An Influence of Oxygen Flow Rate and Spray Distance on the Porosity of HVOF Coating and Its Effects on Corrosion—A Review
Abstract
:1. Introduction
2. Hot Corrosion in Gas Turbine Blades

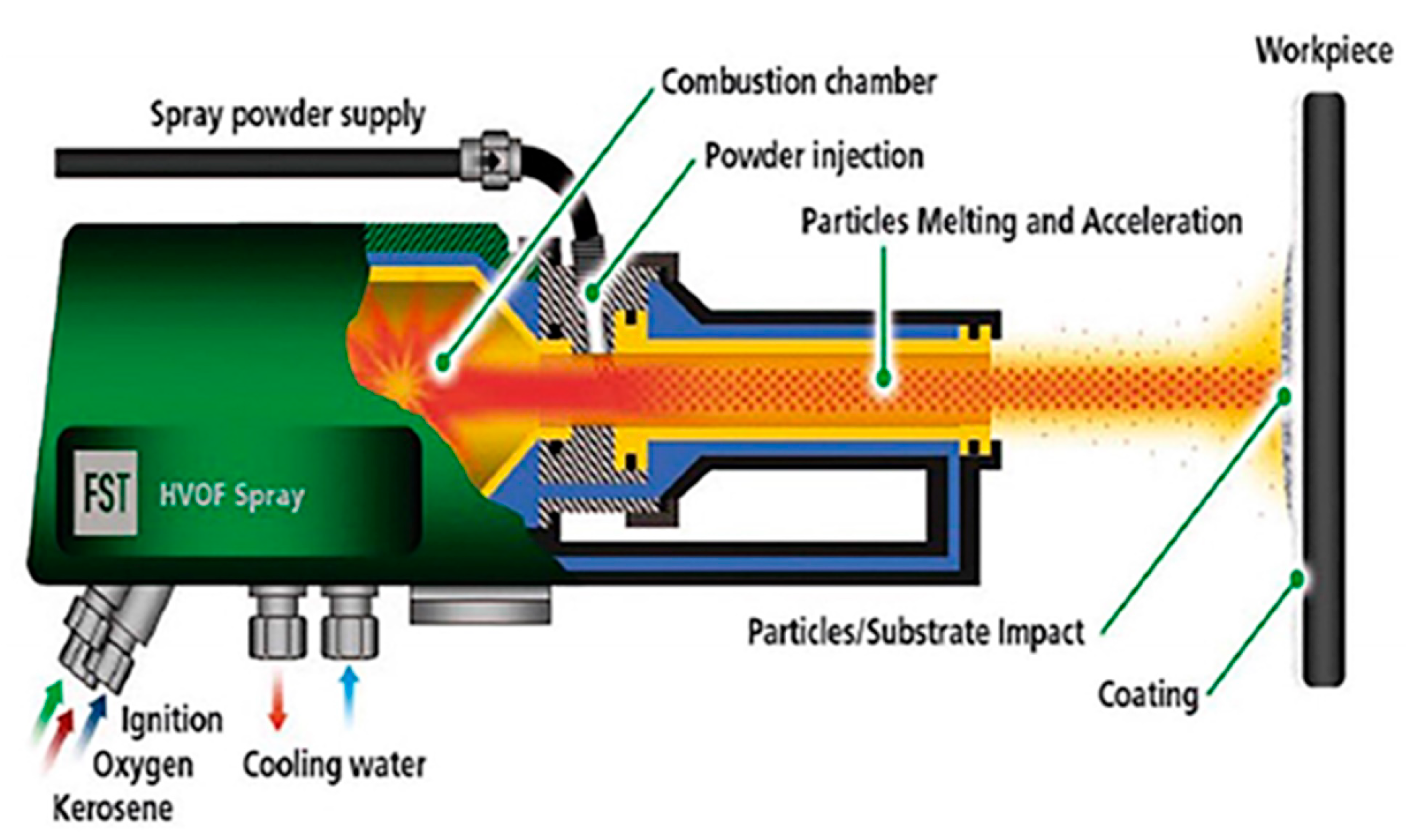
3. High-Velocity Oxy-Fuel
4. Effect of Oxygen Flowrate on Corrosion Resistance
5. Effect of Spray Distance on Corrosion Resistance
6. Recommendations and Discussion
7. Conclusions
- (1)
- Corrosion resistance can be improved by increasing the chromium content, which helps extend the incubation period.
- (2)
- The porosity of coating mainly influences the corrosion resistance, and the porosity is primarily affected by the oxygen-to-fuel ratio. The coating porosity is also reduced by short and moderate spray distances.
- (3)
- Porosity depends on the thermal energy and kinetic energy of particles. Less porous coatings can be obtained with less thermal energy and high kinetic energy.
- (4)
- The porosity reduces by increasing the oxygen-to-fuel ratio, and decreased porosity is the reason for improved corrosion resistance.
- (5)
- The oxygen flow rate depends on the fuel and spray gun used during the coating process. Likewise, the gas flow rate depends on the coating material used.
- (6)
- The gas flow rate would be comparatively low for material if the size of the particles is fine or if it is more disposed to oxidation than other alloys.
- (7)
- For different materials, different spray distances are used. The standoff distance for a thermal spray process depends on the spray gun used.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chagnon, P.; Fauchais, P. Thermal spraying of ceramics. Ceram. Int. 1984, 10, 119–131. [Google Scholar] [CrossRef]
- Sampath, S.; Jiang, X.; Matejicek, J.; Prchlik, L.; Kulkarni, A.; Vaidya, A. Role of thermal spray processing method on the microstructure, residual stress and properties of coatings: An integrated study for Ni–5 wt.% Al bond coats. Mater. Sci. Eng. A 2004, 364, 216–231. [Google Scholar] [CrossRef]
- Cizek, J.; Matejicek, J. Medicine meets thermal spray technology: A review of patents. J. Therm. Spray Technol. 2018, 27, 1251–1279. [Google Scholar] [CrossRef]
- Fanicchia, F.; Axinte, D.; Kell, J.; McIntyre, R.; Brewster, G.; Norton, A. Combustion flame spray of CoNiCrAlY & YSZ coatings. Surf. Coat. Technol. 2017, 315, 546–557. [Google Scholar] [CrossRef]
- Kang, Y.; Bai, Y.; Fan, W.; Yuan, T.; Gao, Y.; Bao, C.; Li, B. Thermal cycling performance of La2Ce2O7/50 vol.% YSZ composite thermal barrier coating with CMAS corrosion. J. Eur. Ceram. Soc. 2018, 38, 2851–2862. [Google Scholar] [CrossRef]
- Bose, S. Chapter 5—High-Temperature Corrosion. In High Temperature Coatings; Bose, S., Ed.; Butterworth-Heinemann: Burlington, VT, USA, 2007; pp. 53–70. [Google Scholar]
- Mehboob, G.; Liu, M.-J.; Xu, T.; Hussain, S.; Mehboob, G.; Tahir, A. A review on failure mechanism of thermal barrier coatings and strategies to extend their lifetime. Ceram. Int. 2020, 46, 8497–8521. [Google Scholar] [CrossRef]
- Meng, G.-H.; Zhang, B.-Y.; Liu, H.; Yang, G.-J.; Xu, T.; Li, C.-X.; Li, C.-J. Highly oxidation resistant and cost effective MCrAlY bond coats prepared by controlled atmosphere heat treatment. Surf. Coat. Technol. 2018, 347, 54–65. [Google Scholar] [CrossRef]
- Zhang, B.-Y.; Yang, G.-J.; Li, C.-X.; Li, C.-J. Non-parabolic isothermal oxidation kinetics of low pressure plasma sprayed MCrAlY bond coat. Appl. Surf. Sci. 2017, 406, 99–109. [Google Scholar] [CrossRef]
- Masoule, S.T.; Valefi, Z.; Ehsani, N.; Lavasani, H.Q. Thermal insulation and thermal shock behavior of conventional and nanostructured plasma-sprayed TBCs. J. Therm. Spray Technol. 2016, 25, 1684–1691. [Google Scholar] [CrossRef]
- Sivakumar, G.; Banerjee, S.; Raja, V.; Joshi, S.V. Hot corrosion behavior of plasma sprayed powder-solution precursor hybrid thermal barrier coatings. Surf. Coat. Technol. 2018, 349, 452–461. [Google Scholar] [CrossRef]
- Sivakumar, S.; Praveen, K.; Shanmugavelayutham, G.; Yugeswaran, S.; Mostaghimi, J. Thermo-physical behavior of atmospheric plasma sprayed high porosity lanthanum zirconate coatings. Surf. Coat. Technol. 2017, 326, 173–182. [Google Scholar] [CrossRef]
- Meng, G.-H.; Zhang, B.-Y.; Liu, H.; Yang, G.-J.; Xu, T.; Li, C.-X.; Li, C.-J. Vacuum heat treatment mechanisms promoting the adhesion strength of thermally sprayed metallic coatings. Surf. Coat. Technol. 2018, 344, 102–110. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Y.; Gu, L.; Zou, B.; Wang, Y.; Cao, X. Hot corrosion behaviour of plasma sprayed YSZ/LaMgAl11O19 composite coatings in molten sulfate–vanadate salt. Corros. Sci. 2011, 53, 2335–2343. [Google Scholar] [CrossRef]
- Afrasiabi, A.; Saremi, M.; Kobayashi, A. A comparative study on hot corrosion resistance of three types of thermal barrier coatings: YSZ, YSZ+Al2O3 and YSZ/Al2O3. Mater. Sci. Eng. A 2008, 478, 264–269. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Q.; Zhou, Y.-C.; Mao, W.; Lu, C. Finite element simulation on thermal fatigue of a turbine blade with thermal barrier coatings. J. Mater. Sci. Technol. 2014, 30, 371–380. [Google Scholar] [CrossRef]
- Bahamirian, M.; Hadavi, S.; Farvizi, M.; Rahimipour, M.; Keyvani, A. Enhancement of hot corrosion resistance of thermal barrier coatings by using nanostructured Gd2Zr2O7 coating. Surf. Coat. Technol. 2019, 360, 1–12. [Google Scholar] [CrossRef]
- Thakare, J.G.; Pandey, C.; Mahapatra, M.M.; Mulik, R.S. Thermal Barrier Coatings—A State of the Art Review. Met. Mater. Int. 2020, 27, 1947–1968. [Google Scholar] [CrossRef]
- Clarke, D.R.; Oechsner, M.; Padture, N.P. Thermal-barrier coatings for more efficient gas-turbine engines. MRS Bull. 2012, 37, 891–898. [Google Scholar] [CrossRef]
- Prashar, G.; Vasudev, H. Structure-property correlation and high-temperature erosion performance of Inconel625-Al2O3 plasma-sprayed bimodal composite coatings. Surf. Coat. Technol. 2022, 439, 128450. [Google Scholar] [CrossRef]
- Vasudev, H.; Thakur, L.; Singh, H.; Bansal, A. Effect of addition of Al2O3 on the high-temperature solid particle erosion behaviour of HVOF sprayed Inconel-718 coatings. Mater. Today Commun. 2022, 30, 103017. [Google Scholar] [CrossRef]
- Prashar, G.; Vasudev, H.; Thakur, L. Influence of heat treatment on surface properties of HVOF deposited WC and Ni-based powder coatings: A review. Surf. Topogr. Metrol. 2021, 9, 043002. [Google Scholar] [CrossRef]
- Prashar, G.; Vasudev, H.; Thakur, L. High-Temperature Oxidation and Erosion Resistance of Ni-Based Thermally-Sprayed Coatings Used in Power Generation Machinery: A Review. Surf. Rev. Lett. 2022, 29, 2230003. [Google Scholar] [CrossRef]
- Prashar, G.; Vasudev, H. A review on the influence of process parameters and heat treatment on the corrosion performance of Ni-based thermal spray coatings. Surf. Rev. Lett. 2022, 29, 2230001. [Google Scholar] [CrossRef]
- Singh, P.; Bansal, A.; Vasudev, H.; Singh, P. In situ surface modification of stainless steel with hydroxyapatite using microwave heating. Surf. Topogr. Metrol. 2021, 9, 035053. [Google Scholar] [CrossRef]
- Vasudev, H.; Thakur, L.; Singh, H.; Bansal, A. Erosion behaviour of HVOF sprayed Alloy718-nano Al2O3 composite coatings on grey cast iron at elevated temperature conditions. Surf. Topogr. Metrol. 2021, 9, 035022. [Google Scholar] [CrossRef]
- Vasudev, H.; Thakur, L.; Singh, H.; Bansal, A. An investigation on oxidation behaviour of high velocity oxy-fuel sprayed Inconel718-Al2O3 composite coatings. Surf. Coat. Technol. 2020, 393, 125770. [Google Scholar] [CrossRef]
- Wu, S.; Zhao, Y.; Li, W.; Liu, W.; Wu, Y.; Liu, F. Research Progresses on Ceramic Materials of Thermal Barrier Coatings on Gas Turbine. Coatings 2021, 11, 79. [Google Scholar] [CrossRef]
- Gopi, R.; Saravanan, I.; Devaraju, A.; Ponnusamy, P. Tribological behaviour of thermal sprayed high velocity oxy-fuel coatings on tungsten carbide–A review. Mater. Today Proc. 2021, 39, 292–295. [Google Scholar] [CrossRef]
- Gupta, N.; Singh, S.K.; Pandey, S.M. Tribological characterisation of thermal sprayed CrC alloyed coating–A review. Adv. Mater. Process. Technol. 2021, 7, 660–683. [Google Scholar] [CrossRef]
- Vats, A.; Kumar, A.; Patnaik, A.; Meena, M. Influence of deposition parameters on Tribological Performance of HVOF Coating: A review. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2021; p. 012015. [Google Scholar] [CrossRef]
- Kumar, R.; Bhandari, D.; Goyal, K.J. A Review of the Mechanical Properties and Erosion Behavior of HVOF Sprayed Nanocomposite Coatings. In Advancement in Materials, Manufacturing Energy Engineering; Springer: Berlin/Heidelberg, Germany, 2022; Volume 1, pp. 277–287. [Google Scholar]
- Puspitasari, W.; Ahmad, F.; Ullah, S.; Raza, M.R.; Hussain, P.; Yusoff, P.; Yasmin, A. The study of corrosion behaviour of intumescent fire retardant coating with structural steel substrate. Int. J. Electrochem. Sci. 2018, 13, 9916–9930. [Google Scholar] [CrossRef]
- Kabeb, S.M.; Hassan, A.; Mohamad, Z.; Sharer, Z.; Mokhtar, M.; Ahmad, F.J.C.E.T. Synergistic effect of graphene oxide/halloysite in anticorrosion performance and flame retardancy properties of epoxy nanocomposite coating. Chem. Eng. Trans. 2020, 78, 529–534. [Google Scholar] [CrossRef]
- Kabeba, S.M.; Hassanb, A.; Mohamadb, Z.; Sharerb, Z.; Mokhtarb, M.; Ahmadc, F. Exploring the effects of nanofillers of epoxy nanocomposite coating for sustainable corrosion protection. Chem. Eng. Trans. 2019, 72, 121–126. [Google Scholar] [CrossRef]
- Leyens, C.; Wright, I.G.; Pint, B.A. Hot Corrosion of an EB-PVD Thermal-Barrier Coating System at 950 °C. Oxid. Met. 2000, 54, 401–424. [Google Scholar] [CrossRef]
- Chatha, S.S.; Sidhu, H.S.; Sidhu, B.S. High temperature hot corrosion behaviour of NiCr and Cr3C2–NiCr coatings on T91 boiler steel in an aggressive environment at 750 °C. Surf. Coat. Technol. 2012, 206, 3839–3850. [Google Scholar] [CrossRef]
- Jonnalagadda, K.P.; Eriksson, R.; Peng, R.; Li, X.-H.; Johansson, S. Factors affecting the performance of thermal barrier coatings in the presence of V2O5 and Na2SO4. J. Ceram. Sci. Technol. 2016, 7, 409–415. [Google Scholar] [CrossRef]
- Jones, R.L. Some aspects of the hot corrosion of thermal barrier coatings. J. Therm. Spray Technol. 1997, 6, 77–84. [Google Scholar] [CrossRef]
- Berger, J.E.; Schulz, R.; Savoie, S.; Gallego, J.; Kiminami, C.S.; Bolfarini, C.; Botta, W.J. Wear and corrosion properties of HVOF coatings from Superduplex alloy modified with addition of boron. Surf. Coat. Technol. 2017, 309, 911–919. [Google Scholar] [CrossRef]
- Conde, J.; Erdös, E.; Rahmel, A. Mechanisms of hot corrosion. In High Temperature Alloys for Gas Turbines; Springer: Dordrecht, The Netherlands, 1982; pp. 99–148. [Google Scholar]
- Khajavi, M.; Shariat, M. Failure of first stage gas turbine blades. Eng. Fail. Anal. 2004, 11, 589–597. [Google Scholar] [CrossRef]
- Vasudev, H.; Prashar, G.; Thakur, L.; Bansal, A. Prevention Electrochemical Corrosion Behavior and Microstructural Characterization of HVOF Sprayed Inconel-718 Coating on Gray Cast Iron. J. Fail. Anal. Prev. 2021, 21, 250–260. [Google Scholar] [CrossRef]
- Pishva, P.; Salehi, M.; Golozar, M.A. Effect of grinding on surface characteristics of HVOF-sprayed WC–10Co–4Cr coatings. Surf. Eng. 2020, 36, 1180–1189. [Google Scholar] [CrossRef]
- Padmavathi, G.; Sarada, B.; Shanmuganathan, S.; Padmini, B.; Mohan, N. Effects of high velocity oxy fuel thermal spray coating on mechanical and tribological properties of materials–A review. Mater. Today Proc. 2020, 27, 2152–2157. [Google Scholar] [CrossRef]
- Balki, M.K.; Sayin, C.; Sarıkaya, M. Optimization of the operating parameters based on Taguchi method in an SI engine used pure gasoline, ethanol and methanol. Fuel 2016, 180, 630–637. [Google Scholar] [CrossRef]
- Qiao, L.; Wu, Y.; Hong, S.; Cheng, J.; Wei, Z. Influence of the high-velocity oxygen-fuel spray parameters on the porosity and corrosion resistance of iron-based amorphous coatings. Surf. Coat. Technol. 2019, 366, 296–302. [Google Scholar] [CrossRef]
- Vignesh, S.; Shanmugam, K.; Balasubramanian, V.; Sridhar, K. Identifying the optimal HVOF spray parameters to attain minimum porosity and maximum hardness in iron based amorphous metallic coatings. Def. Technol. 2017, 13, 101–110. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, S.; Zheng, Y.; Ke, W.; Sun, W.; Chang, X.; Hou, W.; Wang, J. Effect of processing parameters on the microstructures and corrosion behaviour of high-velocity oxy-fuel (HVOF) sprayed Fe-based amorphous metallic coatings. Mater. Corros. 2013, 64, 801–810. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, Y.; Hou, G.; An, Y.; Liu, G. The effect of high-velocity oxy-fuel spraying parameters on microstructure, corrosion and wear resistance of Fe-based metallic glass coatings. J. Non-Cryst. Solids 2014, 406, 37–44. [Google Scholar] [CrossRef]
- Huang, Y.; Ding, X.; Yuan, C.-Q.; Yu, Z.-K.; Ding, Z.-X. Slurry erosion behaviour and mechanism of HVOF sprayed micro-nano structured WC-CoCr coatings in NaCl medium. Tribol. Int. 2020, 148, 106315. [Google Scholar] [CrossRef]
- Li, C.; Gao, X.; Zhang, D.; Gao, H.; Han, X.; Zhang, B.J. Numerical Investigation on the Flame Characteristics and Particle Behaviors in a HVOF Spray Process Using Kerosene as Fuel. J. Therm. Spray Technol. 2021, 30, 725–738. [Google Scholar] [CrossRef]
- Zois, D.; Lekatou, A.; Vardavoulias, M.; Vaimakis, T.; Karantzalis, A. Partially amorphous stainless steel coatings: Microstructure, annealing behavior and statistical optimization of spray parameters. Surf. Coat. Technol. 2011, 206, 1469–1483. [Google Scholar] [CrossRef]
- Picas, J.A.; Punset, M.; Baile, M.T.; Martín, E.; Forn, A. Effect of oxygen/fuel ratio on the in-flight particle parameters and properties of HVOF WC-CoCr coatings. Surf. Coat. Technol. 2011, 205, S364–S368. [Google Scholar] [CrossRef]
- Azizpour, M.J.; Tolouei-Rad, M. The effect of spraying temperature on the corrosion and wear behavior of HVOF thermal sprayed WC-Co coatings. Ceram. Int. 2019, 45, 13934–13941. [Google Scholar] [CrossRef]
- Mardali, M.; Salimijazi, H.; Karimzadeh, F.; Luthringer-Feyerabend, B. The effect of an MgO intermediate layer on a nanostructured HA coating fabricated by HVOF on an Mg alloy. Surf. Coat. Technol. 2019, 374, 1071–1077. [Google Scholar] [CrossRef]
- Testa, V.; Morelli, S.; Bolelli, G.; Benedetti, B.; Puddu, P.; Sassatelli, P.; Lusvarghi, L. Alternative metallic matrices for WC-based HVOF coatings. Surf. Coat. Technol. 2020, 402, 126308. [Google Scholar] [CrossRef]
- Murariu, A.C.; Cernescu, A.V.; Perianu, I.-A. The effect of saline environment on the fatigue behaviour of HVOF-sprayed WC–CrC–Ni coatings. Surf. Eng. 2018, 34, 755–761. [Google Scholar] [CrossRef]
- Madhu, G.; Mrityunjaya Swamy, K.; Kumar, D.A.; Prasad, C.D.; Harish, U. Evaluation of Hot Corrosion Behavior of HVOF Thermally Sprayed Cr3C2-35NiCr Coating on SS 304 Boiler Tube Steel; AIP Publishing LLC: Melville, NY, USA, 2021; p. 030014. [Google Scholar]
- Pala, Z.; Bai, M.; Lukac, F.; Hussain, T. Laser clad and HVOF-sprayed stellite 6 coating in chlorine-rich environment with KCl at 700 C. Oxid. Met. 2017, 88, 749–771. [Google Scholar] [CrossRef]
- Vignesh, S.; Shanmugam, K.; Balasubramanian, V.; Sridhar, K.; Thirumalaikumarasamy, D. Electrochemical corrosion behaviour of HVOF sprayed iron-based amorphous metallic coatings on AISI 316 stainless steel in an NaCl solution. J. Mech. Behav. Mater. 2018, 27, 3–4. [Google Scholar] [CrossRef]
- Bolelli, G.; Colella, A.; Lusvarghi, L.; Puddu, P.; Rigon, R.; Sassatelli, P.; Testa, V. Properties of HVOF-sprayed TiC-FeCrAl coatings. Wear 2019, 418, 36–51. [Google Scholar] [CrossRef]
- Ding, X.; Huang, Y.; Yuan, C.; Ding, Z. Deposition and cavitation erosion behavior of multimodal WC-10Co4Cr coatings sprayed by HVOF. Surf. Coat. Technol. 2020, 392, 125757. [Google Scholar] [CrossRef]
- Amudha, A.; Nagaraja, H.; Shashikala, H. Electrochemical Corrosion Behaviour of Nickel Chromium-Chromium Carbide Coating by HVOF Process; AIP Publishing LLC: Melville, NY, USA, 2018; p. 020092. [Google Scholar]
- Yao, S.H.; Su, Y.L.; Shu, H.Y.; You, Z.L.; Lai, Y.C. Corrosive Resistance of HVOF WC Coatings with a Different Binder; Trans Tech Publications Ltd.: Schwyz, Switzerland, 2017; pp. 120–124. [Google Scholar] [CrossRef]
- Sassatelli, P.; Bolelli, G.; Gualtieri, M.L.; Heinonen, E.; Honkanen, M.; Lusvarghi, L.; Manfredini, T.; Rigon, R.; Vippola, M. Properties of HVOF-sprayed Stellite-6 coatings. Surf. Coat. Technol. 2018, 338, 45–62. [Google Scholar] [CrossRef]
- Murariu, A.C.; Pleşu, N.; Perianu, I.A.; Tară-Lungă-Mihali, M. Investigations on corrosion behaviour of WC–CrC–Ni coatings deposited by HVOF thermal spraying process. Int. J. Electrochem. Sci. 2017, 12, 1535–1549. [Google Scholar] [CrossRef]
- Farokhian, G.; Salehnasab, B.; Zat Ajam, H.; Nahidi, H. Influence of WC–20Co–1Ni coating by HVOF on lifespan of the downhole drilling motors. Surf. Eng. 2018, 34, 771–782. [Google Scholar] [CrossRef]
- Sarkar, K.; Rai, P.; Katiyar, P.K.; Satapathy, B.; Pathak, A.S.; Dutta, M.; Banerjee, A.; Mondal, K. Composite (glass+crystalline) coatings from blast furnace pig iron by high velocity oxy-fuel (HVOF) process and their electrochemical behavior. Surf. Coat. Technol. 2019, 372, 72–83. [Google Scholar] [CrossRef]
- Wei, Z.; Wu, Y.; Hong, S.; Cheng, J.; Qiao, L.; Cheng, J.; Zhu, S. Electrochemical properties and cavitation erosion behaviors of HVOF sprayed (AlCoCrFeNi) 1-X (WC-10Co) X composite coatings in NaCl medium. Ceram. Int. 2021, 47, 29410–29422. [Google Scholar] [CrossRef]
- Zouari, S.; Ghorbel, H.; Danlos, Y.; Liao, H.; Elleuch, R. Comparative study of HVOF-sprayed NiCrBSi alloy and 316L stainless steel coatings on a brass substrate. J. Therm. Spray Technol. 2019, 28, 1284–1294. [Google Scholar] [CrossRef]
- Wei, Z.; Wu, Y.; Hong, S.; Cheng, J.; Qiao, L.; Cheng, J.; Zhu, S. Ultrasonic cavitation erosion behaviors of high-velocity oxygen-fuel (HVOF) sprayed AlCoCrFeNi high-entropy alloy coating in different solutions. Surf. Coat. Technol. 2021, 409, 126899. [Google Scholar] [CrossRef]
- Li, D.; Chen, X.; Hui, X.; Wang, J.; Jin, P.; Li, H. Effect of amorphicity of HVOF sprayed Fe-based coatings on their corrosion performances and contacting osteoblast behavior. Surf. Coat. Technol. 2017, 310, 207–213. [Google Scholar] [CrossRef]
- Pukasiewicz, A.; Pukasiewicz, A.; Vaz, R.; Araújo, I. Corrosion resistance of iron-based alloy coatings deposited by HVOF process. In Proceedings of the ITSC 2019—International Thermal Spray Conference and Exposition, Yokohama, Japan, 26–29 May 2019. [Google Scholar]
- Aoudia, K.; Retraint, D.; Verdy, C.; Langlade, C.; Creus, J.; Sanchette, F. Enhancement of Mechanical Properties and Corrosion Resistance of HVOF-Sprayed NiCrBSi Coatings Through Mechanical Attrition Treatment (SMAT). J. Therm. Spray Technol. 2020, 29, 2065–2079. [Google Scholar] [CrossRef]
- Alnaser, I.A.; Yunus, M.; Alfattani, R.; Alamro, T. High-Temperature Corrosion of APS-and HVOF-Coated Nickel-Based Super Alloy under Air Oxidation and Melted Salt Domains. Materials 2021, 14, 5119. [Google Scholar] [CrossRef]
- Liu, Y.W.; Sun, W.C.; Tian, S.S.; Xiao, Y.; Jia, Y.P. Microstructures and corrosion behavior of HVOF-sprayed WC-12% Cr3C2-6% Ni coatings before and after sealing. Int. J. Appl. Ceram. Technol. 2021, 19, 383–396. [Google Scholar] [CrossRef]
- Verdian, M. Corrosion behaviour of HVOF-sprayed Co–Cr–W–C coating in NaCl solution. Trans. IMF 2018, 96, 206–211. [Google Scholar] [CrossRef]
- Chakradhar, R.; Prasad, G.; Venkateswarlu, K.; Srivastava, M. An Investigation on the Wear and Corrosion Behavior of HVOF-Sprayed WC-12Co-Al2O3 Cermet Coating. J. Mater. Eng. Perform. 2018, 27, 1241–1248. [Google Scholar] [CrossRef]
- Sidhu, V.P.S.; Goyal, K.; Goyal, R. Hot corrosion behaviour of HVOF-sprayed 93 (WC-Cr3C2)-7Ni and 83WC-17Co coatings on boiler tube steel in coal fired boiler. Aust. J. Mech. Eng. 2019, 17, 127–132. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, C.; Song, J.; Deng, C.; Liu, M.; Dai, M. Electrochemical corrosive behaviors of Fe-based amorphous/nanocrystalline coating on stainless steel prepared by HVOF-sprayed. Coatings 2019, 9, 226. [Google Scholar] [CrossRef]
- Qiao, L.; Wu, Y.; Hong, S.; Qin, Y.; Shi, W.; Li, G. Performance Corrosion behavior of HVOF-sprayed Fe-based alloy coating in various solutions. J. Mater. Eng. Perform. 2017, 26, 3813–3820. [Google Scholar] [CrossRef]
- Akkaş, M. The mechanical and corrosion properties of WCCo–Al coatings formed on AA2024 using the HVOF method. Mater. Res. Express 2020, 7, 076515. [Google Scholar] [CrossRef]
- Singh, G.; Bala, N.; Chawla, V.; Singla, Y.K. Hot corrosion behavior of HVOF-sprayed carbide based composite coatings for boiler steel in Na2SO4–60% V2O5 environment at 900° C under cyclic conditions. Corros. Sci. 2021, 190, 109666. [Google Scholar] [CrossRef]
- Wang, K.; Hong, S.; Wei, Z.; Hu, N.; Cheng, J.; Wu, Y. Long-term corrosion behavior of HVOF sprayed Cr3C2–NiCr coatings in sulfide-containing 3.5 wt.% NaCl solution. J. Mater. Res. Technol. 2021, 15, 3122–3132. [Google Scholar] [CrossRef]
- Zhou, Y.-K.; Liu, X.-B.; Kang, J.-J.; Yue, W.; Qin, W.-B.; Ma, G.-Z.; Fu, Z.-Q.; Zhu, L.-N.; She, D.-S.; Wang, H.-D. Corrosion behavior of HVOF sprayed WC-10Co4Cr coatings in the simulated seawater drilling fluid under the high pressure. Eng. Fail. Anal. 2020, 109, 104338. [Google Scholar] [CrossRef]
- Sidhu, V.P.S.; Goyal, K.; Goyal, R. Materials An investigation of corrosion resistance of HVOF coated ASME SA213 T91 boiler steel in an actual boiler environment. Anti-Corros Methods Mater. 2017, 64, 499–507. [Google Scholar] [CrossRef]
- Li, S.; Guo, Z.; Xiong, J.; Lei, Y.; Li, Y.; Tang, J.; Liu, J.; Ye, J. Corrosion behavior of HVOF sprayed hard face coatings in alkaline-sulfide solution. Appl. Surf. Sci. 2017, 416, 69–77. [Google Scholar] [CrossRef]
- Fantozzi, D.; Matikainen, V.; Uusitalo, M.; Koivuluoto, H.; Vuoristo, P.J.C.S. Chlorine induced high-temperature corrosion mechanisms in HVOF and HVAF sprayed Cr3C2-based hardmetal coatings. Corros. Sci. 2019, 160, 108166. [Google Scholar] [CrossRef]
- Sidhu, V.P.S.; Goyal, K.; Goyal, R. Comparative study of corrosion behaviour of HVOF-coated boiler steel in actual boiler environment of a thermal power plant. J. Aust. Ceram. Soc. 2017, 53, 925–932. [Google Scholar] [CrossRef]
- Melero, H.C.; Sakai, R.T.; Vignatti, C.A.; Benedetti, A.V.; Fernández, J.; Guilemany, J.M.; Suegama, P.H. Corrosion resistance evaluation of HVOF produced hydroxyapatite and TiO2-hydroxyapatite coatings in Hanks’ solution. Mater. Res. 2018, 21, 2. [Google Scholar] [CrossRef]
- Chi, H.; Pans, M.A.; Bai, M.; Sun, C.; Hussain, T.; Sun, W.; Yao, Y.; Lyu, J.; Liu, H. Experimental investigations on the chlorine-induced corrosion of HVOF thermal sprayed Stellite-6 and NiAl coatings with fluidised bed biomass/anthracite combustion systems. Fuel 2021, 288, 119607. [Google Scholar] [CrossRef]
- García-Rodríguez, S.; López, A.J.; Bonache, V.; Torres, B.; Rams, J. Fabrication, Wear, and Corrosion Resistance of HVOF Sprayed WC-12Co on ZE41 Magnesium Alloy. Coatings 2020, 10, 502. [Google Scholar] [CrossRef]
- Wolf, W.; Koga, G.Y.; Schulz, R.; Savoie, S.; Kiminami, C.S.; Bolfarini, C.; Botta, W.J. Wear and Corrosion Performance of Al-Cu-Fe-(Cr) Quasicrystalline Coatings Produced by HVOF. J. Therm. Spray Technol. 2020, 29, 1195–1207. [Google Scholar] [CrossRef]
- Zhou, W.X.; Zhou, K.S.; Deng, C.M.; Zeng, K.L.; Li, Y.X. Hot corrosion behavior of HVOF-sprayed Cr3C2-WC-NiCoCrMo coating. Ceram. Int. 2017, 43, 9390–9400. [Google Scholar] [CrossRef]
- Wang, Y.J.; Hao, E.K.; An, Y.L.; Hou, G.L.; Zhao, X.Q.; Zhou, H.D. The interaction mechanism of cavitation erosion and corrosion on HVOF sprayed NiCrWMoCuCBFe coating in artificial seawater. Appl. Surf. Sci. 2020, 525, 146499. [Google Scholar] [CrossRef]
- Mayer, A.R.; Bertuol, K.; Siqueira, I.B.; Chicoski, A.; Váz, R.F.; de Sousa, M.J.; Pukasiewicz, A.G. Evaluation of cavitation/corrosion synergy of the Cr3C2-25NiCr coating deposited by HVOF process. Ultrason. Sonochem. 2020, 69, 105271. [Google Scholar] [CrossRef]
- Ludwig, G.A.; Malfatti, C.F.; Schroeder, R.M.; Ferrari, V.Z.; Muller, I.L. WC10Co4Cr coatings deposited by HVOF on martensitic stainless steel for use in hydraulic turbines: Resistance to corrosion and slurry erosion. Surf. Coat. Technol. 2019, 377, 124918. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Z.-W.; Chen, Q.; Liu, L. Effect of hydrostatic pressure on the corrosion behavior of HVOF-sprayed Fe-based amorphous coating. J. Alloys Compd. 2018, 758, 108–115. [Google Scholar] [CrossRef]
- Mohammadi, M.; Kobayashi, A.; Javadpour, S.; Jahromi, S. Evaluation of hot corrosion behaviors of Al2O3-YSZ composite TBC on gradient MCrAlY coatings in the presence of Na2SO4-NaVO3 salt. Vacuum 2019, 167, 547–553. [Google Scholar] [CrossRef]
- Khajezadeh, M.H.; Mohammadi, M.; Ghatee, M. Hot corrosion performance and electrochemical study of CoNiCrAlY/YSZ/YSZ-La2O3 multilayer thermal barrier coatings in the presence of molten salt. Mater. Chem. Phys. 2018, 220, 23–34. [Google Scholar] [CrossRef]
- Al-Mutairi, S.; Hashmi, M.; Yilbas, B.; Stokes, J. Microstructural characterization of HVOF/plasma thermal spray of micro/nano WC–12% Co powders. Surf. Coat. Technol. 2015, 264, 175–186. [Google Scholar] [CrossRef]
- Muthu, S.; Arivarasu, M. Investigations of hot corrosion resistance of HVOF coated Fe based superalloy A-286 in simulated gas turbine environment. Eng. Fail. Anal. 2020, 107, 104224. [Google Scholar] [CrossRef]
- Nag, A.; Bhadu, M.K.; Bijalwan, P.K.; Pathak, A.S.J.C.S. Investigation of selected HVOF and plasma sprayed coatings for sustained performance in molten zinc. Corros. Sci. 2021, 180, 109177. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, R.; Xie, L.; Wang, W.; Li, Y.; Wang, S.; Li, H.; Zhang, J.; Zhang, J. Interfacial bonding mechanism and properties of HVOF-sprayed Fe-based amorphous coatings on LA141 magnesium alloy substrate. Surf. Coat. Technol. 2021, 426, 127801. [Google Scholar] [CrossRef]
- Bîrdeanu, A.V.; Milovanovic, D.S.; Ciganovic, J.; Petronić, S.; Vaida, M.; Bîrdeanu, M. Investigations of Corrosion Behavior on Combined Fast Laser Texturing and HVOF TiO2 Powder Deposition Surface Engineering Treatment; Trans Tech Publications Ltd.: Schwyz, Switzerland, 2019; pp. 119–125. [Google Scholar]
- Zhang, H.; Gong, Y.; Chen, X.; McDonald, A.; Li, H. A comparative study of cavitation erosion resistance of several HVOF-sprayed coatings in deionized water and artificial seawater. J. Therm. Spray Technol. 2019, 28, 1060–1071. [Google Scholar] [CrossRef]
- Asl, S.K.; Rabizadeh, T.; Noori, N.F. The Effects of Heat Treatment on the Corrosion Behavior of HVOF-sprayed WC-17 wt.% Co Coatings. Prot. Met. Phys Chem. Surf. 2019, 55, 936–941. [Google Scholar] [CrossRef]
- Zhang, H.; Gong, Y.; Zhang, B.; Chen, X.; Fang, L.; Jin, P.; Li, H. Corrosion and algal adhesion behaviors of HVOF-sprayed Fe-based amorphous coatings for marine applications. J. Therm. Spray Technol. 2019, 28, 283–290. [Google Scholar] [CrossRef]
- Chen, X.; Li, C.; Bai, X.; Wang, H.; Xu, S.; Song, S. Microstructure, mechanical properties, abrasive wear, and corrosion behavior in molten zinc of boride-based coatings in situ synthesized by an HVOF spraying process. Coatings 2019, 9, 665. [Google Scholar] [CrossRef]
- Reddy, L.; Shipway, P.; Davis, C.; Hussain, T. HVOF and laser cladded Fe-Cr-B coating in simulated biomass combustion: Microstructure and fireside corrosion. Oxid. Met. 2017, 87, 825–835. [Google Scholar] [CrossRef]
- Shankar, R.; Balasubramanian, K.; Sivapirakasam, S. Hot corrosion behavior of nanostructured and conventional HVOF Cr3C2NiCrBSi coatings on superalloy. Mater. Today Proc. 2021, 46, 9529–9536. [Google Scholar] [CrossRef]
- Mousavi, S.E.; Naghshehkesh, N.; Amirnejad, M.; Shammakhi, H.; Sonboli, A. Wear and corrosion properties of stellite-6 coating fabricated by HVOF on nickel–aluminium bronze substrate. Met. Mater. Int. 2021, 27, 3269–3281. [Google Scholar] [CrossRef]
- Hao, E.; Wang, Y.; Zhao, X.; Gao, M.; Chen, J.; An, Y.; Yan, F. Influence of molten salt with or without V2O5 on hot corrosion and high-temperature tribological performance of HVOF-sprayed Ni-based self-lubricating composite coating. Surf. Coat. Technol. 2021, 417, 127210. [Google Scholar] [CrossRef]
- Sreenivasulu, V.; Manikandan, M. Hot corrosion studies of HVOF sprayed carbide and metallic powder coatings on alloy 80A at 900° C. Mater. Res. Express 2018, 6, 036519. [Google Scholar] [CrossRef]
- Ang, A.S.M.; Howse, H.; Wade, S.A.; Berndt, C.C. Development of processing windows for HVOF carbide-based coatings. J. Therm. Spray Technol. 2016, 25, 28–35. [Google Scholar] [CrossRef]
- Ahledel, N.; Schulz, R.; Gariepy, M.; Hermawan, H.; Alamdari, H. Electrochemical corrosion behavior of Fe3Al/TiC and Fe3Al-Cr/TiC coatings prepared by HVOF in NaCl solution. Metals 2019, 9, 437. [Google Scholar] [CrossRef] [Green Version]
- Pougoum, F.; Qian, J.; Martinu, L.; Klemberg-Sapieha, J.; Zhou, Z.; Li, K.Y.; Savoie, S.; Lacasse, R.; Potvin, E.; Schulz, R. Study of corrosion and tribocorrosion of Fe3Al-based duplex PVD/HVOF coatings against alumina in NaCl solution. Surf. Coat. Technol. 2019, 357, 774–783. [Google Scholar] [CrossRef]
- Javed, M.; Ang, A.; Bhadra, C.; Piola, R.; Neil, W.; Berndt, C.; Leigh, M.; Howse, H.; Wade, S. Corrosion and mechanical performance of HVOF WC-based coatings with alloyed nickel binder for use in marine hydraulic applications. Surf. Coat. Technol. 2021, 418, 127239. [Google Scholar] [CrossRef]
- Liu, J.; Chen, T.; Yuan, C.; Bai, X. Performance Analysis of Cavitation Erosion Resistance and Corrosion Behavior of HVOF-Sprayed WC-10Co-4Cr, WC-12Co, and Cr3C2-NiCr Coatings. J. Therm. Spray Technol. 2020, 29, 798–810. [Google Scholar] [CrossRef]
- Reddy, N.C.; Koppad, P.G.; Reddappa, H.; Ramesh, M.; Babu, E.; Varol, T. Hot corrosion behaviour of HVOF sprayed Ni3Ti and Ni3Ti+(Cr3C2+20NiCr) coatings in presence of Na2SO4-40% V2O5 at 650° C. Surf. Topogr: Mterol. Prop. 2019, 7, 025019. [Google Scholar] [CrossRef]
- Liu, Y.; Hang, Z.; Xi, N.; Chen, H.; Ma, C.; Wu, X. Erosion-corrosion behavior of HVOF WC-Co coating in Cl− and SO42− containing solutions. Appl. Surf. Sci. 2018, 431, 55–59. [Google Scholar] [CrossRef]
- Vasudev, H.; Thakur, L.; Singh, H.; Bansal, A. A study on processing and hot corrosion behaviour of HVOF sprayed Inconel718-nano Al2O3. Coatings 2020, 25, 101626. [Google Scholar] [CrossRef]
- Lee, H.-B.; Lin, T.-J.; Lee, C.-Y. Corrosion of high-velocity-oxygen-fuel (HVOF) sprayed non-crystalline alloy coating in marine environment. Surf. Coat. Technol. 2021, 409, 126896. [Google Scholar] [CrossRef]
- Abu-warda, N.; Lopez, A.J.; Lopez, M.D.; Utrilla, M.V. High temperature corrosion and wear behavior of HVOF-sprayed coating of It) Al2O3-NiAl on AISI 304 stainless steel. Surf. Coat. Technol. 2019, 359, 35–46. [Google Scholar] [CrossRef]
- Singh, G.; Bala, N.; Chawla, V. Microstructural analysis and hot corrosion behavior of HVOF-sprayed Ni-22Cr-10Al-1Y and Ni-22Cr-10Al-1Y-SiC (N) coatings on ASTM-SA213-T22 steel. Int. J. Miner. Metall. Mater. 2020, 27, 401–416. [Google Scholar] [CrossRef]
- Ahmed, R.; Vourlias, G.; Algoburi, A.; Vogiatzis, C.; Chaliampalias, D.; Skolianos, S.; Berger, L.M.; Paul, S.; Faisal, N.H.; Toma, F.L.; et al. Comparative Study of Corrosion Performance of HVOF-Sprayed Coatings Produced Using Conventional and Suspension WC-Co Feedstock. J. Therm. Spray Technol. 2018, 27, 1579–1593. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Zhang, J.F.; Xu, J.Y.; Zhang, C. Cavitation-corrosion behaviors of HVOF sprayed WC-25WB-10Co-5NiCr and MoB-25NiCr coatings. Ceram. Int. 2020, 46, 21707–21718. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, H.J.; Ghen, X.Y.; McDonald, A.; Wu, S.J.; Xiao, T.H.; Li, H. Effect of cavitation on corrosion behavior of HVOF-sprayed WC-10Co4Cr coating with post-sealing in artificial seawater. Surf. Coat. Technol. 2020, 397, 126012. [Google Scholar] [CrossRef]
- Tang, P.J.; He, D.Q.; Li, W.S.; Shang, L.L.; Zhai, H.M.; Wang, L.P.; Zhang, G.G. Achieving superior hot corrosion resistance by PVD/HVOF duplex design. Corros. Sci. 2020, 175, 108845. [Google Scholar] [CrossRef]
- Abu-warda, N.; Tomás, L.; López, A.; Utrilla, M. High temperature corrosion behavior of Ni and Co base HVOF coatings exposed to NaCl-KCl salt mixture. Surf. Coat. Technol. 2021, 418, 127277. [Google Scholar] [CrossRef]
- Abu-Warda, N.; López, A.; Pedraza, F.; Utrilla, M. Fireside corrosion on T24 steel pipes and HVOF NiCr coatings exposed to different salt mixtures. Corros. Sci. 2020, 173, 108747. [Google Scholar] [CrossRef]
- Doleker, K.M.; Ozgurluk, Y.; Kahraman, Y.; Karaoglanli, A.C. Oxidation and hot corrosion resistance of HVOF/EB-PVD thermal barrier coating system. Surf. Coat. Technol. 2021, 409, 126862. [Google Scholar] [CrossRef]
- Zhang, Y.; Hong, S.; Lin, J.; Zheng, Y. Influence of ultrasonic excitation sealing on the corrosion resistance of HVOF-sprayed nanostructured WC-CoCr coatings under different corrosive environments. Coatings 2019, 9, 724. [Google Scholar] [CrossRef]
- Bai, M.; Reddy, L.; Hussain, T. Experimental and thermodynamic investigations on the chlorine-induced corrosion of HVOF thermal sprayed NiAl coatings and 304 stainless steels at 700 °C. Corros. Sci. 2018, 135, 147–157. [Google Scholar] [CrossRef]
- Picas, J.; Punset, M.; Rupérez, E.; Menargues, S.; Martin, E.; Baile, M. Corrosion mechanism of HVOF thermal sprayed WC-CoCr coatings in acidic chloride media. Surf. Coat. Technol. 2019, 371, 378–388. [Google Scholar] [CrossRef]
- Hong, S.; Wei, Z.; Wang, K.; Gao, W.; Wu, Y.; Lin, J. The optimization of microbial influenced corrosion resistance of HVOF sprayed nanostructured WC-10Co-4Cr coatings by ultrasound-assisted sealing. Ultrason. Sonochem. 2021, 72, 105438. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, M.; Song, J.; Deng, C.; Deng, C. Compounds Microstructure and corrosion behavior of Fe-based amorphous coating prepared by HVOF. J. Alloys Compd. 2017, 721, 506–511. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, S.; Wang, S.; Wang, H.; Ramachandran, C.S. Wear, erosion and corrosion resistance of HVOF-sprayed WC and Cr3C2 based coatings for electrolytic hard chrome replacement. Int. J. Refract. Met. Hard Mater. 2019, 81, 242–252. [Google Scholar] [CrossRef]
- Cui, X.-Y.; Wang, C.-B.; Kang, J.-J.; Yue, W.; Fu, Z.-Q.; Zhu, L.-N.A. Influence of the corrosion of saturated saltwater drilling fluid on the tribological behavior of HVOF WC-10Co4Cr coatings. Eng. Fail. Anal. 2017, 71, 195–203. [Google Scholar] [CrossRef]
- Hong, S.; Wu, Y.; Gao, W.; Zhang, J.; Zheng, Y.; Zheng, Y. Slurry erosion-corrosion resistance and microbial corrosion electrochemical characteristics of HVOF sprayed WC-10Co-4Cr coating for offshore hydraulic machinery. Int. J. Refractory Met. Hard Mater. 2018, 74, 7–13. [Google Scholar] [CrossRef]
- Zulkifli, I.M.; Yajid, M.M.; Idris, M.; Uday, M.; Daroonparvar, M.; Emadzadeh, A.; Arshad, A. Microstructural evaluation and thermal oxidation behaviors of YSZ/NiCoCrAlYTa coatings deposited by different thermal techniques. Ceram. Int. 2020, 46, 22438–22451. [Google Scholar] [CrossRef]
- Galedari, S.; Jazi, M.S.; Azarmi, F.; Wang, Y. High temperature corrosion and electrochemical behavior of HVOF sprayed Inconel 718 coating using an innovative device: HTCMD. Mater. Corros. 2017, 68, 731–739. [Google Scholar] [CrossRef]

| Fuel | Oxygen Flowrate | Coating Material | Substrate | Remarks | Ref. |
|---|---|---|---|---|---|
| LPG | 250 LPM | WC-12Co-10-15%Al2O3 | Stainless steel 304 | WC-12Co-15Al2O3 coating showed superior corrosion resistance to WC-12Co-10Al2O3 coating due to low porosity. | [79] |
| LPG | 250 LPM | WC-Cr3C2-Ni and WC-17Co | ASME SA213-T22 and ASME SA213-T91 | The corrosion resistance of ASME SA213-T91) alloy coated with 93(WC-Cr3C2)-7Ni was better than other specimens in an actual boiler environment. | [80] |
| Kerosene | 900 LPM | FeCrMnWMoSi amorphous and nanocrystalline | 304 stainless steel | The corrosion performance of the coating was good under acidic and neutral media but not suitable under alkaline conditions. | [81] |
| Kerosene | 967 LPM | Fe-based alloy powder | Low carbon steel | The Fe-based coating’s corrosion resistance was higher than that of the electroplated hard chromium coating in HCL and 3.5% NaCl solution due to fewer defects and a dense structure. | [82] |
| Kerosene | 900 LPM | WCCo-Al | Al alloy AA2024 | The corrosion resistance of the coating increased as the amount of WCCo increased in the coating layer in corrosion tests. | [83] |
| LPG | 250 LPM | NiCrAlY-SiC and NiCrAlY-B4C | T 22 steel | The coating’s hot corrosion behavior was studied at 900 °C in a Na2SO4-V2O5 molten salt environment for 50 cycles. NiCrAlY-SiC brought a better corrosion resistance than NiCrAlY-B4C coating. | [84] |
| Kerosene | 1900 scfh (893LPM) | Cr3C2-NiCr | Q 345 steel | The corrosion behavior of the coating was probed using 3.5% wt. NaCl and 3.5 wt.%NaCl with 20 ppm Na2S solution for 70 days. The coating revealed an improved corrosion resistance in NaCl with 20 ppm Na2S compared to NaCl. | [85] |
| Kerosene | 90 LPM | WC-10Co4Cr with micron-size WC(conventional) and a mixture of nano and micron-sized WC particles (bimodal). | ANSI 4135 steel | The coatings were continuously immersed in drilling fluid simulated seawater at room temperature for 1, 7 and 15 days. The results showed that bimodal coating has a better corrosion resistance and a lower porosity than the conventional coating. | [86] |
| LPG | 250 LPM | 93(WC-Cr3C2)-7Ni, 75Cr3C2-25NiCr, 83WC-17CO and 86WC-10CO-4Cr | ASME SA213 T91 | The coatings were exposed at 900 °C in an actual boiler atmosphere for 10 cycles, including 100 h each. Among all specimens, the 93(WC-Cr3C2)-7Ni coating showed the maximum corrosion resistance. | [87] |
| Kerosene | 920 SLPM | WC-17Co, WC-10Co-4Cr and Cr3C2-25NiCr | AISI 1045 steel | The corrosion behavior of the coatings was studied in an alkaline sulfide solution by immersing the specimens in the solution for 18 days. Corrosion rate analysis showed that the Cr3C2-25NiCr coating exhibited the best resistance against corrosion due to the lowest porosity of 0.87%. | [88] |
| Propane | 240 SLPM | Cr3C2-25NiCr, Cr3C2-50NiCrMoNb and Cr3C2-37WC18NiCoCr | Low carbon steel (grade P92) | The coatings were tested under KCl deposit at two different temperatures of 450 and 550 °C in a furnace for 168 h. The most resistant coating was Cr3C2-37WC-18NiCoCr at 450 °C. | [89] |
| LPG | 250 LPM | Cr3C2-25NiCr and WC-10CO-4Cr | ASME SA213 T91, ASME SA213 T22 | Both bare steel and coated alloys were exposed to cyclic exposure in a coal-fired boiler at 900 °C for 10 cycles. Both coatings showed a better corrosion resistance on ASME SA213 T22 alloy. In contrast, the corrosion resistance of the 86WC-10CO-4Cr coating was higher than that of the 75Cr3C2-25NiCr coating on ASME SA213 T22. | [90] |
| Propylene | 278, 265 LPM | Hydroxyapatite (HA) and 80HA-20TiO2 | Ti-6Al-4V | The electrochemical technique was used to investigate the performance of coatings in natural Hank’s solution in the absence and presence of bovine serum album for 30 days. The polarization studies indicated that 80HA-20TiO2 is the only coating with a narrow passive potential region, −0.4 V to 0 V, showing that adding TiO2 is beneficial for the stability of HA coating. | [91] |
| Kerosene | 800 LPM for NiAl, 920 LPM for stellie-6 | NiAl and Stellite-6 | 304 stainless steel | The flow rates of oxygen and kerosene were low for NiAl powder due to its lower flowability. Testing was done in a biomass combustor and industrial-scale boiler. The corrosion resistance of stellie-6 coating was excellent in both combustion systems. In contrast, the anti-corrosion performance of NiAl coating was depleted because of the production of the Al2O3 layer at the substrate/coating interface. | [92] |
| Hydrogen | O2/H2 ratio: 0.34, 0.31 | WC-12 wt.% Co | Magnesium alloy ZE41 | Two O2-H2 ratio values were used to assess the effect of gas flow transport on the coating morphology. The lowest amount of porosity was gained by the O2/H2 ratio of 0.34. Due to this low porosity, the coating with two layers protected the substrate from 3 wt.% NaCl solution for 96 h. | [93] |
| Kerosene | 566.4 SLPM | Al62.5 Cu25Fe12.5 and Al67Cu20Fe5Cr8 | Ferritic stainless steel | The corrosion resistance was measured in acidic and alkaline media in the presence and absence of chlorides. After the electrochemical test, the Al-Cu-Fe-Cr coating performed better, having a less corroded surface in the presence of chlorides. | [94] |
| Hydrogen | 280 LPM | Cr3C2-WC-NiCoCrMo and Cr3C2-(25NiCr) | Inconel 625, P91 stainless steel | A hot corrosion test was performed under cyclic conditions in an NaCl-KCl-Na2SO4 salt environment at 500 °C. As compared to Cr3C2-(25NiCr), Cr3C2-WC-NiCoCrMo coating exhibited a superior resistance against corrosion due to low porosity. The thickness of the corrosion scale was one-third for the Cr3C2-WC-NiCoCrMo coating compared to Cr3C2-(25NiCr). | [95] |
| Natural gas | 330 LPM | NiCrWMoCuCBFe | 316 stainless steel | The corrosion resistance of the coating was evaluated in artificial seawater after 3, 8, 14, and 20 h of cavitation erosion. With the increase in time, the cavitation erosion rate slowed down and the corrosion performance improved. | [96] |
| Propane | 253 LPM | Cr3C2 25(Ni-20Cr) | SAE 1020 carbon steel | To determine the corrosion rate, 3.5 wt.% NaCl solution was used at 25 °C. There was more of an impact of cavitation on the kinetics of corrosion than an impact of corrosion on the cavitation resistance of the coating. | [97] |
| Propane | 215, 220, 250 LPM | WC-10Co-4Cr | AISI 410 stainless steel | The temperature of the particles increased during deposition due to a decrease in the oxygen fraction of the gas mixture composition, which contributed to the reduction of porosity. The coating layer with a lower porosity had a better performance in terms of corrosion resistance. | [98] |
| Kerosene | 533 LPM | Fe48Mo14Cr15Y2C15B6 | 316 SS | The corrosion behavior of the coating was examined under atmospheric pressure (1 atm) and high hydrostatic pressure (80 atm). Concerning 1 atm, the general corrosion rate of the coating increased at 80 atm. | [99] |
| Kerosene | 900 LPM | CoNiCrAlYSi and Al2O3- YSZ | Inconel 738 | A hot corrosion test was performed for 220 h at 880 °C in an electrical tube furnace. The results showed that the central protective oxide was α-Al2O3 on the coating. | [100] |
| Kerosene | 850 LPM | CoNiCrAlY/YSZ/YSZ-10 wt.%La2O3 | Inconel 738 | The results indicated that the topcoat reduced the infiltration of corrosive components during the hot corrosion test at 880 °C for 400 h. | [101] |
| Kerosene | 920 LPM | WC-12%Co | Carbon steel | The coating produced by the nanostructure HVOF procedure had the best microstructure due to a low porosity. | [102] |
| Hydrogen | 14 LPM | Ni-20%Cr and Cr3C2-25% | A 286 superalloy | The uncoated superalloy severely suffered from hot corrosion under cyclic conditions at 700 °C compared to the coating. | [103] |
| Substrate | Coating Material | Spray Distance (mm) | Remarks | Study |
|---|---|---|---|---|
| Mild steel | Fe3Al/TiC and Fe3Al-Cr/TiC | 380 | The corrosion resistance of Fe3Al/TiC coating increased during the electrochemical test after adding chromium. | [117] |
| 304 stainless steel | Fe3Al | 380 | Electrochemical impedance spectroscopy revealed that the coating corrosion resistance is inferior because of porosity and cracks. | [118] |
| Monel K500 | WC-10 wt.% Ni-5 wt.% Cr, WC-18 wt.% Hastelloy C | 330 | HVOF-coated samples showed a better corrosion resistance for 1533 h than that for the uncoated substrate. | [119] |
| Stainless steel | WC-10Co-4Cr, WC-12Co, Cr3C2-NiCr | 450, 420, 400 | WC-10Co-4Cr coating with a low porosity and high toughness showed the best erosion resistance. | [120] |
| AISI 420 stainless steel | Ni3Ti and Ni3Ti+(Cr3C2 + 20NiCr) | 200–250 | The thermocyclic hot corrosion test was conducted in a molten salt environment for nearly 50 cycles at 650 °C. | [121] |
| - | WC-12Co and CeO2 modified WC-12Co | 350 | The coating was tested in 1 mol/L H2SO4 and 3.5 wt.% NaCl solution for 168 h. The corrosion resistance was better in the NaCl solution than that in the H2SO4 solution for both CeO2 modified WC-12Co and WC-12Co coatings. | [122] |
| Grey cast iron | Inconel 718-nano-Al2O3 | 200 | The hot corrosion performance of coated and bare specimens was evaluated in a high-temperature furnace at 900 °C for 50 h. The composite coating containing 30 wt.% of Al2O3 content exhibited maximum corrosion resistance and hardness. | [123] |
| SUS 316 | Fe40-Cr19-Mo18-C15-B8 alloy | 330–360 | A rotating corrosion tester was used for the corrosion test. The testing duration was 2400 s, and the rotating speed of the spindle was 200 rpm. The coating’s potentiodynamic polarization curves were measured in four solutions: seawater, 0.5 M H2SO4, 3.5 wt.% NaCl, and 1 M NaOH. The coating revealed an excellent corrosion resistance, with a 185 nm depth in the coating surface. | [124] |
| AISI 304 stainless steel | Al2O3-30(Ni20Al) | 200 | Both the coated and uncoated samples were subjected to thermal cycling tests at 750 and 850 °C in an air atmosphere for 2, 6, 10, and 15 days of exposure time. There was no oxidation on the coated samples, but the uncoated samples were harshly corroded. | [125] |
| T 22 boiler tube steel | Ni22Cr10Al-1Y and Ni–22Cr–10Al–1Y–20 wt.% SiC | 200 | The hot corrosion behavior of coated and bare steel was determined in a molten salt environment for 50 cycles at 900 °C. The performance of the composite coating Ni–22Cr–10Al–1Y–20 wt.% SiC, with a low porosity of 0.97%, was better than that of the Ni22Cr10Al-1Y coating, with a porosity of 1.7%. | [126] |
| AISI 440C stainless steel | WC-12 wt.% Co | 380 | The corrosion performance of conventional and suspension WC-Co powders was compared by an electrochemical test. Compared to traditional HVOF, the suspension HVOF technique showed a lower corrosion resistance. | [127] |
| Low carbon martensitic stainless steel. | Cr3C2-25NiCr, WC-25WB-10Co-5NiCr, WC-10Co-4Cr and MoB-25NiCr | 350 | Compared to other coatings, the WC-25WB-10Co-5NiCr coating was the most compact, with no micro defects, and it had the highest corrosion resistance. Cr3C2-25NiCr had the lowest corrosion resistance in a NaCl solution and deionized water. | [128] |
| 316 SS | WC-10Co4Cr | 300 | The sealed, treated WC-10Co4Cr coating showed a higher corrosion resistance than the sprayed WC-10Co4Cr coating. | [129] |
| 316 SS | CrN film and Cr3C2-20NiCr interlayer | 160 | The hot corrosion behaviors of single CrN film, Cr3C2-20NiCr, and duplex CrN/Cr3C2-20NiCr coatings were compared at 550 °C for 50 h. The duplex coating exhibited the lowest weight gain after 50 h. | [130] |
| T24 and T92 steel | NiMoCrW and CoNiCrAlY | 250 | The coatings were analyzed in a salt mixture of NaCl-KCl and dry air atmosphere for 360 h at 650 °C. Pointedly, the corrosion resistance of both coatings was lower in the salt mixture due to the active oxidation induced by chlorine. However, compared to NiCr coating, these coatings presented a higher corrosion resistance. | [131] |
| T 24 steel | Ni20Cr | 250 | The samples were subjected to deposits of KCl, NaCl, K2SO4, and Na2SO4 for 360 h in dry air at 650 °C. Sulfate-based salts were not the cause of severe damage; while chloride-based deposits were responsible for aggressive damage. However, the coating maintained the substrate integrity. | [132] |
| Inconel 718 | Co-32Ni-21Cr-8Al-0.8Y and Gd2Zr2O7 (topcoat) | 200 | A hot corrosion test was conducted in molten salts with 5-, 10-, 15-, and 20-h cycles at 1000 °C. The performance of Gd2Zr2O7 deposited by EB-PVD was superior in hot corrosion compared to that of the conventional YSZ coating system. | [133] |
| AISI 1045 steel | WC-CoCr | 330 | HVOF-sprayed coating was sealed with an aluminum phosphate sealing agent, and then the corrosion behavior of the sealed and unsealed coatings was compared in different environments. It was demonstrated that sealant improved the corrosion resistance of the coating. | [134] |
| 304 stainless steels | Ni69Al31 | 356 | A corrosion test was conducted at 700 °C for 250 h. Severe corrosion was observed due to Al growth. It was concluded that direct exposure of O/Cl2 gases to the steel/coating interface should be avoided | [135] |
| AISI 4340 steel | WC-10Co4Cr | 300 and 260 | The corrosion resistance of a hydrogen-fueled HVOF gun with a standoff distance of 260 mm was slightly higher than that of a kerosene fuel gun with a standoff distance of 300 mm. This happened due to the decarburization of WC particles at higher temperatures and a lower porosity. | [136] |
| AISI 1045 steel | WC-10Co-4Cr | 330 | The coating’s microbial-influenced corrosion behavior was studied using ultrasound-assisted sealing of the aluminum phosphate. The ultrasound-assisted sealing enhanced the corrosion resistance of the coating. | [137] |
| 316 L stainless steel | FeCrMnWMoSi | 380 | The Fe-based coating performed better against pit corrosion in seawater than stainless steel. | [138] |
| Carbon steel | WC-40Cr3C2-25NiCr, WC-10Co4Cr and Cr3C2-25NiCr | 380 | The corrosion resistances of two powders—except Cr3C2-25NiCr—were higher than that of the electrolytic hard chrome coating. | [139] |
| 1045 steel substrate | WC-10Co4Cr | 420 | The results showed that the coating showed a dense microstructure, a low porosity, and no negative transformation in saturated saltwater. | [140] |
| Stainless steel | WC-10Co-4Cr | 300 | The coating influenced the corrosion resistance in sea water compared to the steel substrate. | [141] |
| Inconel 625 | NiCoCrAlYTa and YSZ | 250 | The hot corrosion test was conducted for 52 h at 1000 °C. The oxidation compounds formed can damage the coating deposited by APS more than those using the HVOF technique. | [142] |
| Low carbon steel | Inconel 718 | 254 | Most oxide contents were iron and chromium. The chromium oxide layer was not stable at high temperatures after the corrosion test. | [143] |
| Sr No. | Coating Material | Oxygen Flowrate (L/min) | Spray Distance (mm) | Porosity (%) | Ref. |
|---|---|---|---|---|---|
| 1 | CrCNiCr | 253 | 250 | 0.9 | [97] |
| 2 | CrCNiCr | 935 | 380 | 0.9 | [139] |
| 3 | CrCNiCr | 950 | 400 | 0.74 | [120] |
| 4 | FeMoCrYCB | 533 | 350 | 0.3 | [99] |
| 5 | PWD1008 | 355 | 25.4 | 1.22 | [143] |
| 6 | TiCFeCrAl | 214 | 240 | 0.82 | [62] |
| 7 | TiCFeCrAl | 188 | 240 | 0.96 | [62] |
| 8 | NiCrAlY | 250 | 200 | 1.64 | [84] |
| 9 | NiCrAlY | 245 | 210 | 1.5 | [123] |
| 10 | NiCrAlYSiC | 250 | 200 | 0.95 | [84] |
| 11 | NiCrAlYSiC | 250 | 200 | 1.57 | [84] |
| 12 | NiCrAlYSiC | 250 | 200 | 1.63 | [84] |
| 13 | WCCoCr | 215 | 200 | 1.43 | [98] |
| 14 | WCCoCr | 215 | 230 | 1.98 | [98] |
| 15 | WCCoCr | 220 | 200 | 0.75 | [98] |
| 16 | WCCoCr | 220 | 230 | 3.01 | [98] |
| 17 | WCCoCr | 250 | 230 | 0.3 | [98] |
| 18 | WCCoCr | 943 | 330 | 0.53 | [137] |
| 19 | WCCoCr | 943 | 370 | 0.31 | [137] |
| 20 | WCCoCr | 100 | 240 | 1.28 | [63] |
| 21 | WCCoCr | 935 | 380 | 0.13 | [63] |
| 22 | WCCoCr | 835 | 350 | 0.5 | [44] |
| 23 | WCCoCr | 950 | 450 | 0.87 | [120] |
| 25 | In718Al2O3 | 270 | 200 | 1.2 | [123] |
| 26 | YSZ | 130 | 250 | 6.5 | [142] |
| 27 | Woka400 | 900 | 300 | 0.76 | [54] |
| 28 | DJ2600 | 280 | 260 | 0.62 | [54] |
| 29 | WCCo | 950 | 420 | 0.99 | [120] |
| 30 | CoNiCrAlY | 250 | 200 | 2.5 | [133] |
| 31 | HC | 893 | 300 | 1.19 | [85] |
| 32 | Fe alloy | 864 | 280 | 1.66 | [82] |
| 33 | Fe alloy | 940 | 280 | 1.74 | [82] |
| 34 | Fe alloy | 1015 | 280 | 1.78 | [82] |
| 35 | Fe alloy | 864 | 330 | 0.49 | [82] |
| 36 | Fe alloy | 940 | 330 | 1.06 | [82] |
| 37 | Fe alloy | 1015 | 330 | 3.19 | [82] |
| 38 | Fe alloy | 864 | 380 | 1.42 | [82] |
| 39 | Fe alloy | 940 | 380 | 1.76 | [82] |
| 40 | Fe alloy | 1015 | 380 | 0.53 | [82] |
| 41 | Fe alloy | 967 | 330 | 0.48 | [47] |
| 42 | HAS | 850 | 380 | 3.37 | [119] |
| 43 | WCN | 950 | 300 | 4.18 | [119] |
| 44 | CoNiCrAlY/YSZ/La2O3 | 850 | 200 | 1.5 | [118] |
| 45 | Al2O3-NiAl | 231 | 250 | 2 | [125] |
| 46 | Stellite6 | 830 | 250 | 1.5 | [113] |
| 47 | Stellite6 | 214 | 250 | 0.51 | [66] |
| 48 | Stellite6 | 201 | 250 | 0.89 | [66] |
| 49 | Stellite6 | 188 | 280 | 4.09 | [66] |
| 50 | WCCrCNiCr | 935 | 380 | 0.87 | [139] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raza, A.; Ahmad, F.; Badri, T.M.; Raza, M.R.; Malik, K. An Influence of Oxygen Flow Rate and Spray Distance on the Porosity of HVOF Coating and Its Effects on Corrosion—A Review. Materials 2022, 15, 6329. https://doi.org/10.3390/ma15186329
Raza A, Ahmad F, Badri TM, Raza MR, Malik K. An Influence of Oxygen Flow Rate and Spray Distance on the Porosity of HVOF Coating and Its Effects on Corrosion—A Review. Materials. 2022; 15(18):6329. https://doi.org/10.3390/ma15186329
Chicago/Turabian StyleRaza, Ali, Faiz Ahmad, Thar M. Badri, M. R. Raza, and Khurshid Malik. 2022. "An Influence of Oxygen Flow Rate and Spray Distance on the Porosity of HVOF Coating and Its Effects on Corrosion—A Review" Materials 15, no. 18: 6329. https://doi.org/10.3390/ma15186329
APA StyleRaza, A., Ahmad, F., Badri, T. M., Raza, M. R., & Malik, K. (2022). An Influence of Oxygen Flow Rate and Spray Distance on the Porosity of HVOF Coating and Its Effects on Corrosion—A Review. Materials, 15(18), 6329. https://doi.org/10.3390/ma15186329








