Synthesis of Pyrochlore Oxides Containing Ir and Ru for Efficient Oxygen Evolution Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of A2Ru2O7
2.3. Synthesis of A2Ir2O7
2.4. Synthesis of Pr2(RuxIr1−x)2O7
2.5. Electrochemical Characterization
2.6. Structural Characterization
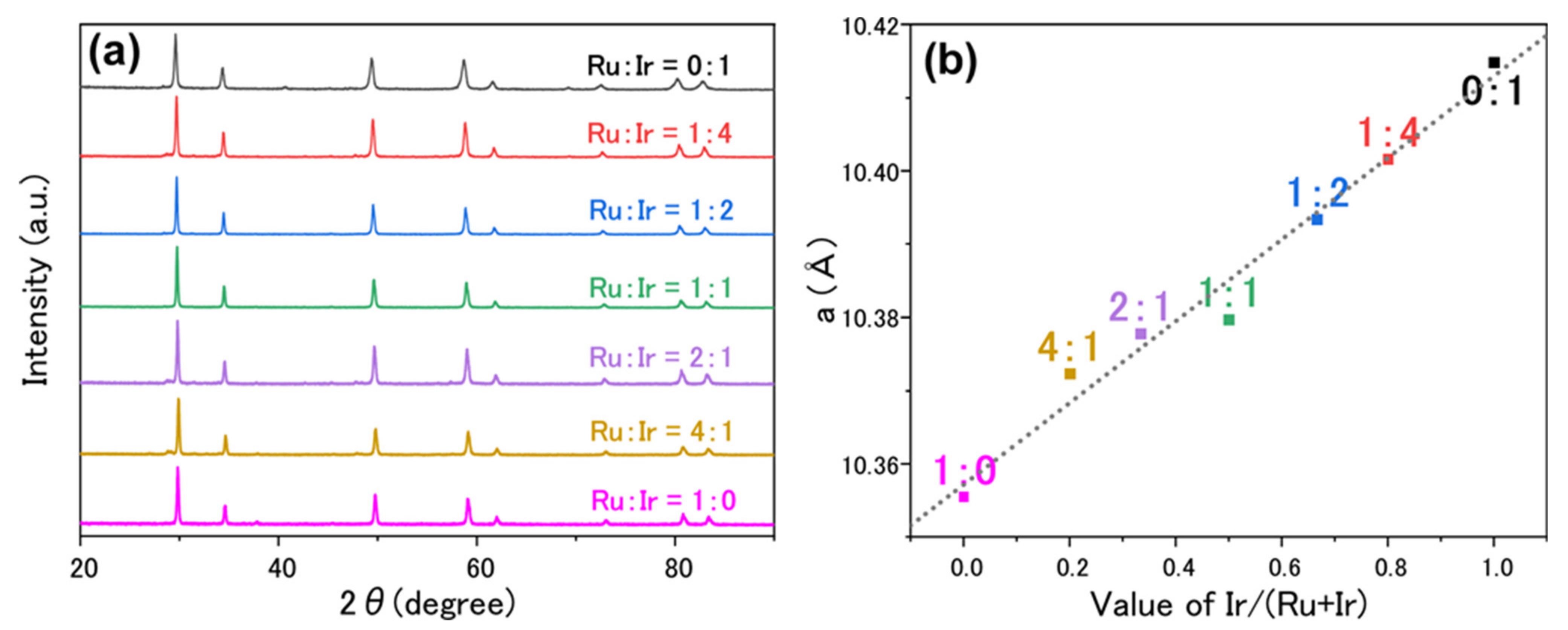
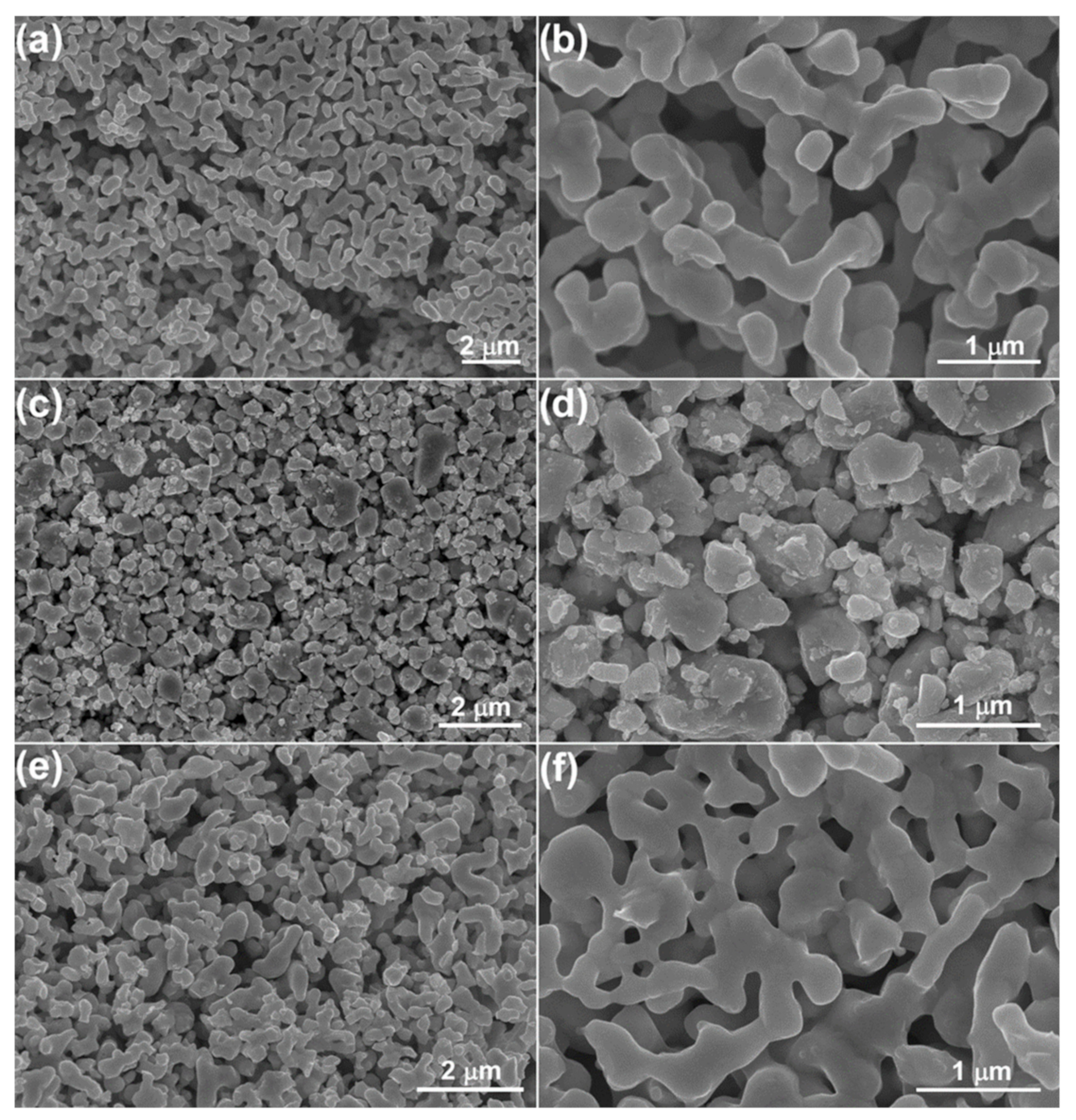
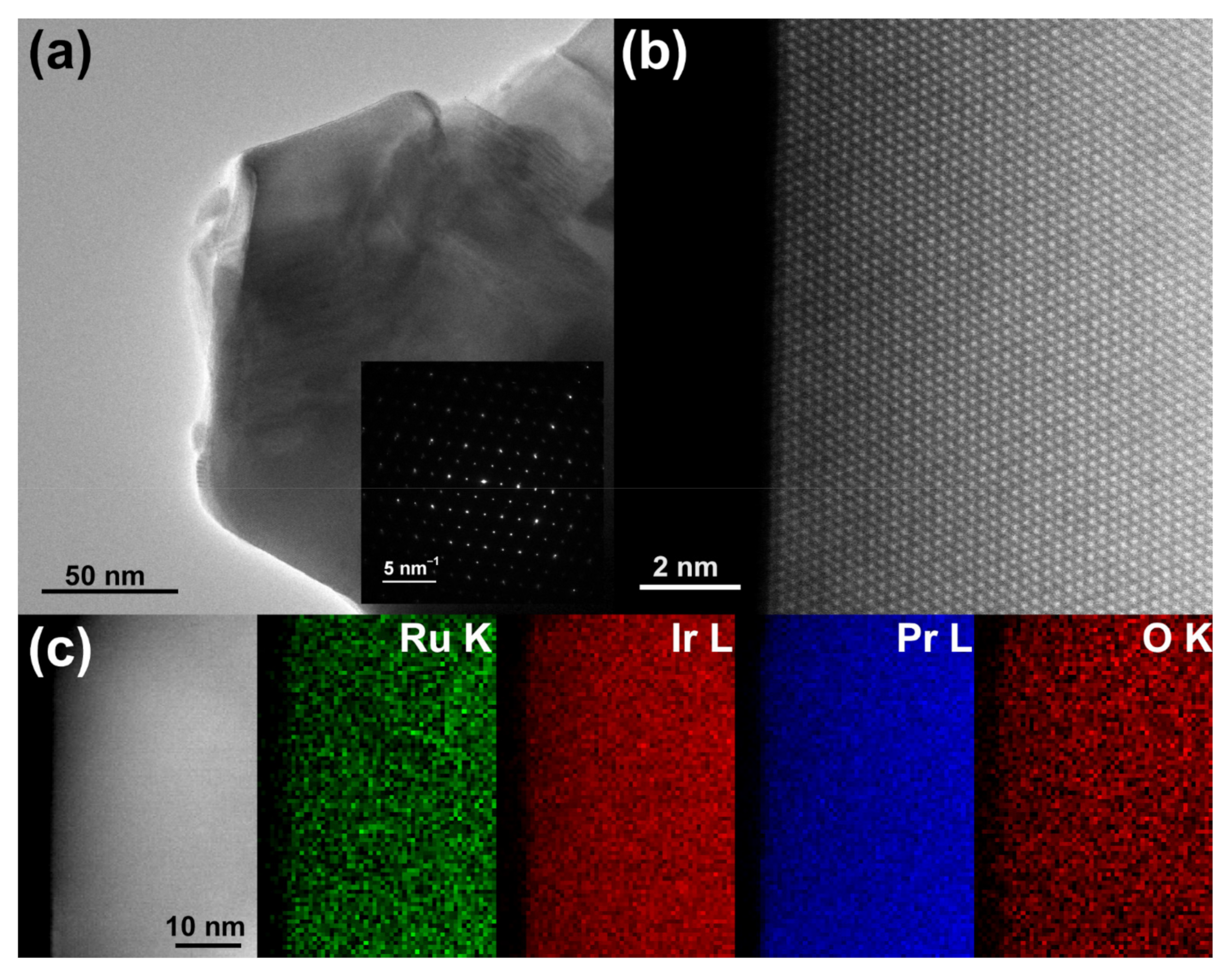
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- You, B.; Sun, Y. Innovative Strategies for Electrocatalytic Water Splitting. Acc. Chem. Res. 2018, 51, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, Y.; Qin, Y.; Zhang, W.; Wang, L.; Luo, M.; Yang, H.; Guo, S. Recent Advances on Water-Splitting Electrocatalysis Mediated by Noble-Metal-Based Nanostructured Materials. Adv. Energy Mater. 2020, 10, 1903120. [Google Scholar] [CrossRef]
- Li, X.; Zhao, L.; Yu, J.; Liu, X.; Zhang, X.; Liu, H.; Zhou, W. Water Splitting: From Electrode to Green Energy System. Nanomicro Lett. 2020, 12, 131. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Xia, B.Y.; Zhao, B.; Wang, X. A review on noble-metal-free bifunctional heterogeneous catalysts for overall electrochemical water splitting. J. Mater. Chem. A 2016, 4, 17587–17603. [Google Scholar] [CrossRef]
- Cook, T.R.; Dogutan, D.K.; Reece, S.Y.; Surendranath, Y.; Teets, T.S.; Nocera, D.G. Solar Energy Supply and Storage for the Legacy and Nonlegacy Worlds. Chem. Rev. 2010, 110, 6474–6502. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.T.; Sunde, S.; Tsypkin, M.; Tunold, R. Performance of a PEM water electrolysis cell using IrxRuyTazO2 electrocatalysts for the oxygen evolution electrode. Int. J. Hydrogen Energy 2007, 32, 2320–2324. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, H.; Chen, G.; Zhang, Y. Study of IrxRu1−xO2 oxides as anodic electrocatalysts for solid polymer electrolyte water electrolysis. Electrochim. Acta 2009, 54, 6250–6256. [Google Scholar] [CrossRef]
- Audichon, T.; Mayousse, E.; Morisset, S.; Morais, C.; Comminges, C.; Napporn, T.W.; Kokoh, K.B. Electroactivity of RuO2–IrO2 mixed nanocatalysts toward the oxygen evolution reaction in a water electrolyzer supplied by a solar profile. Int. J. Hydrogen Energy 2014, 39, 16785–16796. [Google Scholar] [CrossRef]
- Kokoh, K.B.; Mayousse, E.; Napporn, T.W.; Servat, K.; Guillet, N.; Soyez, E.; Grosjean, A.; Rakotondrainibé, A.; Paul-Joseph, J. Efficient multi-metallic anode catalysts in a PEM water electrolyzer. Int. J. Hydrogen Energy 2014, 39, 1924–1931. [Google Scholar] [CrossRef]
- Siracusano, S.; Van Dijk, N.; Payne-Johnson, E.; Baglio, V.; Aricò, A.S. Nanosized IrOx and IrRuOx electrocatalysts for the O2 evolution reaction in PEM water electrolysers. Appl. Catal. B Environ. 2015, 164, 488–495. [Google Scholar] [CrossRef]
- Siracusano, S.; Baglio, V.; Moukheiber, E.; Merlo, L.; Aricò, A.S. Performance of a PEM water electrolyser combining an IrRu-oxide anode electrocatalyst and a short-side chain Aquivion membrane. Int. J. Hydrogen Energy 2015, 40, 14430–14435. [Google Scholar] [CrossRef]
- Siracusano, S.; Baglio, V.; Van Dijk, N.; Merlo, L.; Aricò, A.S. Enhanced performance and durability of low catalyst loading PEM water electrolyser based on a short-side chain perfluorosulfonic ionomer. Appl. Energy 2017, 192, 477–489. [Google Scholar] [CrossRef]
- Wang, L.; Saveleva, V.A.; Zafeiratos, S.; Savinova, E.R.; Lettenmeier, P.; Gazdzicki, P.; Gago, A.S.; Friedrich, K.A. Highly active anode electrocatalysts derived from electrochemical leaching of Ru from metallic Ir0.7Ru0.3 for proton exchange membrane electrolyzers. Nano Energy 2017, 34, 385–391. [Google Scholar] [CrossRef]
- Siracusano, S.; Trocino, S.; Briguglio, N.; Pantò, F.; Aricò, A.S. Analysis of performance degradation during steady-state and load-thermal cycles of proton exchange membrane water electrolysis cells. J. Power Source 2020, 468, 228390. [Google Scholar] [CrossRef]
- Escalera-López, D.; Czioska, S.; Geppert, J.; Boubnov, A.; Röse, P.; Saraçi, E.; Krewer, U.; Grunwaldt, J.-D.; Cherevko, S. Phase- and Surface Composition-Dependent Electrochemical Stability of Ir-Ru Nanoparticles during Oxygen Evolution Reaction. ACS Catal. 2021, 11, 9300–9316. [Google Scholar] [CrossRef]
- Parrondo, J.; George, M.; Capuano, C.; Ayers, K.E.; Ramani, V. Pyrochlore electrocatalysts for efficient alkaline water electrolysis. J. Mater. Chem. A 2015, 3, 10819–10828. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Z.; Li, M.; Wang, Z.; Zhang, X.; Li, T.; Li, Y.; Tian, S.; Kuang, Y.; Sun, X. Iridium Doped Pyrochlore Ruthenates for Efficient and Durable Electrocatalytic Oxygen Evolution in Acidic Media. Small 2022, 18, 2202513. [Google Scholar] [CrossRef]
- Pitike, K.C.; Macias, A.; Eisenbach, M.; Bridges, C.A.; Cooper, V.R. Computationally Accelerated Discovery of High Entropy Pyrochlore Oxides. Chem. Mater. 2022, 34, 1459–1472. [Google Scholar] [CrossRef]
- Jiang, B.; Bridges, C.A.; Unocic, R.R.; Pitike, K.C.; Cooper, V.R.; Zhang, Y.; Lin, D.-Y.; Page, K. Probing the Local Site Disorder and Distortion in Pyrochlore High-Entropy Oxides. J. Am. Chem. Soc. 2021, 143, 4193–4204. [Google Scholar] [CrossRef]
- Kinsler-Fedon, C.; Zheng, Q.; Huang, Q.; Choi, E.S.; Yan, J.; Zhou, H.; Mandrus, D.; Keppens, V. Synthesis, characterization, and single-crystal growth of a high-entropy rare-earth pyrochlore oxide. Phys. Rev. Mater. 2020, 4, 104411. [Google Scholar] [CrossRef]
- Teng, Z.; Zhu, L.; Tan, Y.; Zeng, S.; Xia, Y.; Wang, Y.; Zhang, H. Synthesis and structures of high-entropy pyrochlore oxides. J. Eur. Ceram. Soc. 2020, 40, 1639–1643. [Google Scholar] [CrossRef]
- Shang, C.; Cao, C.; Yu, D.; Yan, Y.; Lin, Y.; Li, H.; Zheng, T.; Yan, X.; Yu, W.; Zhou, S.; et al. Electron Correlations Engineer Catalytic Activity of Pyrochlore Iridates for Acidic Water Oxidation. Adv. Mater. 2019, 31, 1805104. [Google Scholar] [CrossRef]
- Sugahara, K.; Kamata, K.; Muratsugu, S.; Hara, M. Amino Acid-Aided Synthesis of a Hexagonal SrMnO3 Nanoperovskite Catalyst for Aerobic Oxidation. ACS Omega 2017, 2, 1608–1616. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Risch, M.; Nam, G.; Park, M.; Shin, T.J.; Park, S.; Kim, M.G.; Shao-Horn, Y.; Cho, J. Single crystalline pyrochlore nanoparticles with metallic conduction as efficient bi-functional oxygen electrocatalysts for Zn–air batteries. Energy Environ. Sci. 2017, 10, 129–136. [Google Scholar] [CrossRef]
- Park, J.; Park, M.; Nam, G.; Kim, M.G.; Cho, J. Unveiling the Catalytic Origin of Nanocrystalline Yttrium Ruthenate Pyrochlore as a Bifunctional Electrocatalyst for Zn–Air Batteries. Nano Lett. 2017, 17, 3974–3981. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Ju, H.; Kim, J. Dihydrogen phosphate ion functionalized nanocrystalline thallium ruthenium oxide pyrochlore as a bifunctional electrocatalyst for aqueous Na-air batteries. Appl. Catal. B Environ. 2019, 245, 29–39. [Google Scholar] [CrossRef]
- Kim, M.; Ju, H.; Kim, J. Single crystalline Bi2Ru2O7 pyrochlore oxide nanoparticles as efficient bifunctional oxygen electrocatalyst for hybrid Na-air batteries. Chem. Eng. J. 2019, 358, 11–19. [Google Scholar] [CrossRef]
- Kim, M.; Ju, H.; Kim, J. Highly efficient bifunctional catalytic activity of bismuth rhodium oxide pyrochlore through tuning the covalent character for rechargeable aqueous Na–air batteries. J. Mater. Chem. A 2018, 6, 8523–8530. [Google Scholar] [CrossRef]
- Kim, M.; Ju, H.; Kim, J. Single crystalline thallium rhodium oxide pyrochlore for highly improved round trip efficiency of hybrid Na–air batteries. Dalton Trans. 2018, 47, 15217–15225. [Google Scholar] [CrossRef]

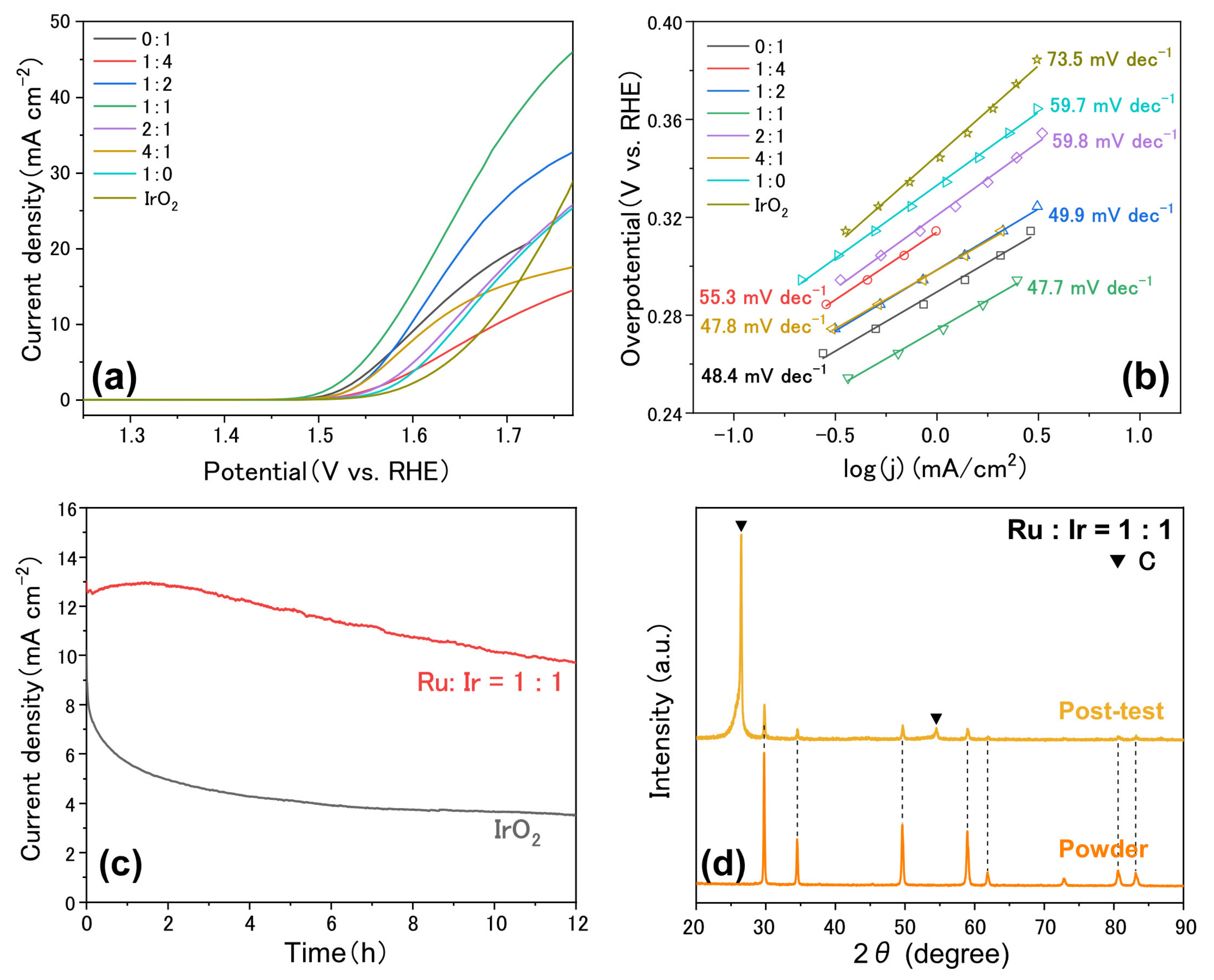
| Catalysts | Electrolyte | Overpotential (mV) | Reference |
|---|---|---|---|
| Pr2Ru1Ir1O7 | 1 M KOH | 350@10 mA cm−2 | This work |
| Bi2.4Ru1.6O7 | 0.1 M KOH | 370 | [16] |
| Pb2Ru2O6.5 | 0.1 M KOH | 418 | [24] |
| Sm2Ru2O7 | 0.1 M KOH | 440@2.5 mA cm–2 | |
| Y2Ru2-XYXO7 | 0.1 M KOH | 490 | [25] |
| P-Tl2Ru2O7 | 0.1 M KOH | 274 | [26] |
| Bi2Ru2O7 | 0.1 M KOH | 448 | [27] |
| P-Bi2Rh2O6.8 | 0.1 M KOH | 290 | [28] |
| Tl2Rh2O7 | 0.1 M KOH | 423 | [29] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumoto, A.; Cai, Z.-X.; Fujita, T. Synthesis of Pyrochlore Oxides Containing Ir and Ru for Efficient Oxygen Evolution Reaction. Materials 2022, 15, 6107. https://doi.org/10.3390/ma15176107
Matsumoto A, Cai Z-X, Fujita T. Synthesis of Pyrochlore Oxides Containing Ir and Ru for Efficient Oxygen Evolution Reaction. Materials. 2022; 15(17):6107. https://doi.org/10.3390/ma15176107
Chicago/Turabian StyleMatsumoto, Aika, Ze-Xing Cai, and Takeshi Fujita. 2022. "Synthesis of Pyrochlore Oxides Containing Ir and Ru for Efficient Oxygen Evolution Reaction" Materials 15, no. 17: 6107. https://doi.org/10.3390/ma15176107
APA StyleMatsumoto, A., Cai, Z.-X., & Fujita, T. (2022). Synthesis of Pyrochlore Oxides Containing Ir and Ru for Efficient Oxygen Evolution Reaction. Materials, 15(17), 6107. https://doi.org/10.3390/ma15176107







