Strength Characteristics and Microstructure Analysis of Alkali-Activated Slag–Fly Ash Cementitious Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Scheme and Mix Design
2.2. Raw Materials
2.2.1. Powdered Ore
2.2.2. Fly Ash
2.2.3. Fine Sand
2.2.4. Water Glass
2.2.5. Standard Sand
2.2.6. Mixing Water
2.3. Test Method
2.3.1. Preparation of Specimens
2.3.2. Test Method for Mechanical Properties
2.3.3. Microstructural Analysis Methods
3. Results and Discussion
3.1. Mechanical Properties of Alkali Slag Cementitious Materials
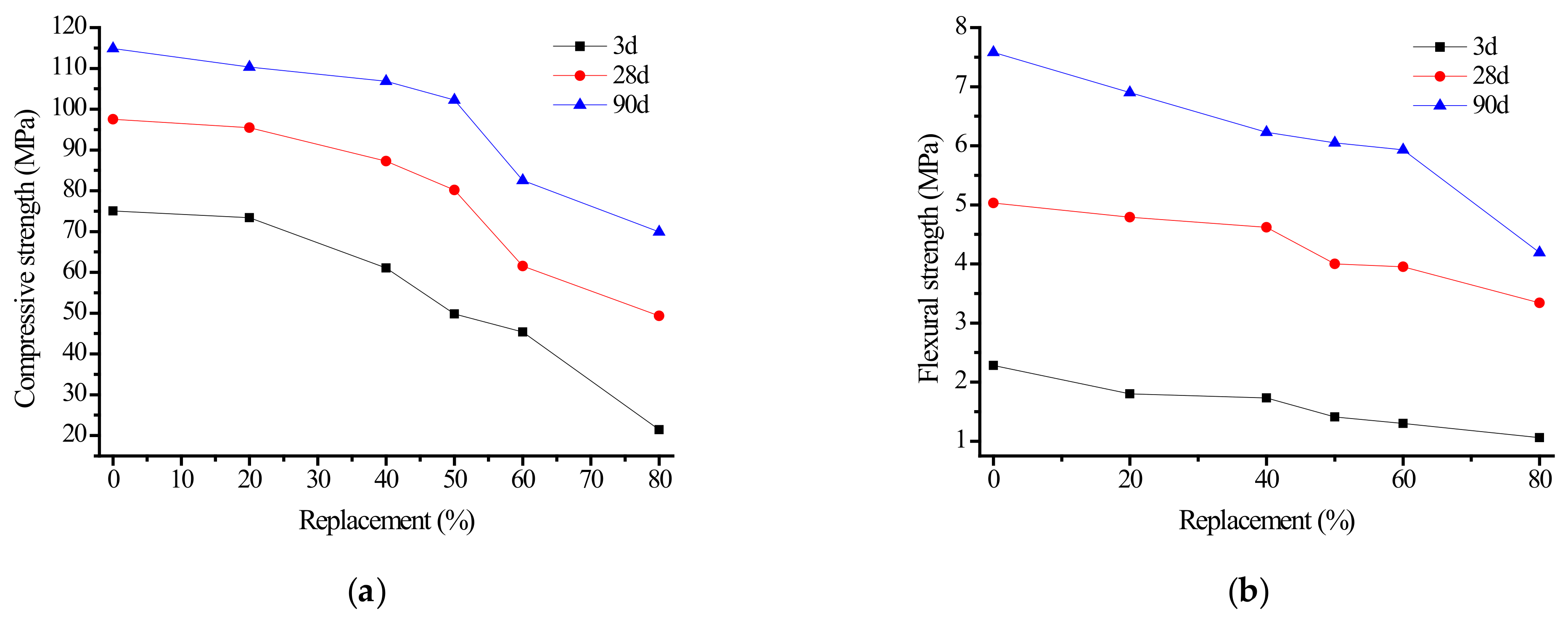
3.1.1. Effect of Fly Ash Content on Strength of Alkali Slag Cement Paste
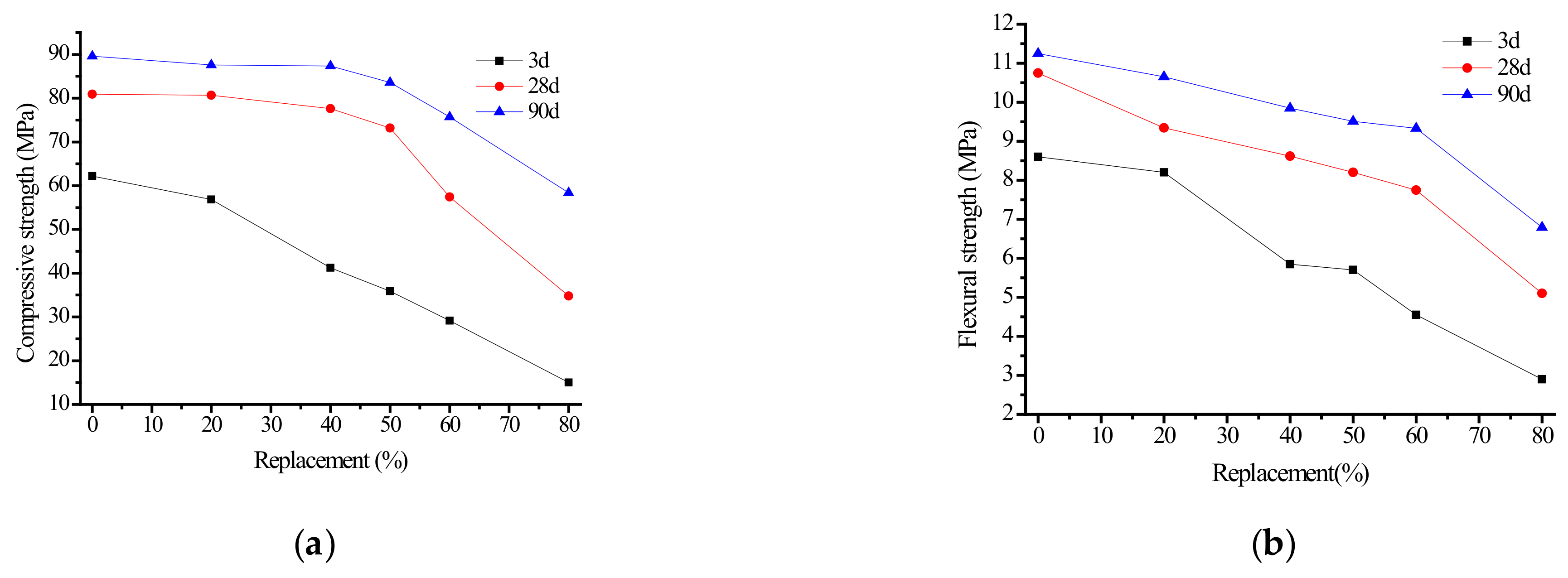
3.1.2. Effect of Fly Ash Content on Strength of Alkali Slag Cement Mortar
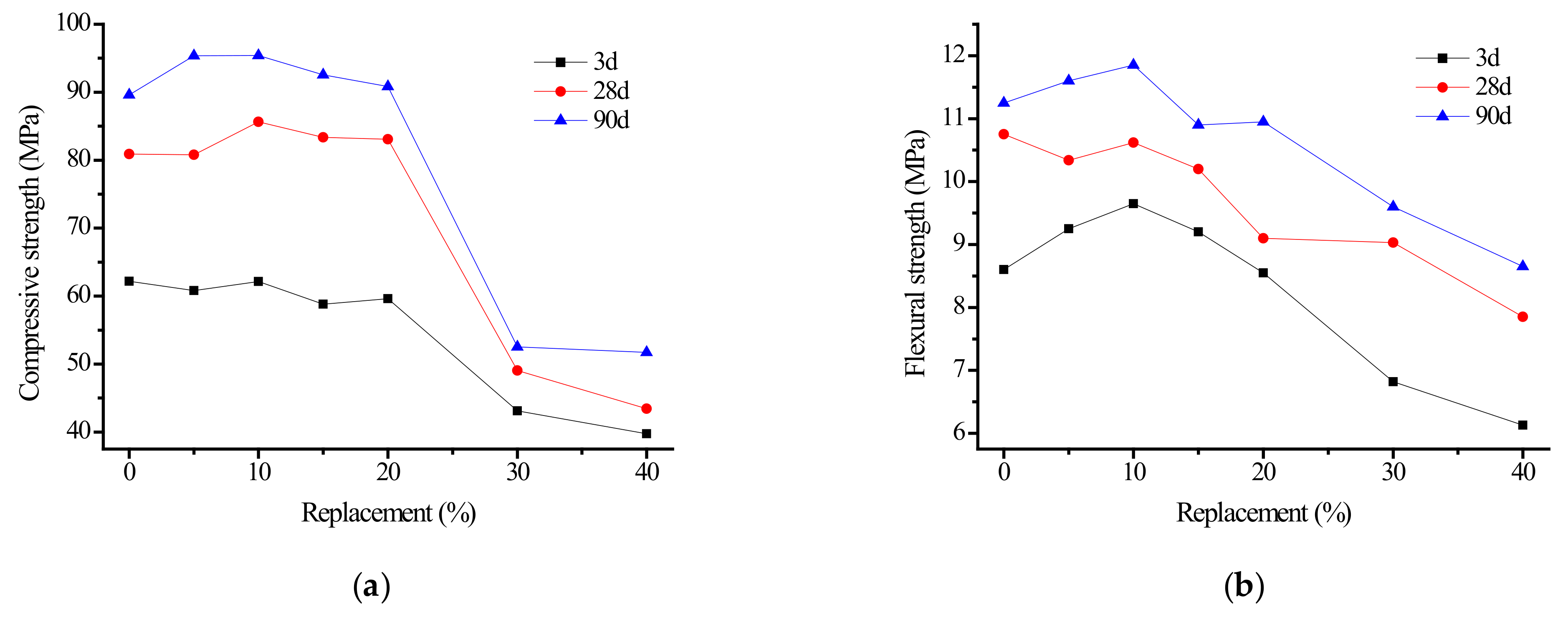
3.1.3. Effect of Sand Content on Strength of Alkali Slag Cement Mortar
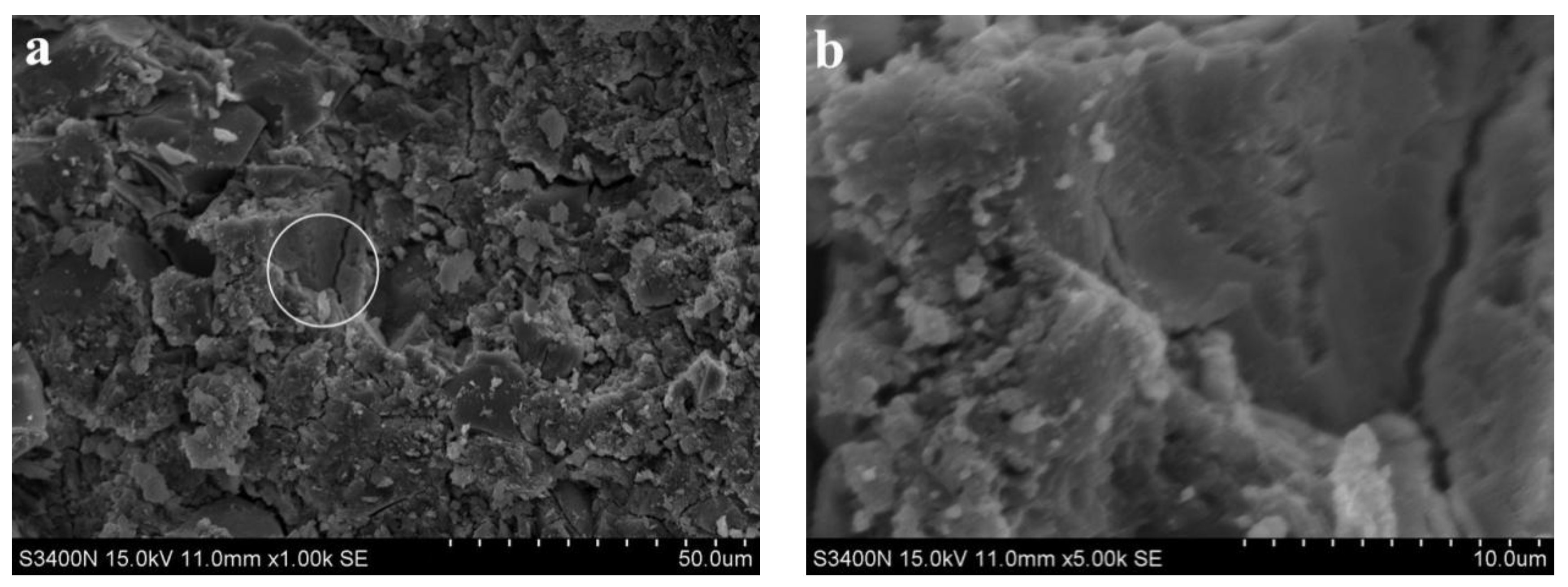
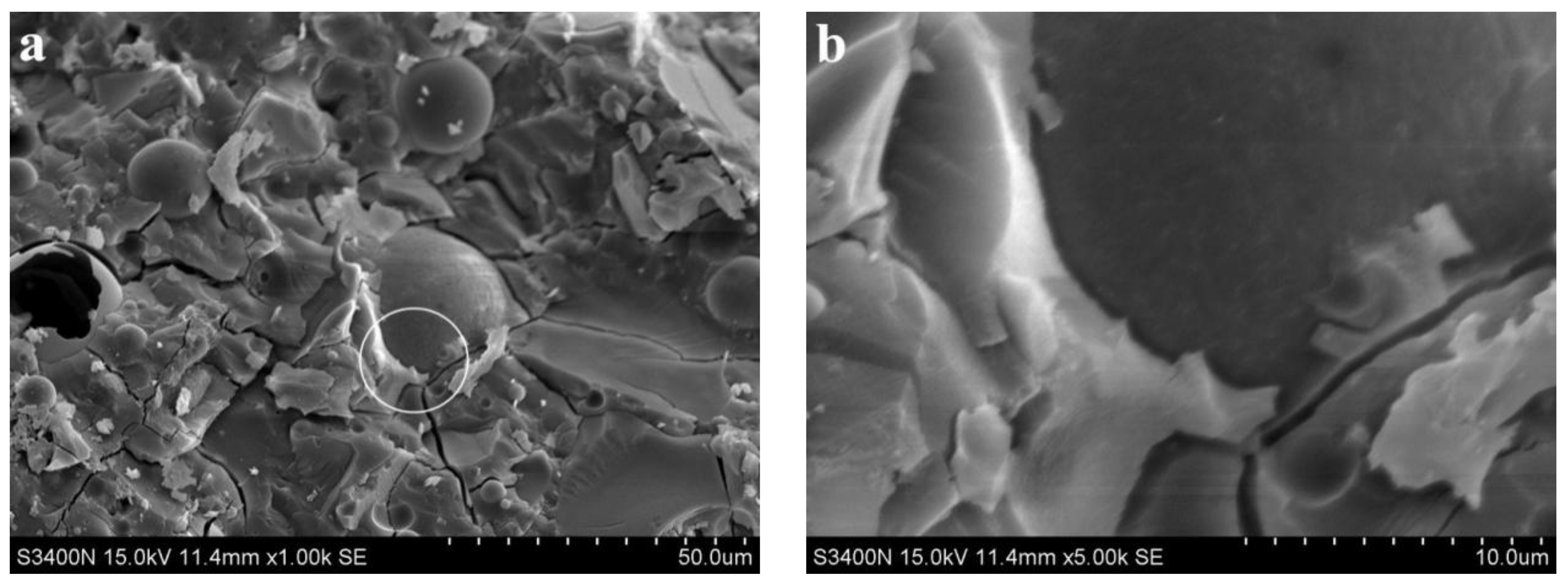
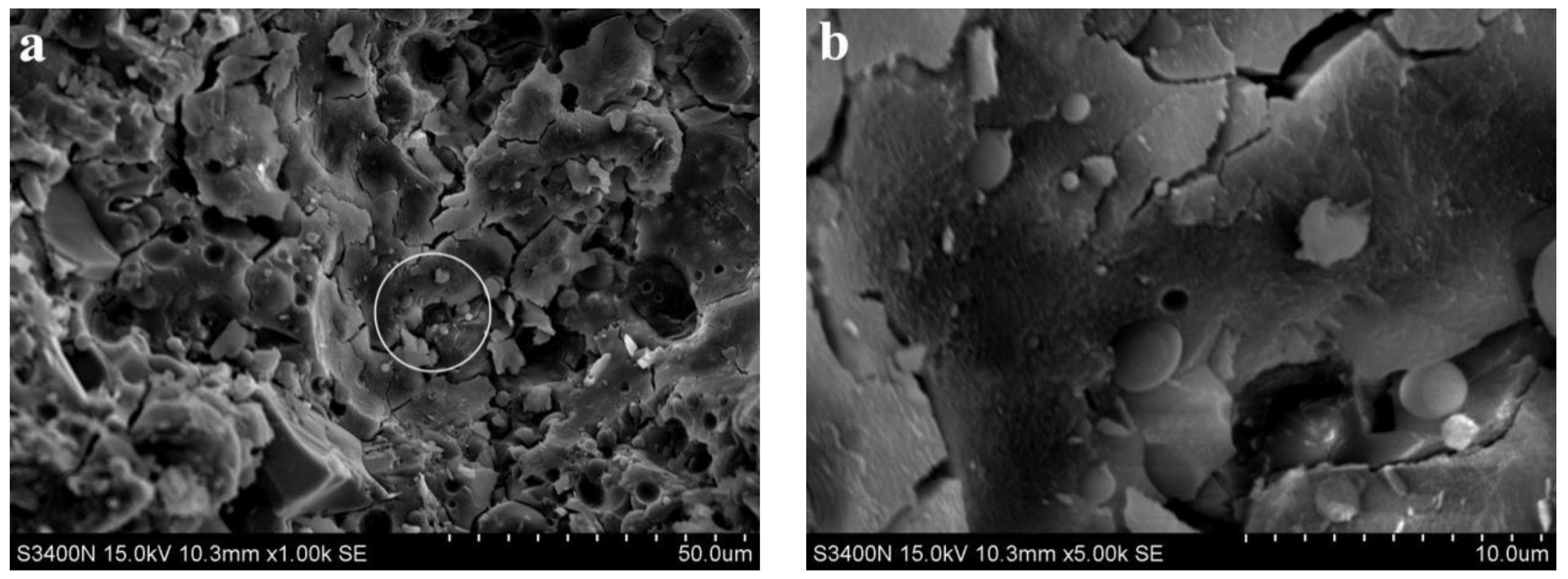
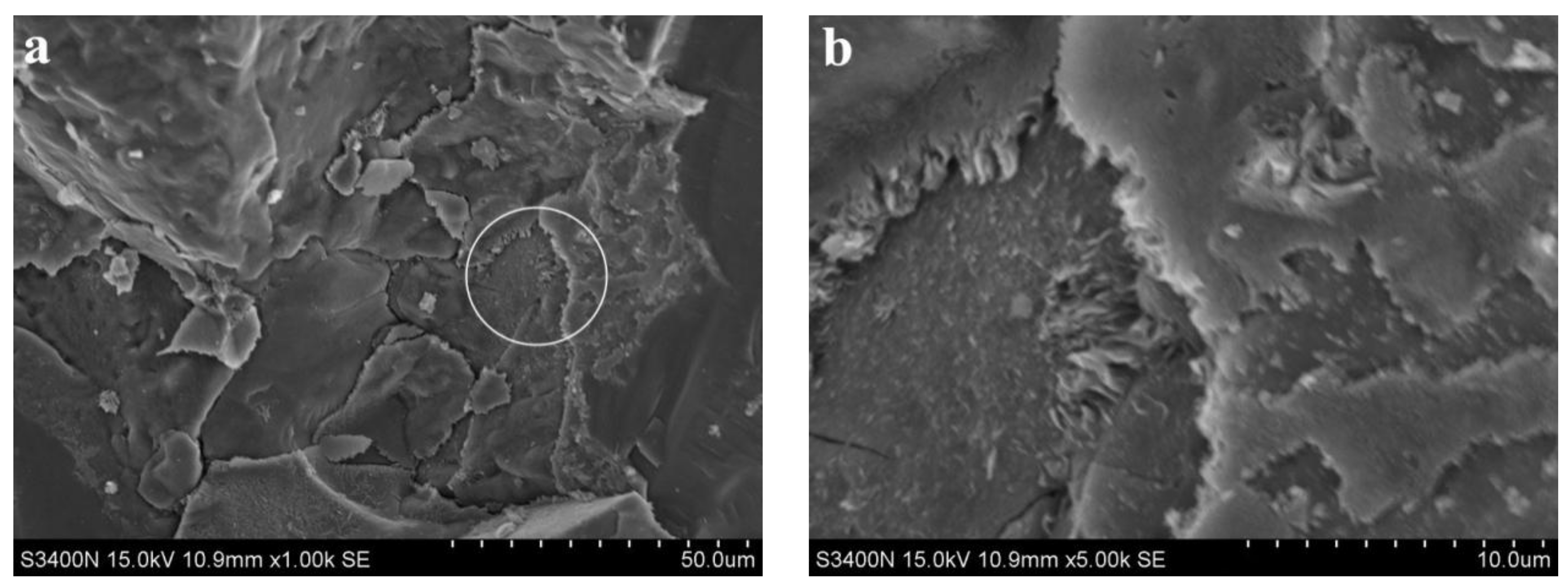
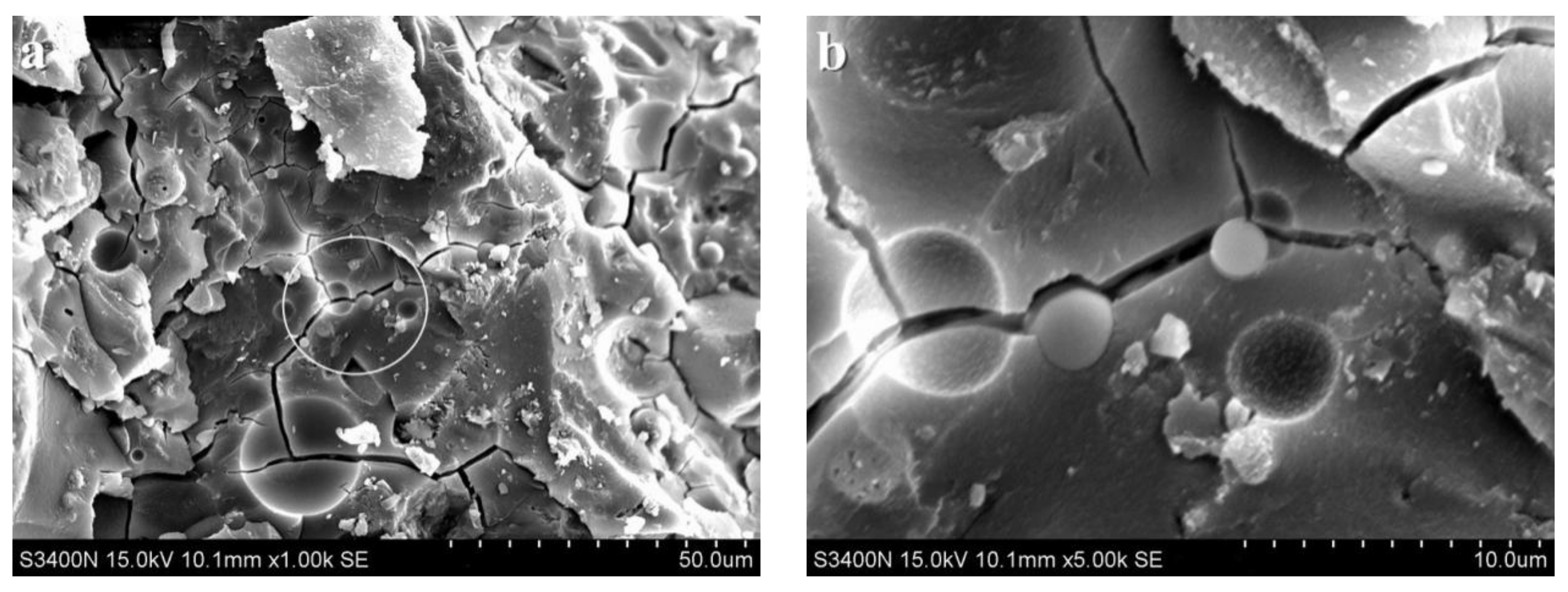
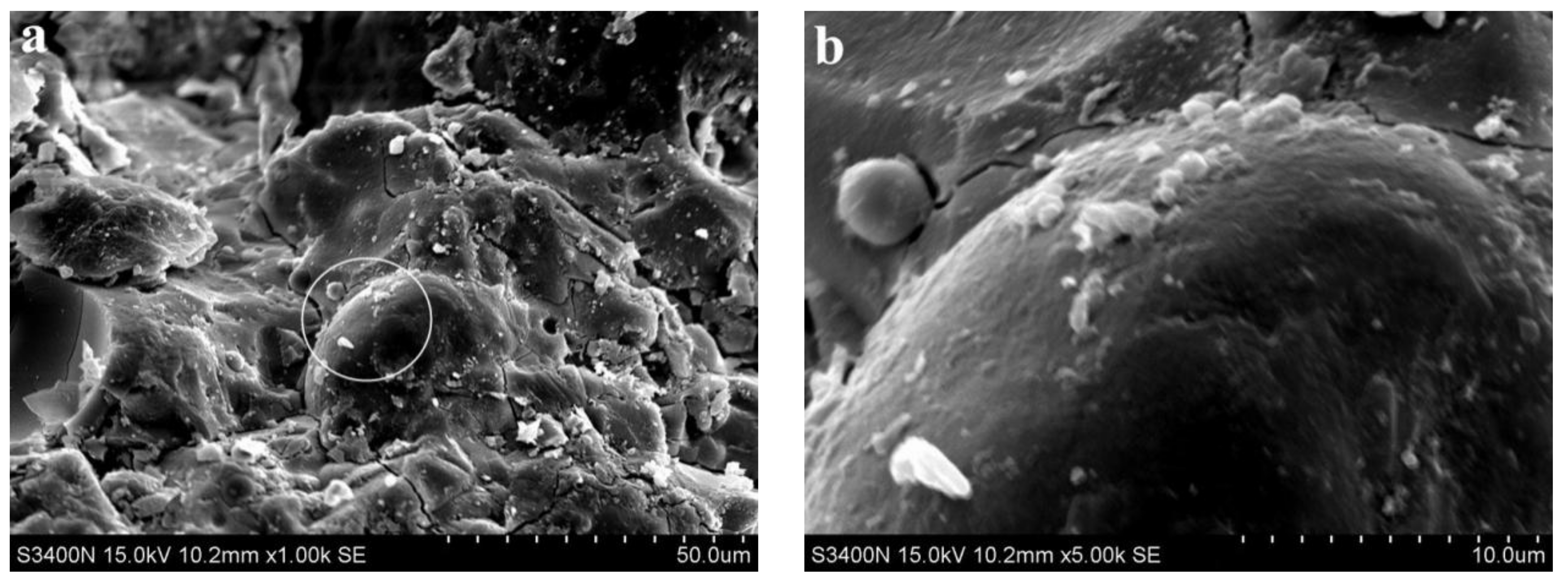
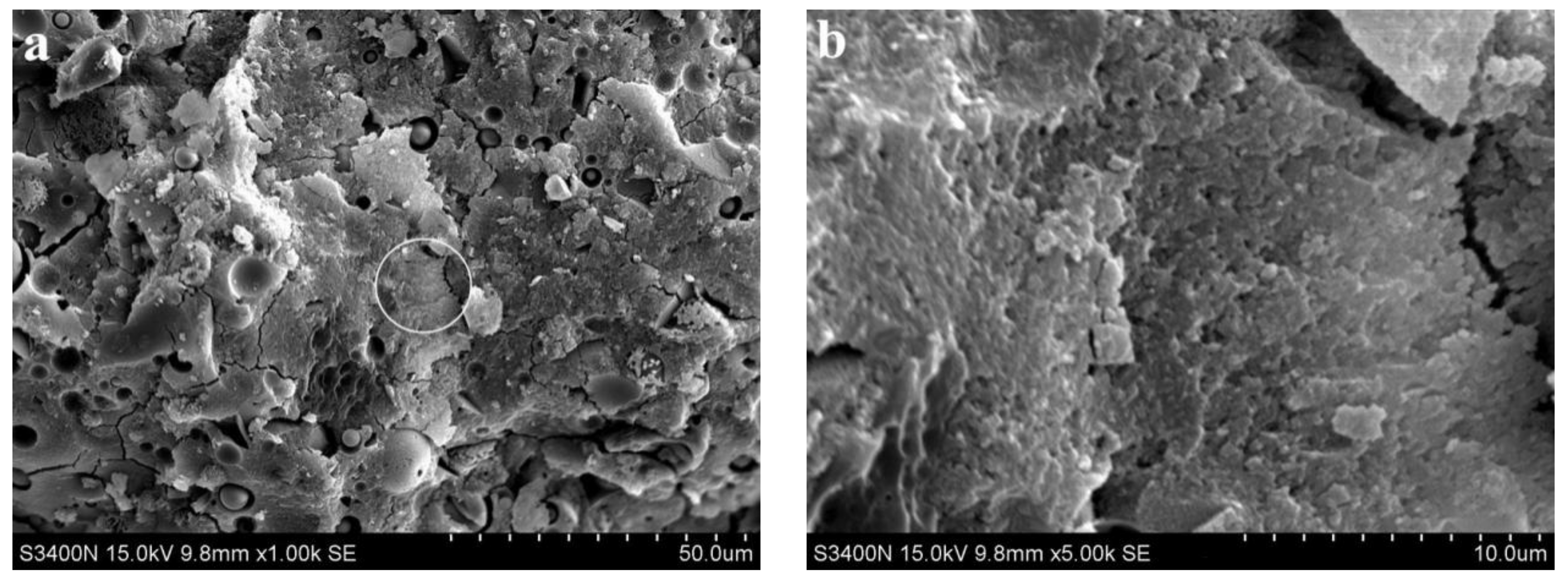
3.2. Analysis of Microscopic Results
3.3. Discussion
4. Conclusions
- (1)
- The substitution of fly ash for ore powder progressively reduces the compressive and flexural strength of alkali slag cement slurry and mortar. When the fly ash content was less than 50%, it had little influence on the strength of the cementing materials. When the fly ash content exceeded 50%, the compressive and bending strength of the specimens decreased significantly. Considering the practical application, economic benefits, and energy conservation, it is suggested that the fly ash content should be approximately 50%.
- (2)
- Fine sand may be used to replace ore powder. When the content of fine sand was less than 20%, the strength of the alkali slag cement mortar changed little. When the content of fine sand exceeded 30%, the compressive and flexural strength of the alkali slag cement mortar decreased significantly. The alkali slag cement specimens with different proportions of fine sand all had higher early strength. With the extension of curing age, the alkali slag cement specimens exhibited no late strength shrinkage phenomenon.
- (3)
- SEM analysis showed that the cementing material of alkali slag with hydration age of 3 d has been hydrated to a great extent, and the structure of the early hardened slurry was already very dense. The density of the slurry increased with the extension of hydration age.
- (4)
- With increasing fly ash content, the density of the slurry decreased, and a large number of unhydrated fly ash particles appeared in the SEM images of 3 d hydration age, and these particles still existed until 28 d. With increasing fine sand content, the density of the slurry decreased, and a large number of microcracks with a width of about 1 μm appeared in the SEM image of 3 d hydration age. With the extension of hydration age, the microcracks gradually decreased.
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Feng, X.Z.; Zou, J. Economic analysis of CO2 emission trends in China. China Popul. Resour. Environ. 2008, 18, 43–47. [Google Scholar]
- Fengming, X.; Tiemao, S.; Jiaoyue, W.; Longfei, B.; Ying, Z.; Zhu, L.; Davis, S.J. Review of cement materials carbon sink. Progress. Inquisitiones Mutat. Clim. 2015, 11, 288–296. [Google Scholar]
- Puertas, F.; Martínez-Ramírez, S.; Alonso, S.; Vázquez, T. Alkali-activated fly ash/slag cements: Strength behaviour and hydration products. Cem. Concr. Res. 2000, 30, 1625–1632. [Google Scholar] [CrossRef]
- Hojati, M.; Radlińska, A. Shrinkage and strength development of alkali-activated fly ash-slag binary cements. Constr. Build. Mater. 2017, 150, 808–816. [Google Scholar] [CrossRef]
- Shen, W.; Wang, Y.; Zhang, T.; Zhou, M.; Li, J.; Cui, X. Magnesia modification of alkali-activated slag fly ash cement. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2011, 26, 5. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, C.; Zhang, Z. Chloride binding of alkali-activated slag/fly ash cements. Constr. Build. Mater. 2019, 226, 21–31. [Google Scholar] [CrossRef]
- Sun, B.; Ye, G.; de Schutter, G. A review: Reaction mechanism and strength of slag and fly ash-based alkali-activated materials. Constr. Build. Mater. 2022, 326, 126843. [Google Scholar] [CrossRef]
- White, C.E.; Daemen, L.L.; Hartl, M.; Page, K. Intrinsic differences in atomic ordering of calcium (alumino) silicate hydrates in conventional and alkali-activated cements. Cem. Concr. Res. 2015, 67, 66–73. [Google Scholar] [CrossRef]
- Glukhovsky, V.D. Soil Silicates; Gosstroi Publishers: Kiev, Ukraine, 1959. [Google Scholar]
- Roy, D.M.; Silsbee, M.R.; Wolfe-Confer, D. New rapid setting alkali activated cement compositions. MRS Proc. 1990, 179, 203–220. [Google Scholar] [CrossRef]
- Wang, S.D.; Scrivener, K.L. Hydration products of alkali activated slag cement. Cem. Concr. Res. 1995, 25, 561–571. [Google Scholar] [CrossRef]
- Pu, X.C.; Wang, C.; Liu, F.; Wang, W.Y.; Wan, C.J.; Yang, C.H. Development and looking forword of ultra high strength high performace concrete(UHSHPC). China Concr. Cem. Prod. 2008, 1–5. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Y.; Zhu, H.; Zhang, Y.; Provis, J.L.; Wang, H. Effect of drying procedures on pore structure and phase evolution of alkali-activated cements. Cem. Concr. Compos. 2018, 96, 194–203. [Google Scholar] [CrossRef]
- Xue, L.; Zhang, Z.; Wang, H. Early hydration kinetics and microstructure development of hybrid alkali activated cements (HAACs) at room temperature. Cem. Concr. Compos. 2021, 123, 104200. [Google Scholar] [CrossRef]
- Fang, Y.H.; Gu, Y.M.; Kang, Q.B. Effect of Fly Ash MgO and Curing Solution on the Chemical Shrinkage of Alkali-Activated Slag Cement. In Advances in Building Materials Pts 1–3; Li, L.J., Ed.; Trans Tech Publications LTD: Stafa-Zurich, Switzerland, 2011; pp. 2008–2012. [Google Scholar]
- Fang, Y.; Zhu, Q.; Cen, Y.; Bian, L. Properties and structure of high volume superfine blast furnace slag powder cementing materials and the effect of phosphorous gypsum addition. J. Chin. Ceram. Soc. 2008, 36, 444–450. [Google Scholar]
- Gu, Y.; Fang, Y. Shrinkage, cracking, shrinkage-reducing and toughening of alkali-activated slag cement-A short review. J. Chin. Ceram. Soc. 2012, 40, 76–84. [Google Scholar]
- Gu, Y.M.; Fang, Y.H.; You, D.; Gong, Y.F.; Zhu, C.H. Properties and microstructure of alkali-activated slag cement cured at below-and about-normal temperature. Constr. Build. Mater. 2015, 79, 1–8. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Fang, Y.H.; Jia, L.L.; Lu, F.F. Hardened paste of alkali-activated phosphorous slag cement and structure of paste-aggregate interface. J. Hohai Univ. (Nat. Sci.) 2010, 38, 72–75. [Google Scholar]
- Jia, L.L.; Fang, Y.H.; Chen, Y.Q. Study on Shrinkage-reduction and Cracking-resistance of Alkali-activated Phosphor Slag Cement. Mater. Rep. 2008, 22, 153–156. [Google Scholar]
- Rosales, J.; Agrela, F.; Díaz-López, J.L.; Cabrera, M. Alkali-activated stainless steel slag as a cementitious material in the manufacture of self-compacting concrete. Materials 2021, 14, 3945. [Google Scholar] [CrossRef]
- Schade, T.; Bellmann, F.; Middendorf, B. Quantitative analysis of C-(K)-A-S-H-amount and hydrotalcite phase content in finely ground highly alkali-activated slag/silica fume blended cementitious material. Cem. Concr. Res. 2022, 153, 106706. [Google Scholar] [CrossRef]
- Tuinukuafe, A.; Kaub, T.; Weiss, C.A., Jr.; Allison, P.G.; Thompson, G.B.; Amirkhanian, A. Atom probe tomography of an alkali activated fly ash concrete. Cem. Concr. Res. 2019, 121, 37–41. [Google Scholar] [CrossRef]
- Zhang, Y.J.; He, P.Y.; Chen, H.; Liu, L.C. Green transforming metallurgical residue into alkali-activated silicomanganese slag-based cementitious material as photocatalyst. Materials 2018, 11, 1773. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhi, S.; Li, T.; Zhou, Z.; Li, M.; Han, J.; Wu, Z. Alkali activation of copper and nickel slag composite cementitious materials. Materials 2020, 13, 1155. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, N.; Zhang, J.; Song, S.; Zhang, Y. C-A-S-H gel and pore structure characteristics of alkali-activated red mud–iron tailings cementitious mortar. Materials 2022, 15, 112. [Google Scholar] [CrossRef]
- Singh, J.; Singh, S. Development of alkali-activated cementitious material using copper slag. Constr. Build. Mater. 2019, 211, 73–79. [Google Scholar] [CrossRef]
- Zhang, Q.; Ji, T.; Yang, Z.; Wang, C.; Wu, H.C. Influence of different activators on microstructure and strength of alkali-activated nickel slag cementitious materials—Science Direct. Constr. Build. Mater. 2020, 235, 117449. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Q.; Zhuang, S.; Yang, J. Evaluation of alkali-activated blast furnace ferronickel slag as a cementitious material: Reaction mechanism, engineering properties and leaching behaviors. Constr. Build. Mater. 2018, 188, 860–873. [Google Scholar] [CrossRef]
- Kumar, S.; García-Triñanes, P.; Teixeira-Pinto, A.; Bao, M. Development of alkali activated cement from mechanically activated silico-manganese (SiMn) slag. Cem. Concr. Compos. 2013, 40, 7–13. [Google Scholar] [CrossRef]
- Hanehara, S.; Tomosawa, F.; Kobayakawa, M.; Hwang, K. Effects of water/powder ratio, mixing ratio of fly ash, and curing temperature on pozzolanic reaction of fly ash in cement paste. Cem. Concr. Res. 2001, 31, 31–39. [Google Scholar] [CrossRef]
- Meguro, T.; Oyanagi, H.; Saeki, T.; Saito, T. Effect of glass composition on pozzolanic reaction of fly ash. Cem. Sci. Concr. Technol. 2017, 71, 24–31. [Google Scholar] [CrossRef][Green Version]



| No. | Slag Powder | Fly Ash | Fine Sand | Standard Sand | Water Glass | Water | Total Water |
|---|---|---|---|---|---|---|---|
| A1 | 400 | 0 | 0 | 0 | 136 | 40 | 120 |
| A2 | 320 | 80 | 0 | 0 | 136 | 40 | 120 |
| A3 | 240 | 160 | 0 | 0 | 136 | 40 | 120 |
| A4 | 200 | 200 | 0 | 0 | 136 | 40 | 120 |
| A5 | 160 | 240 | 0 | 0 | 136 | 40 | 120 |
| A6 | 80 | 320 | 0 | 0 | 136 | 40 | 120 |
| B1 | 450 | 0 | 0 | 1350 | 153 | 135 | 225 |
| B2 | 360 | 90 | 0 | 1350 | 153 | 131 | 220 |
| B3 | 270 | 180 | 0 | 1350 | 153 | 126 | 216 |
| B4 | 225 | 225 | 0 | 1350 | 153 | 124 | 214 |
| B5 | 180 | 270 | 0 | 1350 | 153 | 122 | 211 |
| B6 | 90 | 360 | 0 | 1350 | 153 | 117 | 207 |
| C1 | 427.5 | 0 | 22.5 | 1350 | 146 | 130 | 216 |
| C2 | 405 | 0 | 45 | 1350 | 138 | 125 | 206 |
| C3 | 382.5 | 0 | 67.5 | 1350 | 130 | 120 | 197 |
| C4 | 360 | 0 | 90 | 1350 | 123 | 115 | 187 |
| C5 | 315 | 0 | 135 | 1350 | 107 | 105 | 168 |
| C6 | 270 | 0 | 180 | 1350 | 92 | 95 | 149 |
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | MnO | TiO2 | LOI |
|---|---|---|---|---|---|---|---|---|
| 33.23 | 17.76 | 0.416 | 37.17 | 7.53 | 3.10 | 0.404 | 0.992 | — |
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Na2O | P2O5 | LOI |
|---|---|---|---|---|---|---|---|---|
| 51.07 | 36.24 | 2.88 | 1.25 | 0.744 | 0.394 | 0.438 | 0.125 | 3.72 |
| Solid Content | Na2O | SiO2 |
|---|---|---|
| 36.07 | 8.64 | 27.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.; Wan, Y.; Wang, L.; Ye, Y.; Yu, H.; Yang, J. Strength Characteristics and Microstructure Analysis of Alkali-Activated Slag–Fly Ash Cementitious Material. Materials 2022, 15, 6169. https://doi.org/10.3390/ma15176169
Zhu C, Wan Y, Wang L, Ye Y, Yu H, Yang J. Strength Characteristics and Microstructure Analysis of Alkali-Activated Slag–Fly Ash Cementitious Material. Materials. 2022; 15(17):6169. https://doi.org/10.3390/ma15176169
Chicago/Turabian StyleZhu, Chenhui, Yuanyuan Wan, Lei Wang, Yuchen Ye, Houjun Yu, and Jie Yang. 2022. "Strength Characteristics and Microstructure Analysis of Alkali-Activated Slag–Fly Ash Cementitious Material" Materials 15, no. 17: 6169. https://doi.org/10.3390/ma15176169
APA StyleZhu, C., Wan, Y., Wang, L., Ye, Y., Yu, H., & Yang, J. (2022). Strength Characteristics and Microstructure Analysis of Alkali-Activated Slag–Fly Ash Cementitious Material. Materials, 15(17), 6169. https://doi.org/10.3390/ma15176169





