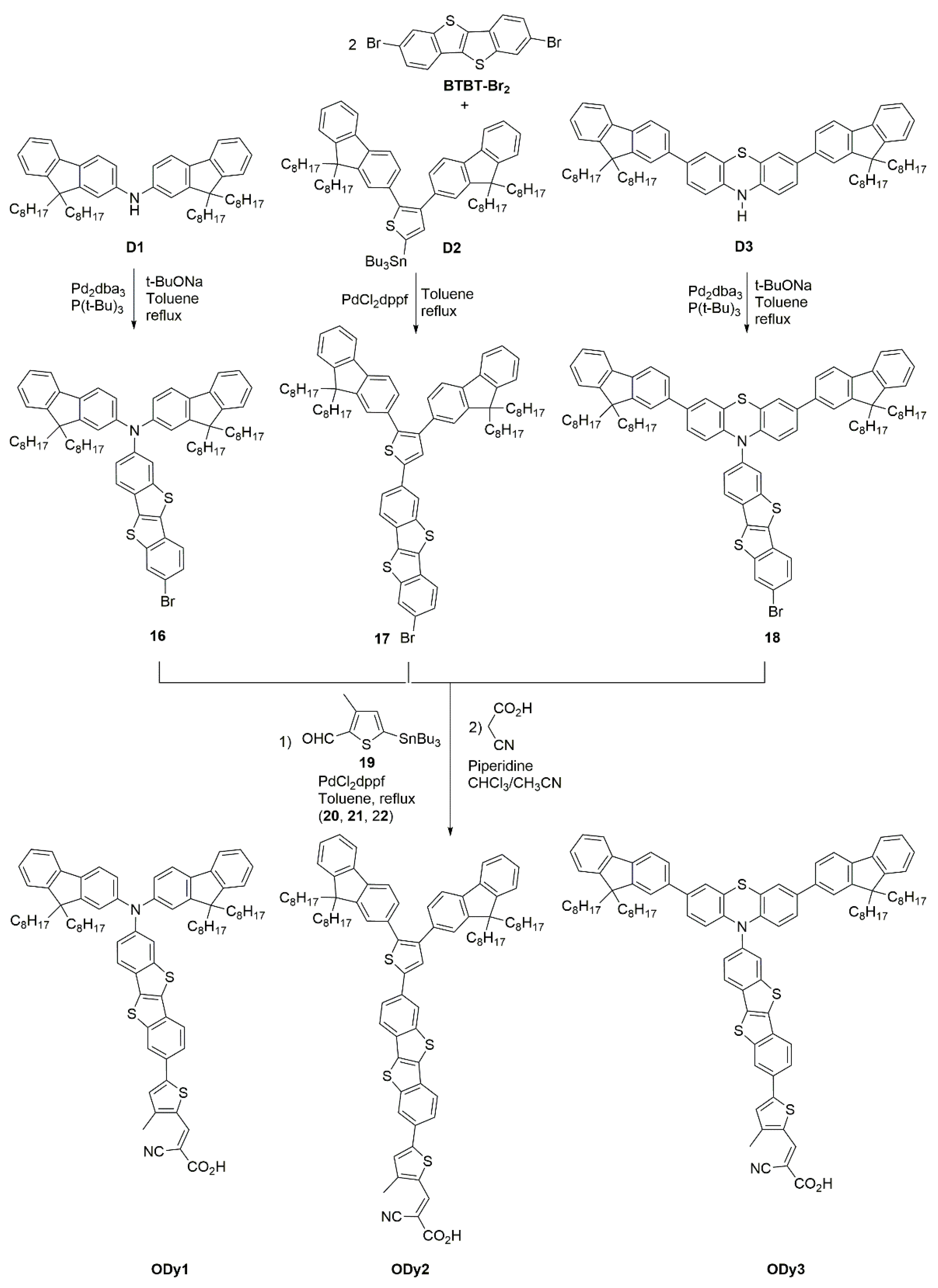
Synthesis of BTBT-Based Organic Dyes
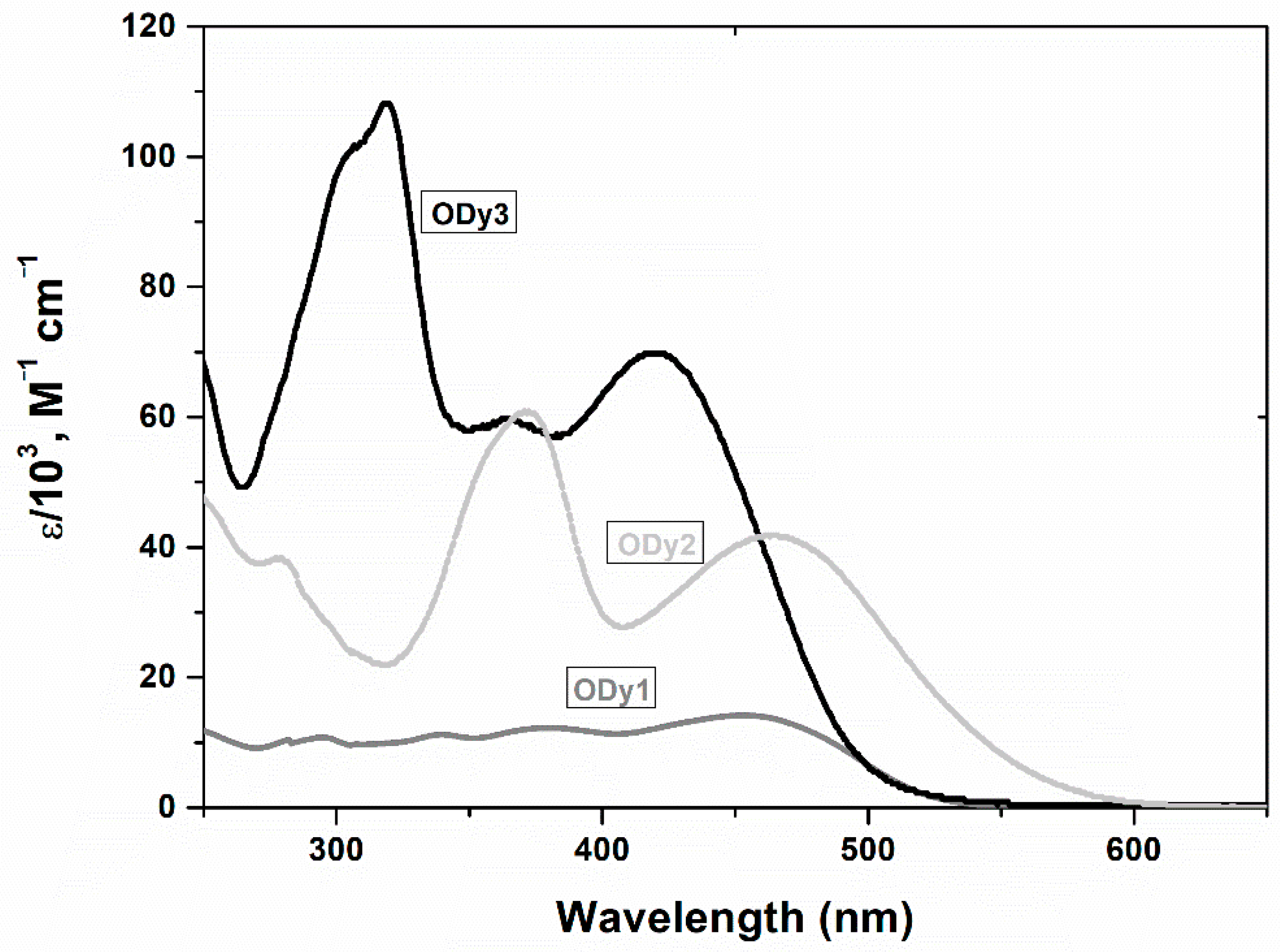
Optical and Electrochemical Properties, Photovoltaic Performance of BTBT-Based Organic Dyes
2.2.2. Dibenzofulvene (DBF)-Based Organic Materials for Dye-Sensitized Solar Cells DSSCs: Synthesis, Structure-Properties Correlation and Cell Performance
Synthesis of Dibenzofulvene (DBF)-Based Organic Dyes
Electro-Optical Properties, Structure-Properties Correlation and Photovoltaic Performance of Dibenzofulvene (DBF)-Based Organic Dyes
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Facchetti, A.; Marks, T.J.; Katz, H.E.; Veinot, J. Organic Semiconductor Materials. In Printed Organic and Molecular Electronics; Gamota, D., Brazis, P., Kalyanasundaram, K., Zhang, J., Eds.; Springer: Boston, MA, USA, 2004; pp. 83–159. [Google Scholar]
- Bronstein, H.; Nielsen, C.B.; Schroeder, B.C.; McCulloch, I. The role of chemical design in the performance of organic semiconductors. Nat. Rev. Chem. 2020, 4, 66–77. [Google Scholar] [CrossRef]
- Oksana, O. Handbook of Organic Materials for Electronic and Photonic Devices, 2nd ed.; Woodhead Publishing: Sawston, UK; Elsevier: Amsterdam, The Netherlands, 2019; pp. 875–891. [Google Scholar]
- Zou, S.-J.; Shen, Y.; Xie, F.-M.; Chen, J.-D.; Li, Y.-Q.; Tang, J.-X. Recent advances in organic light-emitting diodes: Toward smart lighting and displays. Mater. Chem. Front. 2020, 4, 788–820. [Google Scholar] [CrossRef]
- Inganas, O. Organic Photovoltaics over Three Decades. Adv. Mater. 2018, 30, 1800388. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Q.; Gao, J.; Wang, J.; Xu, C.; Ma, X.; Zhang, F. Recent Progress of Organic Photovoltaics with Efficiency over 17%. Energies 2021, 14, 4200. [Google Scholar] [CrossRef]
- Iqbal, J.; Enevold, J.; Larsen, C.; Wang, J.; Revoju, S.; Barzegar, R.H.; Wagberg, T.; Eliasson, B.; Edman, L. An Arylene-vinylene Based Donor-Acceptor-Donor Small Molecule for the Donor Compound in High-Voltage Organic Solar Cells. Sol. Energy Mater. Sol. Cells 2022, 155, 348–355. [Google Scholar] [CrossRef]
- Romero, N.A.; Nicewicz, D.A. Organic Photoredox Catalysis. Chem. Rev. 2016, 17, 10075–10166. [Google Scholar] [CrossRef] [PubMed]
- Melchionna, M.; Fornasiero, P. Updates on the Roadmap for Photocatalysis. ACS Catal. 2020, 10, 5493–5501. [Google Scholar] [CrossRef]
- Huang, T.; Long, M.; Xiao, J.; Liu, H.; Wang, G. Recent research on emerging organic electrode materials for energy storage. Energy Mater. 2021, 1, 100009. [Google Scholar] [CrossRef]
- Lee, S.; Hong, J.; Kang, K. Redox-Active Organic Compounds for Future Sustainable Energy Storage System. Adv. Energy Mater. 2020, 10, 2001445. [Google Scholar] [CrossRef]
- Khandelwal, H.; Schenning, A.P.H.J.; Debije, M.G. Infrared regulating smart window based on organic materials. Adv. Energy Mater. 2017, 7, 1602209. [Google Scholar] [CrossRef] [Green Version]
- Meng, H. Organic Electronics for Electrochromic Materials and Devices; Wiley-VCH GmbH: Weinheim, Germany, 2021; pp. 1–528. [Google Scholar]
- Yu, G.; Gao, J.; Hummelen, J.C.; Wudl, F.; Heeger, A.J. Polymer Photovoltaic Cells: Enhanced Efficiencies via a Network of Internal Donor-Acceptor Heterojunctions. Science 1995, 270, 1789–1791. [Google Scholar] [CrossRef]
- Halls, J.J.M.; Walsh, C.A.; Greenham, N.C.; Marseglia, E.A.; Friend, R.H.; Moratti, S.C.; Holmes, A.B. Efficient photodiodes from interpenetrating polymer networks. Nature 1995, 376, 498–500. [Google Scholar] [CrossRef]
- Günes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated polymer-based organic solar cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.C.; Fréchet, J.M.J. Polymer-fullerene composite solar cells. Angew. Chem. Int. 2007, 47, 58–77. [Google Scholar] [CrossRef]
- Dennler, G.; Scharber, M.C.; Brabec, C.J. Polyer-fullerene bulk-heterojunction solar cells. Adv. Mater. 2009, 21, 1323–1338. [Google Scholar] [CrossRef]
- Sariciftci, N.S.; Smilowitz, L.; Heeger, A.J.; Wudl, F. Photoinduced electron transfer from a conducting polymer to buckminsterfullerene. Science 1992, 258, 1474–1476. [Google Scholar] [CrossRef]
- Van Duren, J.K.J.; Yang, X.; Loos, J.; Bulle-Lieuwma, C.W.T.; Sieval, A.B.; Hummelen, J.C.; Janssen, R.A.J. Relating the morphology of poly(p-phenylenevinylene)/methanofullerene blends to salar-cell performance. Adv. Funct. Mater. 2004, 14, 425–434. [Google Scholar] [CrossRef]
- Babudri, F.; Cardone, A.; De Cola, L.; Farinola, G.M.; Kottas, G.; Martinelli, C.; Naso, F. Synthesis of oligoarylenevinylenes with fluorinated double bonds. Synthesis 2008, 10, 1580–1588. [Google Scholar]
- Babudri, F.; Cardone, A.; Farinola, G.M.; Martinelli, C.; Mendichi, R.; Naso, F.; Striccoli, M. Synthesis of poly(arylenevinylene)s with fluorinated vinylene units. Eur. J. Org. Chem. 2008, 2008, 1977–1982. [Google Scholar] [CrossRef]
- Cardone, A.; Martinelli, C.; Pinto, V.; Babudri, F.; Losurdo, M.; Bruno, G.; Cosma, P.; Naso, F.; Farinola, G.M. Synthesis and characterization of perfluorinated arylenevinylene polymers. J. Polym. Sci. Part. A Polym. Chem. 2010, 48, 285–291. [Google Scholar] [CrossRef]
- Cardone, A.; Martinelli, A.; Babudri, F.; Naso, F.; Pinto, V.; Farinola, G.M. Synthesis of fluorinated (electro)luminescent arylenevinylene polymers and oligomers. Curr. Org. Synth. 2012, 9, 150–162. [Google Scholar] [CrossRef] [Green Version]
- Martinelli, C.; Giovanella, U.; Cardone, A.; Destri, S.; Farinola, G.M. A white emitting poly(phenylenevinylene). Polymer 2014, 55, 5125–5131. [Google Scholar] [CrossRef]
- Losurdo, M.; Giangregorio, M.M.; Capezzuto, P.; Bruno, G.; Babudri, F.; Cardone, A.; Martinelli, C.; Farinola, G.M.; Naso, F.; Büchel, M. Impact of fluorinated vinylene units on supramolecular organization and optical properties of poly(phenylenevinylene) thin film as a class of blue band gap conjugated polymers. Polymer 2008, 49, 4133–4140. [Google Scholar] [CrossRef]
- Piacenza, M.; Comoretto, D.; Burger, M.; Morandi, V.; Marabelli, F.; Martinelli, C.; Farinola, G.M.; Cardone, A.; Gigli, G.; Della Sala, F. Raman spectra of poly(p-phenylenevinylene)s with fluorinated vinylene units: Evidence of inter-ring distorsion. Chem. Phys. Chem. 2009, 10, 1284–1290. [Google Scholar] [CrossRef]
- Losurdo, M.; Giangregorio, M.M.; Capezzuto, P.; Cardone, A.; Martinelli, C.; Farinola, G.M.; Babudri, F.; Naso, F.; Buchel, M.; Bruno, G. Blue-gap poly(p-phenylenevinylene)s with fluorinated double bonds: Interplay between supramolecular organization and optical properties in thin films. Adv. Mater. 2009, 21, 1115–1120. [Google Scholar] [CrossRef]
- Milad, R.; Shi, J.; Aguirre, A.; Cardone, A.; Milián-Medina, B.; Farinola, G.M.; Abderrabba, M.; Gierschner, J. Effective conjugation in conjugated polymers with strongly twisted backbones: A case study on fluorinated MEHPPV. J. Mater. Chem. C 2016, 4, 6900–6906. [Google Scholar] [CrossRef]
- Burger, M.; Floris, F.; Cardone, A.; Farinola, G.M.; Morandi, V.; Marabelli, F.; Comoretto, D. Photo-induced absorption spectra of a poly(p-phenylenevinylene) polymer with fluorinated double bonds. Org. Electr. 2017, 43, 214–221. [Google Scholar] [CrossRef]
- Cardone, A.; Martinelle, C.; Losurdo, M.; Dilonardo, E.; Bruno, G.; Scavia, G.; Destri, S.; Cosma, P.; Salamandra, L.; Reale, A.; et al. Fluoro-functionalization of vinylene units in a polyarylenevinylene for polymer solar cells. J. Mater. Chem. A 2013, 1, 715–727. [Google Scholar] [CrossRef]
- Gourley, K.D.; Lillya, C.P.; Reynolds, J.R.; Chien, J.C.W. Electrically conducting polymers: Arsenic pentafluoride-doped poly(phenylenevinylene) and its analogs. Macromolecules 1984, 17, 1025–1033. [Google Scholar] [CrossRef]
- Chen, Z.-K.; Meng, H.; Lai, Y.-H.; Huang, W. Photoluminescent poly(p-phenylenevinylene)s with an aromatic oxadiazole moiety as the side chain: Synthesis, electrochemistry and spectroscopy study. Macromolecules 1999, 32, 4351–4358. [Google Scholar] [CrossRef]
- Ahn, T.; Song, S.-Y.; Shim, H.-K. Highly photoluminescent and blue-green electroluminescent polymers: New silyl- and alkoxy-substituted poly(p-phenylenevinylene) related copolymers containing carbazole or fluorene groups. Macromolecules 2000, 33, 6764–6771. [Google Scholar] [CrossRef]
- Greenham, N.C.; Moratti, S.C.; Bradley, D.D.C.; Friend, R.H.; Holmes, A.B. Efficient light-emitting diodes based on polymers with high electron affinities. Nature 1993, 365, 628–630. [Google Scholar] [CrossRef]
- Moratti, S.C.; Cervini, R.; Holmes, A.B.; Baigent, D.R.; Friend, R.H.; Greenham, N.C.; Gruner, J.; Hamer, P.J. High electron affinity polymers for LEDs. Synth. Met. 1995, 71, 2117–2120. [Google Scholar] [CrossRef]
- Chen, S.-A.; Chang, E.-C. Structure and properties of cyano-substituted poly(2,5-dialkoxy-p-phenylene vinylene)s. Macromolecules 1998, 31, 4899–4907. [Google Scholar] [CrossRef]
- Jin, Y.; Ju, J.; Kim, J.; Lee, S.; Kim, J.Y.; Park, S.H.; Son, S.-M.; Jin, S.-H.; Lee, K.; Suh, H. Design, synthesis and electroluminescent property of CN-poly(dihexylfluorenevinylene) for LEDs. Macromolecules 2003, 36, 6970–6975. [Google Scholar] [CrossRef]
- Gilch, H.G.; Wheelwright, W.L. Polymerization of -halogenated p-xylenes with base. J. Polym. Sci. A Polym. Chem. 1966, 4, 1337–1349. [Google Scholar] [CrossRef]
- Wessling, R.A. The polymerization of xylylene bisdialkyl sulfonium salts. J. Polym. Sci. Polym. Symp. 1985, 72, 55–66. [Google Scholar] [CrossRef]
- Babudri, F.; Cicco, S.R.; Farinola, G.; Naso, F. Synthesis, characterization and properties of a soluble polymer with a poly(phenylenevinylene) structure. Macromol. Chem. Commun. 1996, 17, 905–911. [Google Scholar] [CrossRef]
- Babudri, F.; Cardone, A.; Chiavarone, L.; Ciccarella, G.; Farinola, G.M.; Naso, F.; Scamarcio, G. Synthesis and characterization of poly(2,3,5,6-tetrafluoro-1,4-phenylenevinylene). Chem. Commun. 2001, 1940–1941. [Google Scholar] [CrossRef]
- Babudri, F.; Cardone, A.; Farinola, G.M.; Naso, F.; Cassano, T.; Chiavarone, L.; Tommasi, R. Synthesis and optical properties of a copolymer of tetrafluoro- and dialkoxy-substituted poly(p-phenylenevinylene) with a high percentage of fluorinated units. Macromol. Chem. Phys. 2003, 204, 1621–1627. [Google Scholar] [CrossRef]
- Liang, Y.; Feng, D.; Wu, Y.; Tsai, S.-T.; Li, G.; Ray, C.; Yu, L. Highly efficient solar cell polymers developed via fine-tuning of structural and electronic properties. J. Am. Chem. Soc. 2009, 131, 7792–7799. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Yu, L. A new class of semiconducting polymers for bulk heterojunction solar cells with exceptionally high performance. Acc. Chem. Res. 2010, 43, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Price, S.C.; Steuart, A.C.; Yang, L.; Zhou, H.; You, W. Fluorine substituted conjugated polymer of medium band gap yields 7% efficiency in polymer-fullerene solar cells. J. Am. Chem. Soc. 2011, 133, 4625–4631. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.C.; Huang, Z.G.; Ashraf, R.S.; Smith, J.; D’Angelo, P.; Watkins, S.E.; Anthopoulos, T.D.; Durrant, J.R.; McCulloch, I. Silaindacenodithiophene-based low band gap polymers-the effect of fluorine substitution on device performance and film morphologies. Adv. Funct. Mater. 2012, 22, 1663–1670. [Google Scholar] [CrossRef]
- Piacenza, M.; Della Sala, F.; Farinola, G.M.; Martinelli, C.; Gigli, G. Large blue-shift in the optical spectra of fluorinated polyphenylenevinylenes. A combined theoretical and experimental study. J. Phys. Chem. B 2008, 112, 2996–3004. [Google Scholar] [CrossRef] [PubMed]
- Facchetti, A.; Yoon, M.H.; Stern, C.L.; Katz, H.E.; Marks, T.J. Building blocks for n-type organic electronics: Regiochemically modulated inversion of majority carrier sign in perfluoroarene-modified polythiophene semiconductors. Angew. Chem. Int. Ed. 2003, 42, 3900–3903. [Google Scholar] [CrossRef]
- Curti, M.D.; Cao, J.; Kampf, J.W. Solid-state packing of conjugated oligomers: From -stacks to the herringbone structure. J. Am. Chem. Soc. 2004, 126, 4318–4328. [Google Scholar] [CrossRef]
- Reichenbacher, K.; Suss, H.I.; Hullinger, J. Fluorine in crystals engineering-“the little atom that could”. Chem. Soc. Rev. 2005, 34, 22–30. [Google Scholar] [CrossRef]
- Gierschner, J.; Ehni, M.; Egelhaaf, H.-J.; Milián-Medina, B.; Beljonne, D.; Benmansour, H.; Bazan, G.C. Solid-state optical properties of linear polyconjugated molecules: -stack contra herringbone. J. Chem. Phys. 2005, 123, 144914. [Google Scholar] [CrossRef]
- Wang, Y.; Parkin, S.R.; Gierschner, J.; Watson, M.D. Highly fluorinated benzobisbenzothiophenes. Org. Lett. 2008, 10, 3307–3310. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A Low-Cost, High-Efficiency Solar Cell Based on Dye-Sensitized Colloidal TiO2 Films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Benkö, G.; Kallioinen, J.; Korppi-Tommola, J.E.I.; Yartsev, A.P.; Sundström, V. Photoinduced ultrafast dye-to-semiconductor electron injection from nonthermalized and thermalized donor states. J. Am. Chem. Soc. 2002, 124, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Sarto Polo, A.; Itokazu, M.K.; Iha, N.Y.M. Metal complex sensitizers in dye-sensitized solar cells. Coord. Chem. Rev. 2004, 248, 1343–1361. [Google Scholar] [CrossRef]
- Yum, J.-H.; Jung, I.; Baik, C.; Ko, J.; Nazeeruddin, M.K.; Gratzel, M. High efficient donor–acceptor ruthenium complex for dye-sensitized solar cell applications. Energy Environ. Sci. 2009, 2, 100–102. [Google Scholar] [CrossRef]
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; Curchod, B.F.E.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; Nazeeruddin, M.K.; Grätzel, M. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 2014, 6, 242–247. [Google Scholar] [CrossRef]
- Qu, S.Y.; Wu, W.J.; Hua, J.L.; Kong, C.; Long, Y.T.; Tian, H. New Diketopyrrolopyrrole (DPP) Dyes for Efficient Dye-Sensitized Solar Cells. J. Phys. Chem. C 2010, 114, 1343–1349. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.C.; Kloo, L.; Petterson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Kozma, E.; Concina, I.; Braga, A.; Borgese, L.; Depero, L.E.; Vomiero, A.; Sberveglieri, G.; Catellani, M. Metal-free organic sensitizers with a sterically hindered thiophene unit for efficient dye-sensitized solar cells. J. Mater. Chem. 2011, 21, 13785–13788. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhang, F.; Grätzel, M.; Meng, S. Structure–Property Relations in All-Organic Dye-Sensitized Solar Cells. Adv. Funct. Mater. 2013, 23, 424–429. [Google Scholar] [CrossRef]
- Kim, D.; Ghhcov, A.; Albu, S.P.; Schmuki, P. Bamboo-Type TiO2 Nanotubes: Improved Conversion Efficiency in Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2008, 130, 16454–16455. [Google Scholar] [CrossRef]
- Sauvage, F.; Di Fonzo, F.; Bassi, A.L.; Casari, C.S.; Russo, V.; Divitini, G.; Ducati, C.; Bottani, C.E.; Comte, P.; Grätzel, M. Hierarchical TiO2 Photoanode for Dye-Sensitized Solar Cells. Nano Lett. 2010, 10, 2562–2567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.X.; Wu, W.J.; Hua, J.L.; Jiang, Y.H.; Qu, S.Y.; Li, J.; Long, Y.T.; Tian, H. Bithiazole-bridged dyes for dye-sensitized solar cells with high open circuit voltage performance. J. Mater. Chem. 2011, 21, 6054–6062. [Google Scholar] [CrossRef]
- Pei, K.; Wu, Y.; Islam, A.; Zhang, Q.; Han, L.; Tian, H.; Zhu, W. Constructing High-Efficiency D–A−π–A-Featured Solar Cell Sensitizers: A Promising Building Block of 2,3-Diphenylquinoxaline for Antiaggregation and Photostability. ACS Appl. Mater. Interfaces 2013, 5, 4986–4995. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Thomas, K.R.J.; Lee, C.-P.; Ho, K.-C. Organic Dyes Containing Fluorene Decorated with Imidazole Units for Dye-Sensitized Solar Cells. J. Org. Chem. 2014, 79, 3159–3172. [Google Scholar] [CrossRef]
- Huang, Z.-S.; Cai, C.; Zang, X.-F.; Iqbal, Z.; Zeng, H.; Kuang, D.-B.; Wang, L.; Meier, H.; Cao, D. Effect of the linkage location in double branched organic dyes on the photovoltaic performance of DSSCs. J. Mater. Chem. A 2015, 3, 1333–1344. [Google Scholar] [CrossRef]
- Qian, X.; Gao, H.-H.; Zhu, Y.-Z.; Lu, L.; Zheng, J.-Y. 6H-Indolo[2,3-b]quinoxaline-based organic dyes containing different electron-rich conjugated linkers for highly efficient dye-sensitized solar cells. J. Power Sources 2015, 280, 573–580. [Google Scholar] [CrossRef]
- Li, X.; Zheng, Z.; Jiang, W.; Wu, W.; Wang, Z.; Tian, H. New D–A–π–A organic sensitizers for efficient dye-sensitized solar cells. Chem. Commun. 2015, 51, 3590–3592. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Liu, J.-M.; Huang, J.-F.; Tan, L.-L.; Shen, Y.; Xiao, L.-M.; Kuanga, D.-B.; Su, C.-Y. Stable organic dyes based on the benzo[1,2-b:4,5-b′]dithiophene donor for efficient dye-sensitized solar cells. J. Mater. Chem. A 2015, 3, 8083–8090. [Google Scholar] [CrossRef]
- Zhou, N.; Prabakaran, K.; Lee, B.; Chang, S.H.; Harutyunyan, B.; Guo, P.; Butler, M.R.; Timalsina, A.; Bedzyk, M.J.; Ratner, M.A.; et al. Metal-Free Tetrathienoacene Sensitizers for High-Performance Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2015, 137, 4414–4423. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.; Xu, M.; Ma, W.; Li, R.; Wang, P. Design of high-efficiency organic dyes for titania solar cells based on the chromophoric core of cyclopentadithiophene-benzothiadiazole. Energy Environ. Sci. 2013, 6, 2944–2949. [Google Scholar] [CrossRef]
- Yang, L.; Zheng, Z.; Li, Y.; Wu, W.; Tian, H.; Wang, Z. N-Annulated perylene-based metal-free organic sensitizers for dye-sensitized solar cells. Chem. Commun. 2015, 51, 4842–4845. [Google Scholar] [CrossRef] [PubMed]
- Capodilupo, A.L.; De Marco, L.; Fabiano, E.; Giannuzzi, R.; Scrascia, A.; Carlucci, C.; Corrente, G.A.; Cipolla, M.P.; Gigli, G.; Ciccarella, G. New organic dyes based on a dibenzofulvene bridge for highly efficient dye-sensitized solar cells. J. Mater. Chem. A 2014, 2, 14181–14188. [Google Scholar]
- Huang, Z.-S.; Feng, H.-L.; Zang, X.-F.; Iqbal, Z.; Zeng, H.; Kuang, D.-B.; Wang, L.; Meierd, H.; Cao, D. Dithienopyrrolobenzothiadiazole-based organic dyes for efficient dye-sensitized solar cells. J. Mater. Chem. A 2014, 2, 15365–15376. [Google Scholar] [CrossRef]
- Choi, H.; Shin, M.; Song, K.; Kang, M.-S.; Kang, Y.; Ko, J. The impact of an indeno[1,2-b]thiophene spacer on dye-sensitized solar cell performances of cyclic thiourea functionalized organic sensitizers. J. Mater. Chem. A 2014, 2, 12931–12939. [Google Scholar] [CrossRef]
- Su, J.-Y.; Lo, C.-Y.; Tsai, C.-H.; Chen, C.-H.; Chou, S.-H.; Liu, S.-H.; Chou, P.-T.; Wong, K.-T. Indolo[2,3-b]carbazole Synthesized from a Double-Intramolecular Buchwald–Hartwig Reaction: Its Application for a Dianchor DSSC Organic Dye. Org. Lett. 2014, 16, 3176–3179. [Google Scholar] [CrossRef]
- Takimiya, K.; Ebata, H.; Sakamoto, K.; Izawa, T.; Otsubo, T.; Jungi, Y. 2,7-Diphenyl[1]benzothieno[3,2-b]benzothiophene, A New Organic Semiconductor for Air-Stable Organic Field-Effect Transistors with Mobilities up to 2.0 cm2 V−1 s−1. J. Am. Chem. Soc. 2006, 128, 12604–12605. [Google Scholar] [CrossRef]
- Ebata, H.; Izawa, T.; Miyazaki, E.; Takimiya, K.; Ikeda, M.; Kuwabara, H.; Yui, T. Highly Soluble [1]Benzothieno[3,2-b]benzothiophene (BTBT) Derivatives for High-Performance, Solution-Processed Organic Field-Effect Transistors. J. Am. Chem. Soc. 2007, 129, 15732–15733. [Google Scholar] [CrossRef]
- Izawa, T.; Miyazaki, E.; Takimiya, K. Molecular Ordering of High-Performance Soluble Molecular Semiconductors and Re-evaluation of Their Field-Effect Transistor Characteristics. Adv. Mater. 2008, 20, 3388–3392. [Google Scholar] [CrossRef]
- Capodilupo, A.L.; Fabiano, E.; De Marco, L.; Ciccarella, G.; Gigli, G.; Martinelli, C.; Cardone, A. [1]Benzothieno[3,2-b]benzothiophene-Based Organic Dyes for Dye-Sensitized Solar Cells. J. Org. Chem. 2016, 81, 3235–3245. [Google Scholar] [CrossRef]
- Ito, S.; Zakeeruddin, S.M.; Humphry-Baker, R.; Liska, P.; Charvet, R.; Comte, P.; Nazeeruddin, M.K.; Péchy, P.; Takata, M.; Miura, H.; et al. High-Efficiency Organic-Dye- Sensitized Solar Cells Controlled by Nanocrystalline-TiO2 Electrode Thickness. Adv. Mater. 2006, 18, 1202–1205. [Google Scholar] [CrossRef]
- Gao, P.; Tsao, H.N.; Grätzel, M.; Nazeeruddin, M.K. Fine-tuning the electronic structure of organic dyes for dye-sensitized solar cells. Org. Lett. 2012, 14, 4330–4333. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Lee, M.J.; Song, H.M.; Song, B.J.; Seo, K.D.; Pastore, M.; Anselmi, C.; Fantacci, S.; De Angelis, F.; Nazeeruddin, M.K.; et al. Organic dyes incorporating low-band-gap chromophores based on π-extended benzothiadiazole for dye-sensitized solar cells. Dyes Pigm. 2011, 91, 192–198. [Google Scholar] [CrossRef]
- Wu, Y.; Marszalek, M.; Zakeeruddin, S.M.; Zhang, Q.; Tian, H.; Gratzel, M.; Zhu, W. High-conversion-efficiency organic dye-sensitized solar cells: Molecular engineering on D–A–π-A featured organic indoline dyes. Energy Environ. Sci. 2012, 5, 8261–8272. [Google Scholar] [CrossRef]
- Haid, S.; Marszalek, M.; Mishra, A.; Wielopolski, M.; Teuscher, J.; Moser, J.-E.; Humphry-Baker, R.; Zakeeruddin, S.M.; Grätzel, M.; Bauerle, P. Significant Improvement of Dye-Sensitized Solar Cell Performance by Small Structural Modification in π-Conjugated Donor–Acceptor Dyes. Adv. Funct. Mater. 2012, 22, 1291–1302. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, M.; Li, R.; Yang, L.; Qiao, Y.; Wang, P. A Metal-Free N-Annulated Thienocyclopentaperylene Dye: Power Conversion Efficiency of 12% for Dye-Sensitized Solar Cells. Angew. Chem. Int. Ed. 2015, 54, 5994–5998. [Google Scholar] [CrossRef]
- Baldoli, C.; Bertuolo, S.; Licandro, E.; Viglianti, L.; Mussini, P.; Marotta, G.; Salvatori, P.; De Angelis, F.; Manca, P.; Manfredi, N.; et al. Benzodithiophene based organic dyes for DSSC: Effect of alkyl chain substitution on dye efficiency. Dyes Pigm. 2015, 121, 351–362. [Google Scholar] [CrossRef]
- Baheti, A.; Gajjela, S.R.; Balaya, P.; Justin Thomas, K.R. Synthesis, optical, electrochemical and photovoltaic properties of organic dyes containing trifluorenylamine donors. Dyes Pigm. 2015, 113, 78–86. [Google Scholar] [CrossRef]
- Justin Thomas, K.R.; Venkateswararao, A.; Lee, C.-P.; Ho, K.-C. Organic dyes containing fluoreneamine donor and carbazole π-linker for dye-sensitized solar cells. Dyes Pigm. 2015, 123, 154–165. [Google Scholar] [CrossRef]
- Kumar, S.; Justin Thomas, K.R.; Li, C.-T.; Ho, K.-C. Synthesis and photovoltaic properties of organic dyes containing N-fluoren-2-yl dithieno[3,2-b:2′,3′-d]pyrrole and different donors. Org. Electron. 2015, 26, 109–116. [Google Scholar] [CrossRef]
- Liu, J.; Sun, X.; Li, Z.; Jin, B.; Lai, G.; Li, H.; Wang, C.; Shen, Y.; Hua, J. New D–π-A system dye based on dithienosilole and carbazole: Synthesis, photo-electrochemical properties and dye-sensitized solar cell performance. J. Photochem. Photobiol. A 2014, 294, 54–61. [Google Scholar] [CrossRef]
- Chung, C.-L.; Chen, C.-H.; Tsai, C.-H.; Wonga, K.-T. Novel organic dyes containing N-bridged oligothiophene coplanar cores for dye-sensitized solar cells. Org. Electron. 2015, 18, 8–16. [Google Scholar] [CrossRef]
- Kumar, D.; Justin Thomas, K.R.; Lee, C.-P.; Ho, K.-C. Triarylamine-Free Pyrenoimidazole-Containing Organic Dyes with Different π-Linkers for Dye-Sensitized Solar Cells. Asian J. Org. Chem. 2015, 4, 164–172. [Google Scholar] [CrossRef]
- Baheti, A.; Justin Thomas, K.R.; Li, C.-T.; Lee, C.-P.; Ho, K.-C. Fluorene-Based Sensitizers with a Phenothiazine Donor: Effect of Mode of Donor Tethering on the Performance of Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 2249–2262. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ying, W.; Zhang, X.; Hu, Y.; Wu, W.; Hua, J. Near-infrared absorbing isoindigo sensitizers: Synthesis and performance for dye-sensitized solar cells. Dyes Pigm. 2015, 112, 327–334. [Google Scholar] [CrossRef]
- Heeney, M.; Bailey, C.; Giles, M.; Shkunov, M.; Sparrowe, D.; Tierney, S.; Zhang, W.; McCulloch, I. Alkylidene Fluorene Liquid Crystalline Semiconducting Polymers for Organic Field Effect Transistor Devices. Macromolecules 2004, 37, 5250–5256. [Google Scholar] [CrossRef]
- Li, Q.; Blancafort, L. A conical intersection model to explain aggregation induced emission in diphenyl dibenzofulvene. Chem. Commun. 2013, 49, 5966–5968. [Google Scholar] [CrossRef]
- Du, C.; Li, C.; Li, W.; Chen, X.; Bo, Z.; Veit, C.; Ma, Z.; Wuefei, U.; Zhu, H.; Hu, W.; et al. 9-Alkylidene-9H-Fluorene-Containing Polymer for High-Efficiency Polymer Solar Cells. Macromolecules 2011, 44, 7617–7624. [Google Scholar] [CrossRef]
- Liu, L.-Q.; Zhang, G.-C.; Liu, P.; Zhang, J.; Dong, S.; Wang, M.; Ma, Y.-G.; Yip, H.-L.; Huang, F. Donor–Acceptor-Type Copolymers Based on a Naphtho[1,2-c:5,6-c]bis(1,2,5-thiadiazole) Scaffold for High-Efficiency Polymer Solar Cells. Chem. Asian J. 2014, 9, 2104–2112. [Google Scholar] [CrossRef]
- Choi, M.-H.; Song, K.W.; Moon, D.K. Alkylidenefluorene–isoindigo copolymers with an optimized molecular conformation for spacer manipulation, π–π stacking and their application in efficient photovoltaic devices. Polym. Chem. 2015, 6, 2636–2646. [Google Scholar] [CrossRef]
- Capodilupo, A.L.; Giannuzzi, R.; Corrente, G.A.; De Marco, L.; Fabiano, E.; Cardone, A.; Gigli, G.; Ciccarella, G. Synthesis and photovoltaic performance of dibenzofulvene-based organic sensitizers for DSSC. Tetrahedron 2016, 72, 5788–5797. [Google Scholar] [CrossRef]
- Capodilupo, A.L.; De Marco, L.; Corrente, G.A.; Giannuzzi, R.; Fabiano, E.; Cardone, A.; Gigli, G.; Ciccarella, G. Synthesis and characterization of a new series of dibenzofulvene based organic dyes for DSSCs. Dyes Pigm. 2016, 130, 79–89. [Google Scholar] [CrossRef]
- Corrente, G.A.; Fabiano, E.; De Marco, L.; Accorsi, G.; Giannuzzi, R.; Cardone, A.; Gigli, G.; Ciccarella, G.; Capodilupo, A.L. Effects of donor position on dibenzofulvene-based organic dyes for photovoltaics. J. Mater Sci. Mater. Electron. 2017, 28, 8694–8707. [Google Scholar] [CrossRef]
- Mahmood, A.; Wang, J.-L. Machine learning for high performance organic solar cells: Current scenario and future prospects. Energy Environ. Sci. 2021, 14, 90–105. [Google Scholar] [CrossRef]
- Mahmood, A.; Irfan, A.; Wang, J.-L. Machine learning for Organic Photovoltaic Polymers: A Minireview. Chin. J. Polym. Sci. 2022, 40, 870–876. [Google Scholar] [CrossRef]
- Mahmood, A.; Irfan, A.; Wang, J.-L. Machine learning and molecular dynamics simulation-assisted evolutionary design and discovery pipeline to screen efficient small molecule acceptors for PTB7-Th-based organic solar cells with over 15% efficiency. J. Mater. Chem. A 2022, 10, 4170–4180. [Google Scholar] [CrossRef]
- Mahmood, A.; Irfan, A.; Wang, J.-L. Developing Efficient Small Molecule Acceptors with sp2-Hybridized Nitrogen at Different Positions by Density Functional Theory Calculations, Molecular Dynamics Simulations and Machine Learning. Chem. A Eur. J. 2022, 28, e202103712. [Google Scholar] [CrossRef]




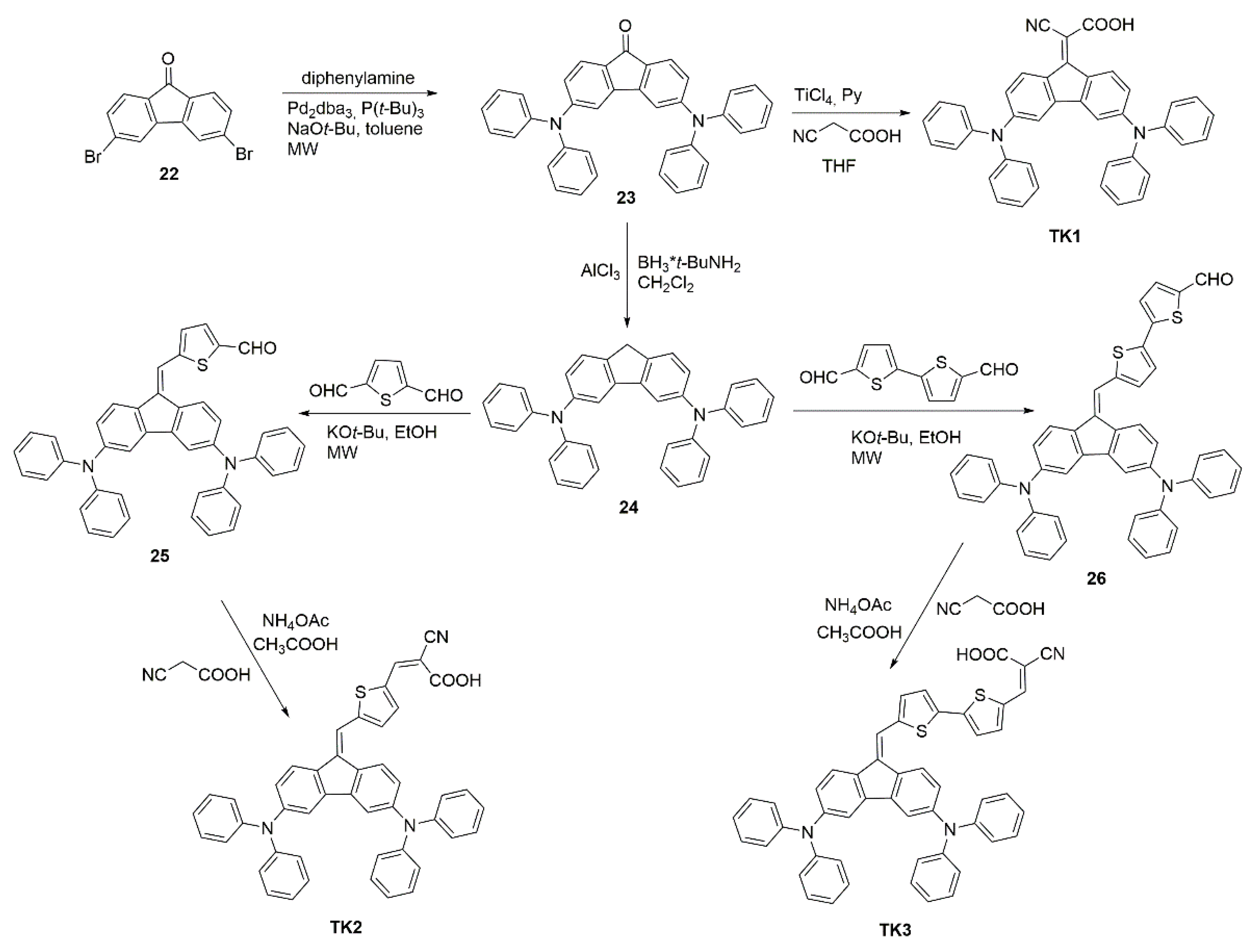
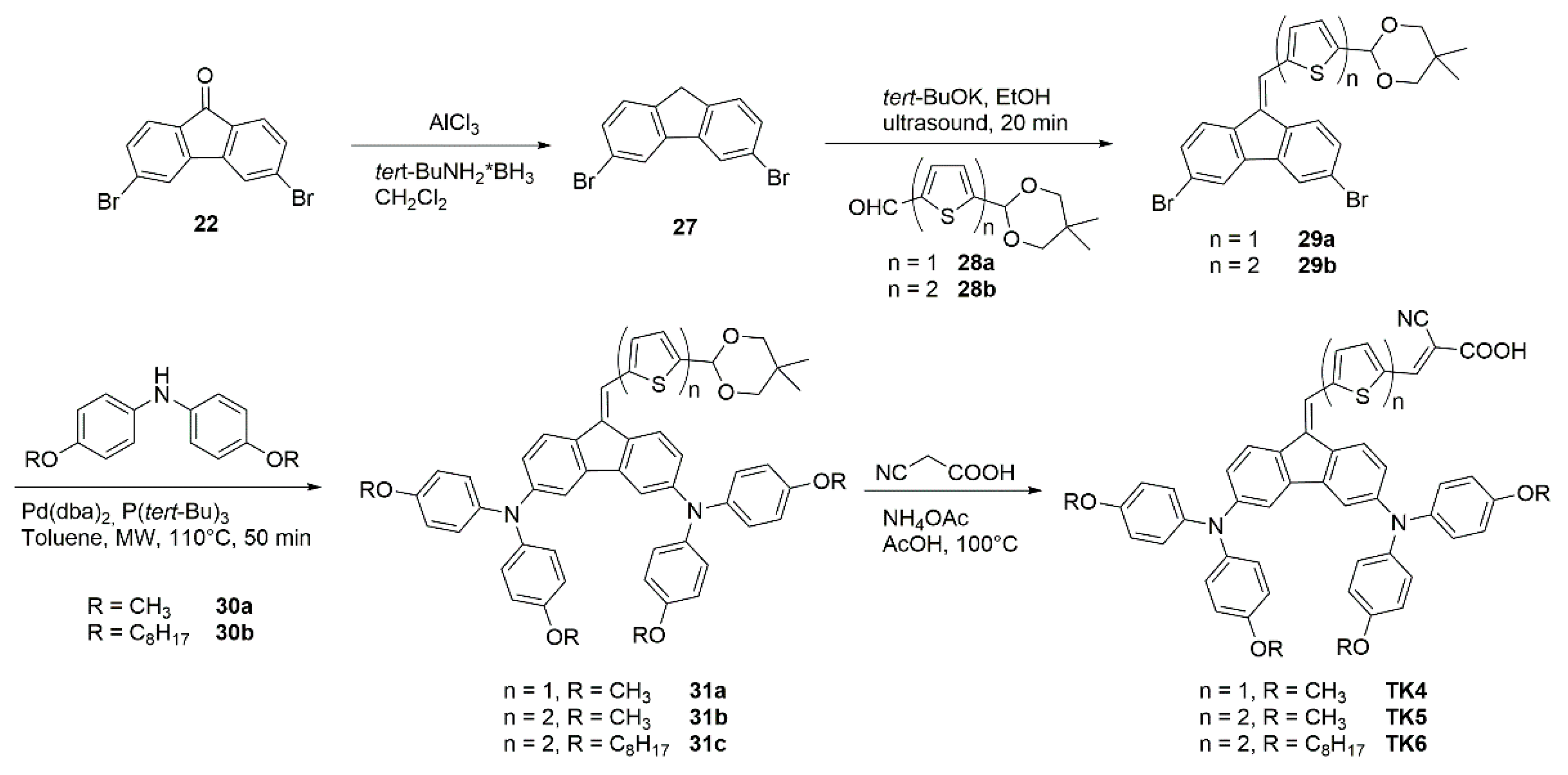
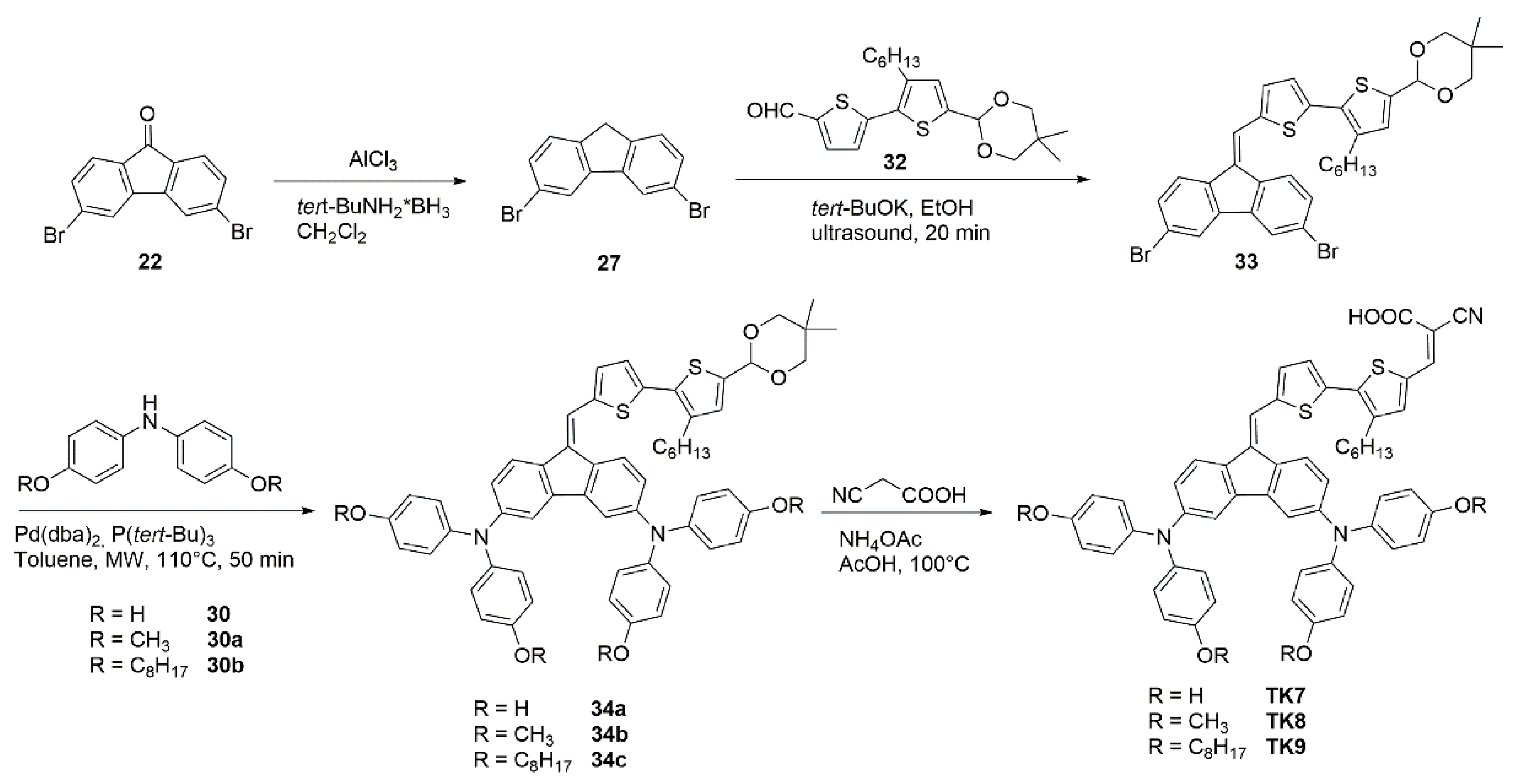
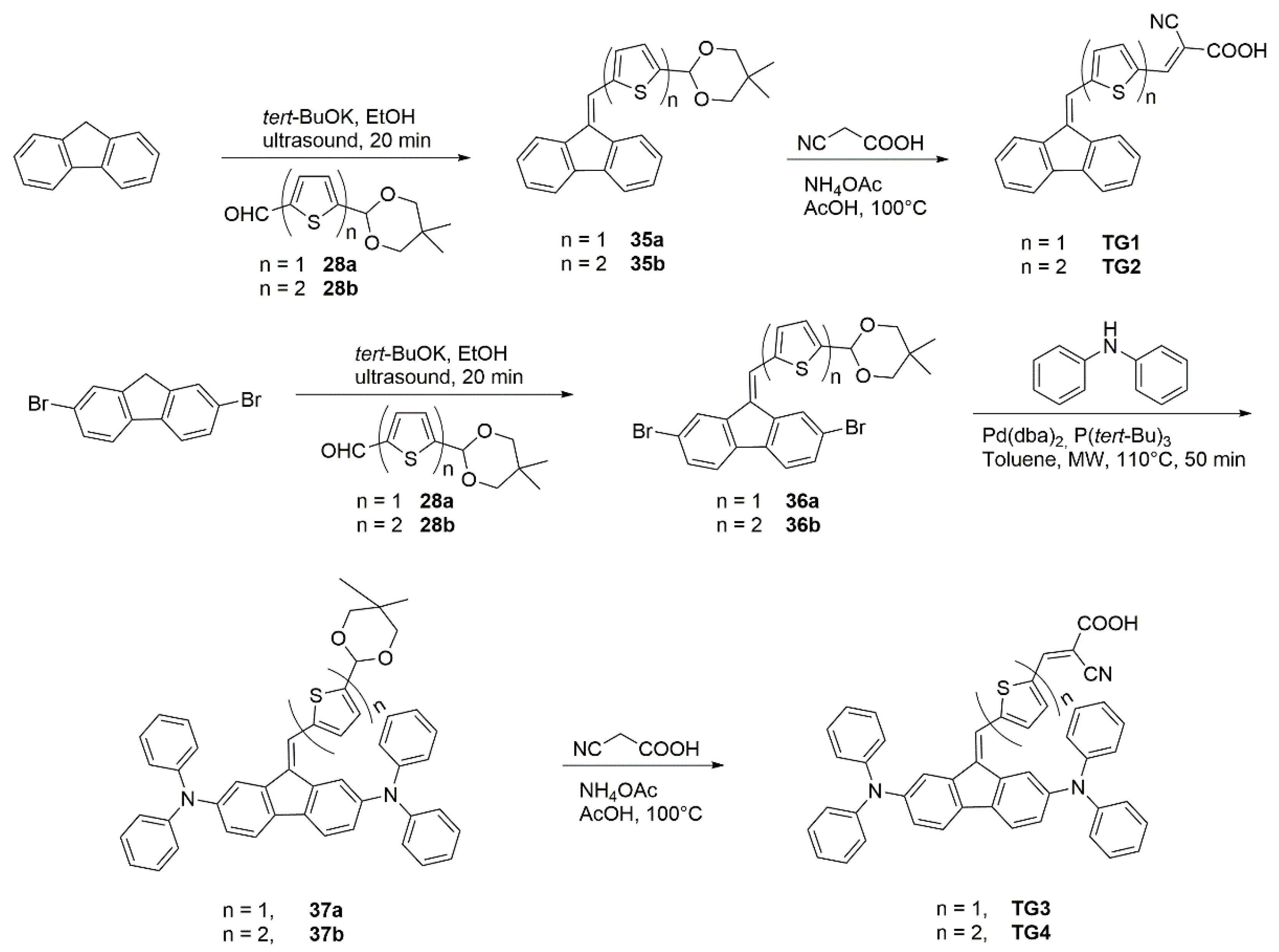

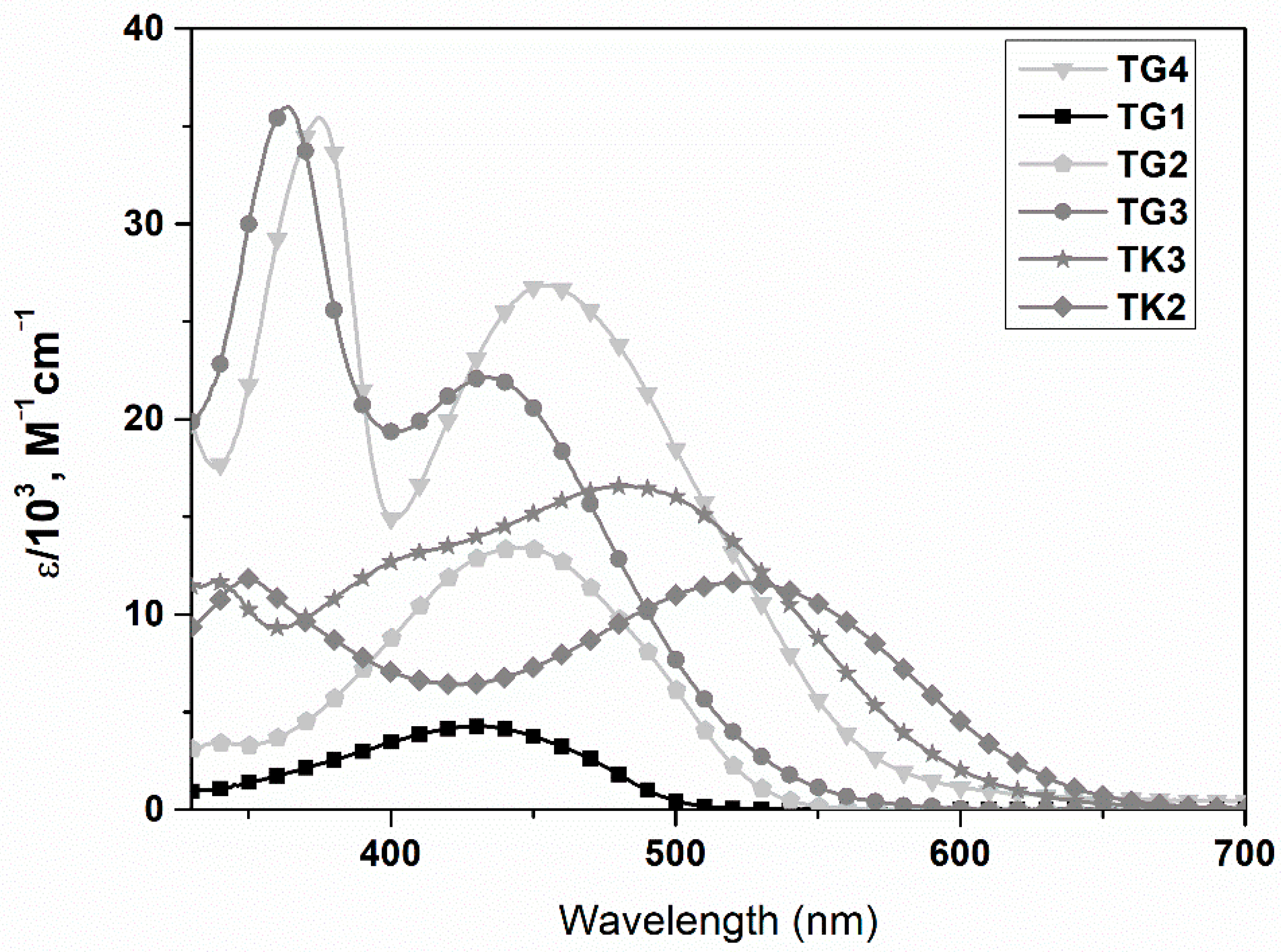
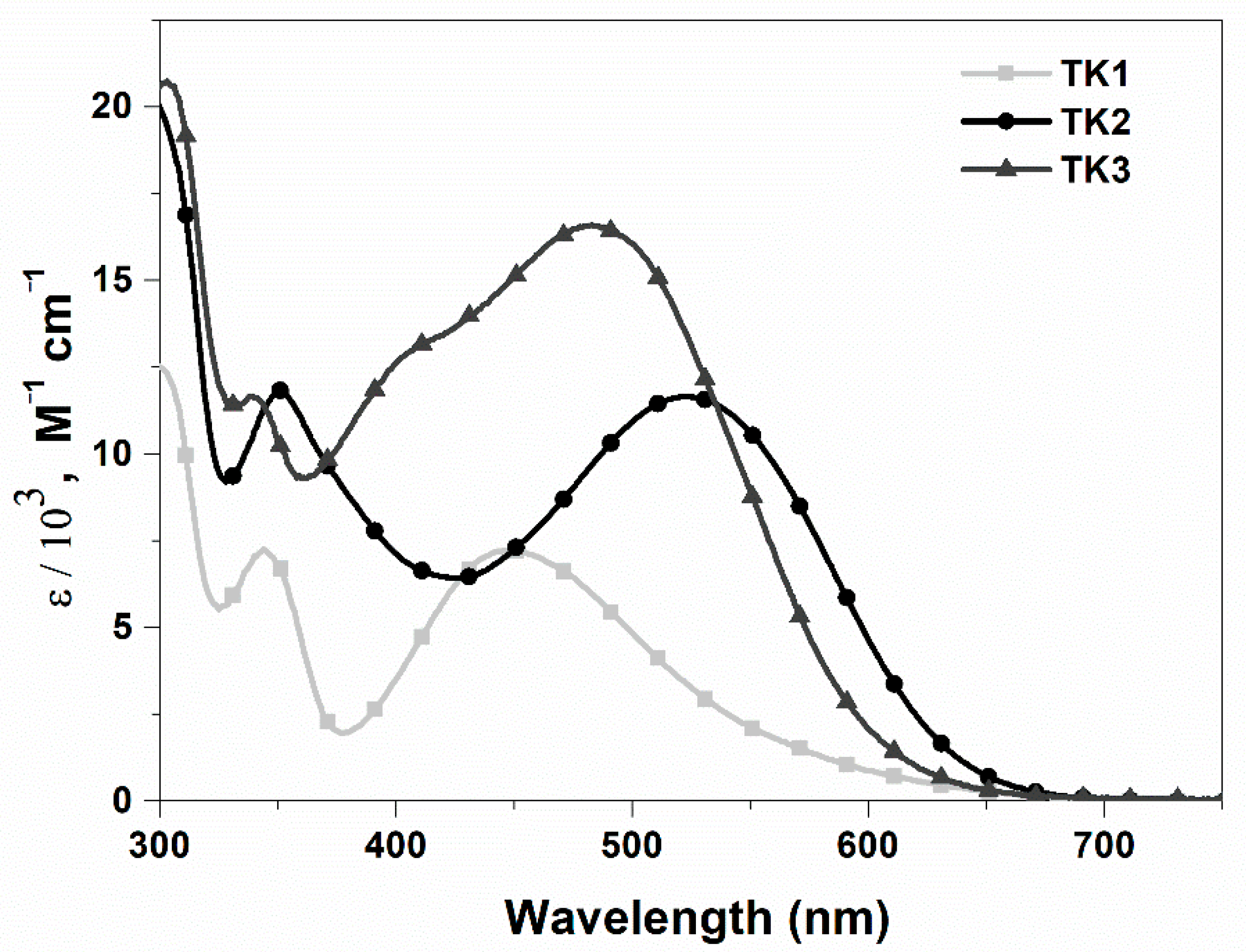

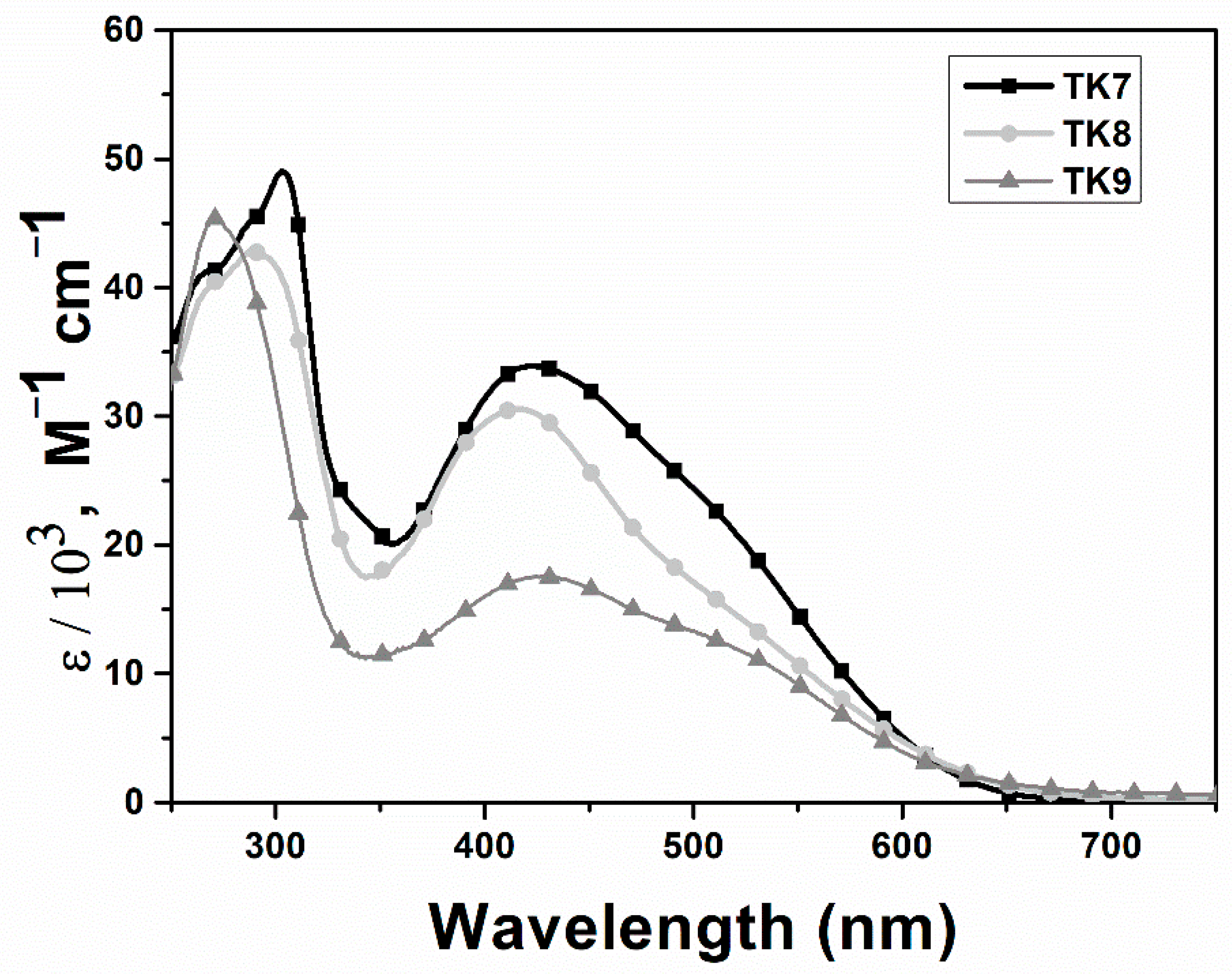
| Polymer | Polymer/PCBM Weight Ratio | Voc [V] | Isc [mA cm−2] | FF [%] | η [%] |
|---|---|---|---|---|---|
| 2:7 | 0.73 | 2.94 | 38.57 | 0.83 | |
| PDTBTFV | 3:7 | 0.75 | 3.55 | 37.97 | 1.05 |
| 1:1 | 0.83 | 4.32 | 34.56 | 1.24 | |
| 2:7 | 0.55 | 3.33 | 28.83 | 0.53 | |
| PDTBTV | 3:7 | 0.56 | 2.42 | 24.50 | 0.33 |
| 1:1 | 0.43 | 3.15 | 33.51 | 0.46 |
| Dye | λmax (nm) a | ε (M−1 cm−1) b | HOMO (V) (vs. NHE) | LUMO (V) (vs. NHE) | Eg (eV) |
|---|---|---|---|---|---|
| ODy1 | 453 | 14,190 | 1.22 | −1.16 | 2.38 |
| ODy2 | 454/372 | 42,130/60,890 | 0.85 | −1.36 | 2.21 |
| ODy3 | 420/320 | 69,800/108,120 | 0.75 | −1.75 | 2.50 |
| Dye | CDCA (mM) | PCE (%) | Voc (V) | Jsc (mA/cm2) | FF | Dye Loading (mol/cm2) |
|---|---|---|---|---|---|---|
| ODy1 | 0 | 4.20 | 0.704 | 9.18 | 0.65 | 2.9 × 10−7 |
| 10 | 5.16 | 0.735 | 10.33 | 0.68 | 2.4 × 10−7 | |
| ODy2 | 0 | 5.76 | 0.736 | 11.50 | 0.68 | 3.0 × 10−7 |
| 10 | 6.25 | 0.765 | 11.85 | 0.69 | 2.4 × 10−7 | |
| ODy3 | 0 | 4.54 | 0.689 | 9.56 | 0.69 | 2.8 × 10−7 |
| 10 | 5.00 | 0.719 | 9.67 | 0.72 | 2.2 × 10−7 |
| Dye | η (%) | Voc (V) | Jsc (mA/cm2) | FF |
|---|---|---|---|---|
| TG1 | 1.23 | 0.56 | 3.09 | 0.71 |
| TG2 | 2.07 | 0.61 | 5.22 | 0.65 |
| TG3 | 3.91 | 0.73 | 7.50 | 0.71 |
| TG4 | 2.68 | 0.65 | 5.37 | 0.77 |
| TK2 | 5.01 | 0.66 | 10.85 | 0.70 |
| TK3 | 5.42 | 0.67 | 11.55 | 0.70 |
| Dye | λabs (nm) ᵃ | ε × 103 (M−1 cm−1) ᵃ | HOMO/LUMO (eV) | E0−0 b | Eox c (V) |
|---|---|---|---|---|---|
| TK1 | 449 | 7.3 | −5.35/−3.17 | 2.18 | 1.29 |
| TK2 | 522 | 12 | −5.08/−3.13 | 1.96 | 1.03 |
| TK3 | 485 | 17 | −4.99/−2.91 | 2.08 | 0.94 |
| Dye | CDCA ᵃ (mM) | η (%) | FF | Voc (V) | Jsc (mA cm−2) | Dye Loading (10−7 mol cm−2) |
|---|---|---|---|---|---|---|
| TK1 | 0 | 2.14 | 0.75 | 0.63 | 4.52 | 2.1 |
| 10 | 1.08 | 0.70 | 0.70 | 2.21 | 1.5 | |
| TK2 | 0 | 5.01 | 0.70 | 0.66 | 10.85 | 2.5 |
| 10 | 4.72 | 0.73 | 0.73 | 8.85 | 1.8 | |
| TK3 | 0 | 5.42 | 0.70 | 0.67 | 11.55 | 2.6 |
| 10 | 7.45 | 0.71 | 0.70 | 14.98 | 2.0 | |
| N719 | 0 | 8.11 | 0.70 | 0.79 | 14.68 | 1.7 |
| Dye | λabs (ε × 103 M−1 cm−1) a (nm) | HOMO b | E0-0 c | LUMO d |
|---|---|---|---|---|
| TK4 | 548 (10.1) | 0.64 | 1.82 | −1.18 |
| TK5 | 513 (17.8) | 0.68 | 1.90 | −1.22 |
| TK6 | 516 (25.1) | 0.73 | 1.88 | −1.15 |
| Dye | η (%) | FF | Voc | Jsc | Dye Loading a |
|---|---|---|---|---|---|
| TK4 | 5.9 | 0.67 | 0.667 | 13.29 | 2.0 |
| TK5 | 7.5 | 0.64 | 0.653 | 17.85 | 1.9 |
| TK6 | 7.8 | 0.69 | 0.663 | 17.19 | 1.6 |
| Dye | λmax (nm) a (ε × 103/M−1 cm−1) | HOMO (V) b (vs. NHE) | LUMO (V) c (vs. NHE) | E0−0 d (eV) |
|---|---|---|---|---|
| TK7 | 422 (33.9) | 0.98 | −1.04 | 2.02 |
| TK8 | 416 (30.6) | 0.94 | −1.04 | 1.98 |
| TK9 | 428 (17.5) | 0.93 | −1.07 | 2.00 |
| Dye | η % | FF | Voc (V) | Jsc (mA/cm2) | Dye Loading (10−7 mol/cm2) |
|---|---|---|---|---|---|
| TK7 | 7.88 | 0.66 | 0.67 | 17.82 | 2.3 |
| TK8 | 6.35 | 0.67 | 0.66 | 14.33 | 2.1 |
| TK9 | 6.14 | 0.67 | 0.68 | 13.48 | 1.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardone, A.; Capodilupo, A.L. Functional Organic Materials for Photovoltaics: The Synthesis as a Tool for Managing Properties for Solid State Applications. Materials 2022, 15, 6333. https://doi.org/10.3390/ma15186333
Cardone A, Capodilupo AL. Functional Organic Materials for Photovoltaics: The Synthesis as a Tool for Managing Properties for Solid State Applications. Materials. 2022; 15(18):6333. https://doi.org/10.3390/ma15186333
Chicago/Turabian StyleCardone, Antonio, and Agostina Lina Capodilupo. 2022. "Functional Organic Materials for Photovoltaics: The Synthesis as a Tool for Managing Properties for Solid State Applications" Materials 15, no. 18: 6333. https://doi.org/10.3390/ma15186333
APA StyleCardone, A., & Capodilupo, A. L. (2022). Functional Organic Materials for Photovoltaics: The Synthesis as a Tool for Managing Properties for Solid State Applications. Materials, 15(18), 6333. https://doi.org/10.3390/ma15186333