Effects of Mono- and Bifunctional Surface Ligands of Cu–In–Se Quantum Dots on Photoelectrochemical Hydrogen Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CISe QDs
2.3. Ligand Exchange Treatment of CISe QDs
2.4. Fabrication of CISe QD-Sensitized TiO2 Photoanodes
2.5. Material Characterization
2.6. Photoelectrochemical Measurements
3. Results and Discussion
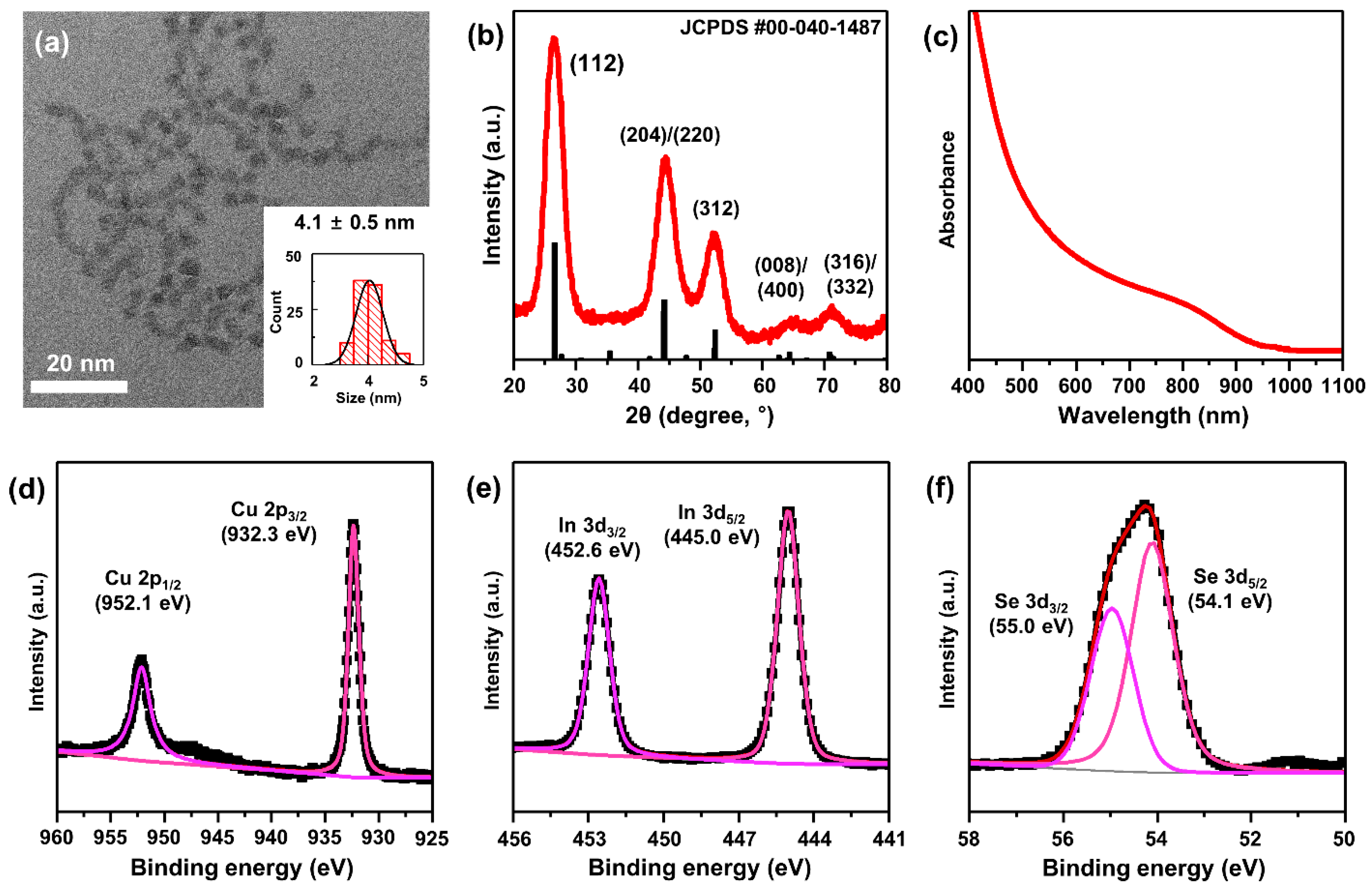
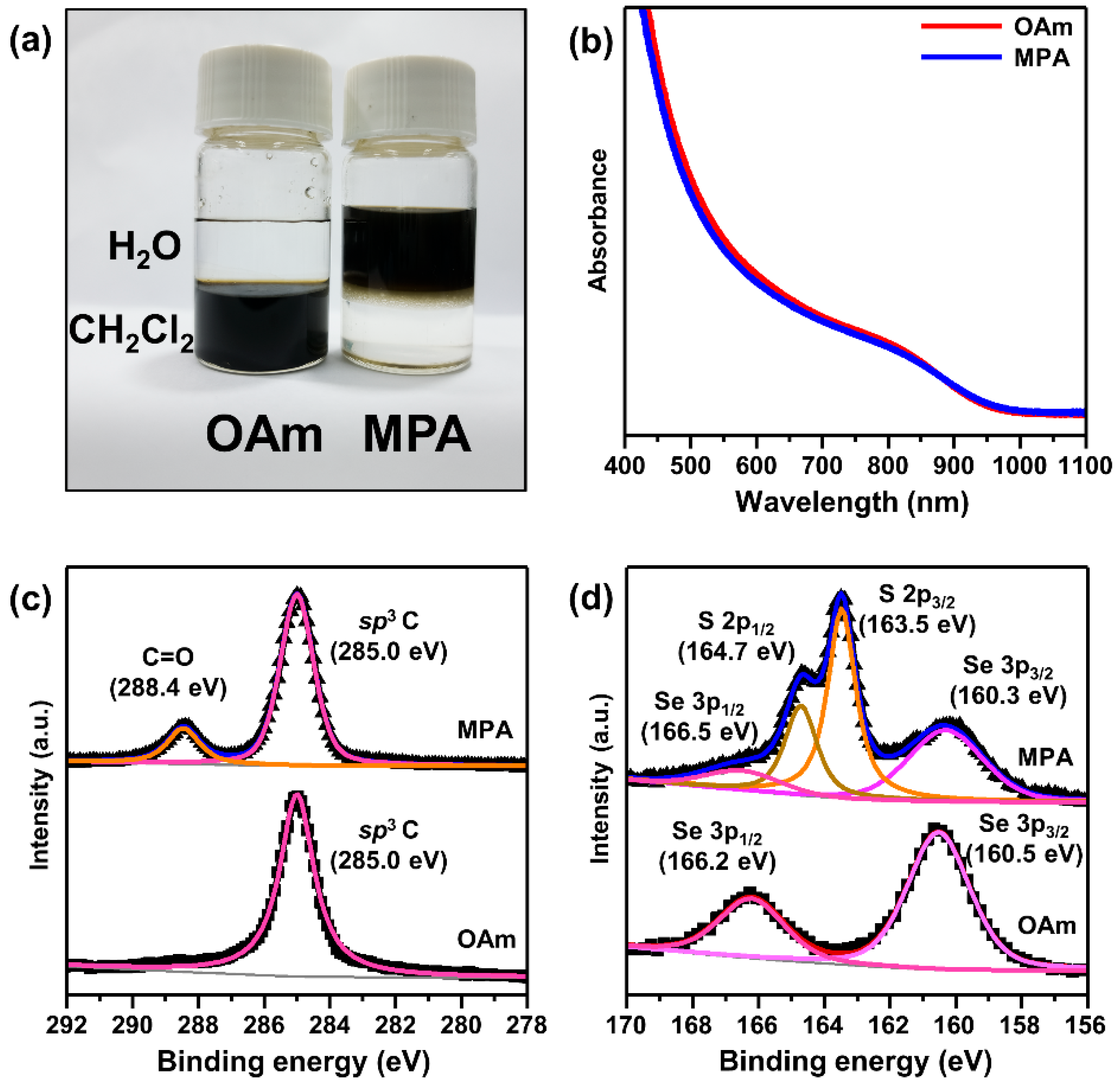
3.1. Preparation of CISe QDs with Different Surface Ligands
3.2. Properties of TiO2–CISe QD Photoanodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nayak, P.K.; Mahesh, S.; Snaith, H.J.; Cahen, D. Photovoltaic Solar Cell Technologies: Analysing the State of the Art. Nat. Rev. Mater. 2019, 4, 269–285. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Xuan, Z.; Chen, T.; Zhang, J.; Hao, X.; Wu, L.; Constantinou, I.; Zhao, D. Progress in Perovskite Solar Cells Towards Commercialization—A Review. Materials 2021, 14, 6569. [Google Scholar] [CrossRef] [PubMed]
- Gong, E.; Ali, S.; Hiragond, C.B.; Kim, H.S.; Powar, N.S.; Kim, D.; Kim, H.; In, S.-I. Solar fuels: Research and Development Strategies to Accelerate Photocatalytic CO2 Conversion into Hydrocarbon Fuels. Energy Environ. Sci. 2022, 15, 880–937. [Google Scholar] [CrossRef]
- Lee, H.; Kim, S.-S.; Bhang, S.H.; Yu, T. Facile Aqueous-Phase Synthesis of Stabilizer-Free Photocatalytic Nanoparticles. Catalysts 2021, 11, 111. [Google Scholar] [CrossRef]
- Hiremath, V.; Deonikar, V.G.; Kim, H.; Seo, J.G. Hierarchically Assembled Porous TiO2 Nanoparticles with Enhanced Photocatalytic Activity Towards Rhodamine-B Degradation. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124199. [Google Scholar] [CrossRef]
- Shuai, H.; Wang, J.; Wang, X.; Du, G. Black Talc-Based TiO2/ZnO Composite for Enhanced UV-Vis Photocatalysis Performance. Materials 2021, 14, 6474. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Jang, Y.J.; Park, Y.B.; Kim, H.E.; Choi, Y.H.; Choi, S.H.; Lee, J.S. Oxygen-Intercalated CuFeO2 Photocathode Fabricated by Hybrid Microwave Annealing for Efficient Solar Hydrogen Production. Chem. Mater. 2016, 28, 6054–6061. [Google Scholar] [CrossRef]
- Sivula, K.; van de Krol, R. Semiconducting Materials for Photoelectrochemical Energy Conversion. Nat. Rev. Mater. 2016, 1, 15010. [Google Scholar] [CrossRef]
- Kim, J.H.; Hansora, D.; Sharma, P.; Jang, J.-W.; Lee, J.S. Toward Practical Solar Hydrogen Production—An Artificial Photosynthetic Leaf-to-Farm Challenge. Chem. Soc. Rev. 2019, 48, 1908–1971. [Google Scholar] [CrossRef]
- Jang, Y.J.; Lee, C.; Moon, Y.H.; Choe, S. Solar-Driven Syngas Production Using Al-Doped ZnTe Nanorod Photocathodes. Materials 2022, 15, 3102. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Jeong, H.; Oh, S.J.; Ma, M.; Zhang, K.; Kwon, J.; Choi, I.T.; Choi, I.Y.; Kim, H.K.; Kim, J.K.; et al. Unassisted Photoelectrochemical Water Splitting Exceeding 7% Solar-to-Hydrogen Conversion Efficiency Using Photon Recycling. Nat. Commun. 2016, 7, 11943. [Google Scholar] [CrossRef]
- Sivasankaran, R.P.; Das, P.K.; Arunachalam, M.; Kanase, R.S.; Park, Y.I.; Seo, J.; Kang, S.H. TiO2 Nanotube Arrays Decorated with Reduced Graphene Oxide and Cu–Tetracyanoquinodimethane as Anode Materials for Photoelectrochemical Water Oxidation. ACS Appl. Nano Mater. 2021, 4, 13218–13233. [Google Scholar] [CrossRef]
- Xin, Y.; Li, Z.; Wu, W.; Fu, B.; Zhang, Z. Pyrite FeS2 Sensitized TiO2 Nanotube Photoanode for Boosting Near-Infrared Light Photoelectrochemical Water Splitting. ACS Sustain. Chem. Eng. 2016, 4, 6659–6667. [Google Scholar] [CrossRef]
- Gao, X.; Liu, X.; Zhu, Z.; Gao, Y.; Wang, Q.; Zhu, F.; Xie, Z. Enhanced Visible Light Photocatalytic Performance of CdS Sensitized TiO2 Nanorod Arrays Decorated with Au Nanoparticles as Electron Sinks. Sci. Rep. 2017, 7, 973. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Liu, Z.; Guo, Z.; Ruan, M.; Li, X. A Promising p-type Co–ZnFe2O4 Nanorod Film as a Photocathode for Photoelectrochemical Water Splitting. Chem. Commun. 2020, 56, 5279–5282. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yang, X.; Qian, F.; Zhang, J.Z.; Li, Y. Double-Sided CdS and CdSe Quantum Dot Co-Sensitized ZnO Nnowire Arrays for Photoelectrochemical Hydrogen Generation. Nano Lett. 2010, 10, 1088–1092. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Jang, Y.J.; Park, J.; Kim, J.; Kang, J.S.; Chung, D.Y.; Sung, Y.E.; Lee, C.; Lee, J.S.; Ko, M.J. Highly Loaded PbS/Mn-Doped CdS Quantum Dots for Dual Application in Solar-to-Electrical and Solar-to-Chemical Energy Conversion. Appl. Catal. B 2018, 227, 409–417. [Google Scholar] [CrossRef]
- Kim, J.H.; Jang, Y.J.; Choi, S.H.; Lee, B.J.; Kim, J.H.; Park, Y.B.; Nam, C.-M.; Kim, H.G.; Lee, J.S. A Multitude of Modifications Strategy of ZnFe2O4 Nanorod Photoanodes for Enhanced Photoelectrochemical Water Splitting Activity. J. Mater. Chem. A 2018, 6, 12693–12700. [Google Scholar] [CrossRef]
- Lim, Y.; Lee, S.Y.; Kim, D.; Han, M.-K.; Han, H.S.; Kang, S.H.; Kim, J.K.; Sim, U.; Park, Y.I. Expanded Solar Absorption Spectrum to Improve Photoelectrochemical Oxygen Evolution Reaction: Synergistic Effect of Upconversion Nanoparticles and ZnFe2O4/TiO2. Chem. Eng. J. 2022, 438, 135503. [Google Scholar] [CrossRef]
- Ali, A.M.; Sayed, M.A.; Algarni, H.; Ganesh, V.; Aslam, M.; Ismail, A.A.; El-Bery, H.M. Synthesis, Characterization and Photoelectric Properties of Fe2O3 Incorporated TiO2 Photocatalyst Nanocomposites. Catalysts 2021, 11, 1062. [Google Scholar] [CrossRef]
- Gawlak, K.; Popiołek, D.; Pisarek, M.; Sulka, G.D.; Zaraska, L. CdS-Decorated Porous Anodic SnOx Photoanodes with Enhanced Performance under Visible Light. Materials 2022, 15, 3848. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.B.; Norris, D.J.; Bawendi, M.G. Synthesis and Characterization of Nearly Monodisperse CdE (E = sulfur, selenium, tellurium) Semiconductor Nanocrystallites. J. Am. Chem. Soc. 1993, 115, 8706–8715. [Google Scholar] [CrossRef]
- Son, J.S.; Park, K.; Kwon, S.G.; Yang, J.; Choi, M.K.; Kim, J.; Yu, J.H.; Joo, J.; Hyeon, T. Dimension-Controlled Synthesis of CdS Nanocrystals: From 0D Quantum Dots to 2D Nanoplates. Small 2012, 8, 2394–2402. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Choi, M.K.; Yang, U.J.; Kim, S.Y.; Kim, Y.S.; Kim, J.H.; Kim, D.-H.; Hyeon, T. Toward Full-Color Electroluminescent Quantum Dot Displays. Nano Lett. 2021, 21, 26–33. [Google Scholar] [CrossRef]
- Yang, J.; Muckel, F.; Choi, B.K.; Lorenz, S.; Kim, I.Y.; Ackermann, J.; Chang, H.; Czerney, T.; Kale, V.S.; Hwang, S.-J.; et al. Co2+-Doping of Magic-Sized CdSe Clusters: Structural Insights via Ligand Field Transitions. Nano Lett. 2018, 18, 7350–7357. [Google Scholar] [CrossRef]
- Xia, C.; Wu, W.; Yu, T.; Xie, X.; van Oversteeg, C.; Gerritsen, H.C.; de Mello Donega, C. Size-Dependent Band-Gap and Molar Absorption Coefficients of Colloidal CuInS2 Quantum Dots. ACS Nano 2018, 12, 8350–8361. [Google Scholar] [CrossRef]
- McGuire, J.A.; Joo, J.; Pietryga, J.M.; Schaller, R.D.; Klimov, V.I. New Aspects of Carrier Multiplication in Semiconductor Nanocrystals. Acc. Chem. Res. 2008, 41, 1810–1819. [Google Scholar] [CrossRef]
- Semonin, O.E.; Luther, J.M.; Choi, S.; Chen, H.-Y.; Gao, J.; Nozik, A.J.; Beard, M.C. Peak External Photocurrent Quantum Efficiency Exceeding 100% via MEG in a Quantum Dot Solar Cell. Science 2011, 334, 1530–1533. [Google Scholar] [CrossRef]
- Panthani, M.G.; Stolle, C.J.; Reid, D.K.; Rhee, D.J.; Harvey, T.B.; Akhavan, V.A.; Yu, Y.; Korgel, B.A. CuInSe2 Quantum Dot Solar Cells with High Open-Circuit Voltage. J. Phys. Chem. Lett. 2013, 4, 2030–2034. [Google Scholar] [CrossRef]
- Yang, J.; Kim, J.-Y.; Yu, J.H.; Ahn, T.Y.; Lee, H.; Choi, T.S.; Kim, Y.W.; Joo, J.; Ko, M.J.; Hyeon, T. Copper-Indium-Selenide Quantum Dot-Sensitized Solar Cells. Phys. Chem. Chem. Phys. 2013, 15, 20517–20525. [Google Scholar] [CrossRef] [PubMed]
- Draguta, S.; McDaniel, H.; Klimov, V.I. Tuning Carrier Mobilities and Polarity of Charge Transport in Films of CuInSexS2-x Quantum Dots. Adv. Mater. 2015, 27, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Yang, J.; Yu, J.H.; Baek, W.; Lee, C.H.; Son, H.J.; Hyeon, T.; Ko, M.J. Highly Efficient Copper-Indium-Selenide Quantum Dot Solar Cells: Suppression of Carrier Recombination by Controlled ZnS Overlayers. ACS Nano 2015, 9, 11286–11295. [Google Scholar] [CrossRef]
- Du, J.; Du, Z.; Hu, J.-S.; Pan, Z.; Shen, Q.; Sun, J.; Long, D.; Dong, H.; Sun, L.; Zhong, X.; et al. Zn–Cu–In–Se Quantum Dot Solar Cells with a Certified Power Conversion Efficiency of 11.6%. J. Am. Chem. Soc. 2016, 138, 4201–4209. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-Y.; Kim, J.-H.; Jang, E.-P.; Lee, S.-H.; Jo, D.-Y.; Kim, Y.; Do, Y.R.; Yang, H. Systematic and Extensive Emission Tuning of Highly Efficient Cu–In–S-Based Quantum Dots from Visible to Near Infrared. Chem. Mater. 2019, 31, 2627–2634. [Google Scholar] [CrossRef]
- Yarema, O.; Yarema, M.; Wood, V. Tuning the Composition of Multicomponent Semiconductor Nanocrystals: The Case of I–III–VI Materials. Chem. Mater. 2018, 30, 1446–1461. [Google Scholar] [CrossRef]
- Tong, X.; Kong, X.T.; Zhou, Y.F.; Navarro-Pardo, F.; Selopal, G.S.; Sun, S.H.; Govorov, A.O.; Zhao, H.G.; Wang, Z.M.M.; Rosei, F. Near-Infrared, Heavy Metal-Free Colloidal “Giant” Core/Shell Quantum Dots. Adv. Energy Mater. 2018, 8, 1701432. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chang, H.-H.; Hsu, Y.-K. Synthesis of CuInS2 Quantum Dots/In2S3/ZnO Nanowire Arrays with High Photoelectrochemical Activity. ACS Sustain. Chem. Eng. 2018, 6, 10861–10868. [Google Scholar] [CrossRef]
- Kim, J.; Jang, Y.J.; Baek, W.; Lee, A.R.; Kim, J.-Y.; Hyeon, T.; Lee, J.S. Highly Efficient Photoelectrochemical Hydrogen Production Using Nontoxic CuIn1.5Se3 Quantum Dots with ZnS/SiO2 Double Overlayers. ACS Appl. Mater. Interfaces 2022, 14, 603–610. [Google Scholar] [CrossRef]
- Lee, J.; Yang, J.; Kwon, S.G.; Hyeon, T. Nonclassical Nucleation and Growth of Inorganic Nanoparticles. Nat. Rev. Mater. 2016, 1, 16034. [Google Scholar] [CrossRef]
- Ban, H.W.; Park, S.; Jeong, H.; Gu, D.H.; Jo, S.; Park, S.H.; Park, J.; Son, J.S. Molybdenum and Tungsten Sulfide Ligands for Versatile Functionalization of All-Inorganic Nanocrystals. J. Phys. Chem. Lett. 2016, 7, 3627–3635. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Fainblat, R.; Kwon, S.G.; Muckel, F.; Yu, J.H.; Terlinden, H.; Kim, B.H.; Iavarone, D.; Choi, M.K.; Kim, I.Y.; et al. Route to the Smallest Doped Semiconductor: Mn2+-Doped (CdSe)13 Clusters. J. Am. Chem. Soc. 2015, 137, 12776–12779. [Google Scholar] [CrossRef] [PubMed]
- Pu, C.; Dai, X.; Shu, Y.; Zhu, M.; Deng, Y.; Jin, Y.; Peng, X. Electrochemically-Stable Ligands Bridge the Photoluminescence-Electroluminescence Gap of Quantum Dots. Nat. Commun. 2020, 11, 937. [Google Scholar] [CrossRef] [PubMed]
- Kagan, C.R.; Lifshitz, E.; Sargent, E.H.; Talapin, D.V. Building Devices from Colloidal Quantum Dots. Science 2016, 353, aac5523. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Choi, M.K.; Kim, D.-H.; Hyeon, T. Designed Assembly and Integration of Colloidal Nanocrystals for Device Applications. Adv. Mater. 2016, 28, 1176–1207. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, K.; Hou, Y.M.; Fang, Z.; Pan, Z.X.; Wu, W.J.; Hua, J.L.; Zhong, X.H. Efficient CdSe Quantum Dot-Sensitized Solar Cells Prepared by Postsynthesis Assembly Approach. Chem. Commun. 2012, 48, 11235–11237. [Google Scholar] [CrossRef]
- Kazmerski, L.L.; Jamjoum, O.; Ireland, P.J.; Deb, S.K.; Mickelsen, R.A.; Chen, W. Initial Oxidation of CuInSe2. J. Vac. Sci. Technol. 1981, 19, 467–471. [Google Scholar] [CrossRef]
- Kuznetsov, M.V.; Shalaeva, E.V.; Yakushev, M.V.; Tomlinson, R.D. Evolution of CuInSe2 (112) Surface Due to Annealing: XPS study. Surf. Sci. 2003, 530, L297–L301. [Google Scholar] [CrossRef]
- Mora-Sero, I.; Gimenez, S.; Fabregat-Santiago, F.; Gomez, R.; Shen, Q.; Toyoda, T.; Bisquert, J. Recombination in Quantum Dot Sensitized Solar Cells. Acc. Chem. Res. 2009, 42, 1848–1857. [Google Scholar] [CrossRef]
- Guijarro, N.; Lana-Villarreal, T.; Mora-Sero, I.; Bisquert, J.; Gomez, R. CdSe Quantum Dot-Sensitized TiO2 Electrodes: Effect of Quantum Dot Coverage and Mode of Attachment. J. Phys. Chem. C 2009, 113, 4208–4214. [Google Scholar] [CrossRef]
- Du, J.; Singh, R.; Fedin, I.; Fuhr, A.S.; Klimov, V.I. Spectroscopic Insights into High Defect Tolerance of Zn: CuInSe2 Quantum-Dot-Sensitized Solar Cells. Nat. Energy 2020, 5, 409–417. [Google Scholar] [CrossRef]
- Biesinger, M.C. Accessing the Robustness of Adventitious Carbon for Charge Referencing (Correction) Purposes in XPS Analysis: Insights from a Multi-User Facility Data Review. Appl. Surf. Sci. 2022, 597, 153681. [Google Scholar] [CrossRef]
- Chang, S.-H.; Chiang, M.-Y.; Chiang, C.-C.; Yuan, F.-W.; Chen, C.-Y.; Chiu, B.-C.; Kao, T.-L.; Lai, C.-H.; Tuan, H.-Y. Facile Colloidal Synthesis of Quinary CuIn1−xGax(SySe1−y)2 (CIGSSe) Nanocrystal Inks with Tunable Band Gaps for Use in Low-Cost Photovoltaics. Energy Environ. Sci. 2011, 4, 4929–4932. [Google Scholar] [CrossRef]
- Pan, Z.; Mora-Seró, I.; Shen, Q.; Zhang, H.; Li, Y.; Zhao, K.; Wang, J.; Zhong, X.; Bisquert, J. High-Efficiency “Green” Quantum Dot Solar Cells. J. Am. Chem. Soc. 2014, 136, 9203–9210. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.R.; Kim, D.; Lunt, R.R.; Zhao, N.; Bawendi, M.G.; Grossman, J.C.; Bulović, V. Energy Level Modification in Lead Sulfide Quantum Dot Thin Films Through Ligand Exchange. ACS Nano 2014, 8, 5863–5872. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jiang, G.; Yu, J.; Wang, W.; Pan, Z.; Nakazawa, N.; Shen, Q.; Zhong, X. High Efficiency Quantum Dot Sensitized Solar Cells Based on Direct Adsorption of Quantum Dots on Photoanodes. ACS Appl. Mater. Interfaces 2017, 9, 22549–22559. [Google Scholar] [CrossRef]
- Wang, K.; Tong, X.; Zhou, Y.F.; Zhang, H.; Navarro-Pardo, F.; Selopal, G.S.; Liu, G.J.; Tang, J.; Wang, Y.Q.; Sun, S.H.; et al. Efficient Solar-Driven Hydrogen Generation Using Colloidal Heterostructured Quantum Dots. J. Mater. Chem. A 2019, 7, 14079–14088. [Google Scholar] [CrossRef]
- Li, F.; Zhang, M.; Benetti, D.; Shi, L.; Besteiro, L.V.; Zhang, H.; Liu, J.; Selopal, G.S.; Sun, S.; Wang, Z.; et al. “Green”, Gradient Multi-Shell CuInSe2/(CuInSexS1-x)5/CuInS2 Quantum Dots for Photo-Electrochemical Hydrogen Generation. Appl. Catal. B 2021, 280, 119402. [Google Scholar] [CrossRef]
- Hines, D.A.; Kamat, P.V. Quantum Dot Surface Chemistry: Ligand Effects and Electron Transfer Reactions. J. Phys. Chem. C 2013, 117, 14418–14426. [Google Scholar] [CrossRef]
- Liu, D.; Liu, J.-C.; Cai, W.; Ma, J.; Yang, H.B.; Xiao, H.; Li, J.; Xiong, Y.; Huang, Y.; Liu, B. Selective Photoelectrochemical Oxidation of Glycerol to High Value-Added Dihydroxyacetone. Nat. Commun. 2019, 10, 1779. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.H.; Kim, J.-Y.; Kim, Y.; Kim, H.S.; Sung, Y.-E. Surface Modification of Stretched TiO2 Nanotubes for Sold-State Dye-Sensitized Solar Cells. J. Phys. Chem. C 2007, 111, 9614–9623. [Google Scholar] [CrossRef]
- Nevins, J.S.; Coughlin, K.M.; Watson, D.F. Attachment of CdSe Nanoparticles to TiO2 via Aqueous Linker-Assisted Assembly: Influence of Molecular Linkers on Electronic Properties and Interfacial Electron Transfer. ACS Appl. Mater. Interfaces 2011, 3, 4242–4253. [Google Scholar] [CrossRef] [PubMed]

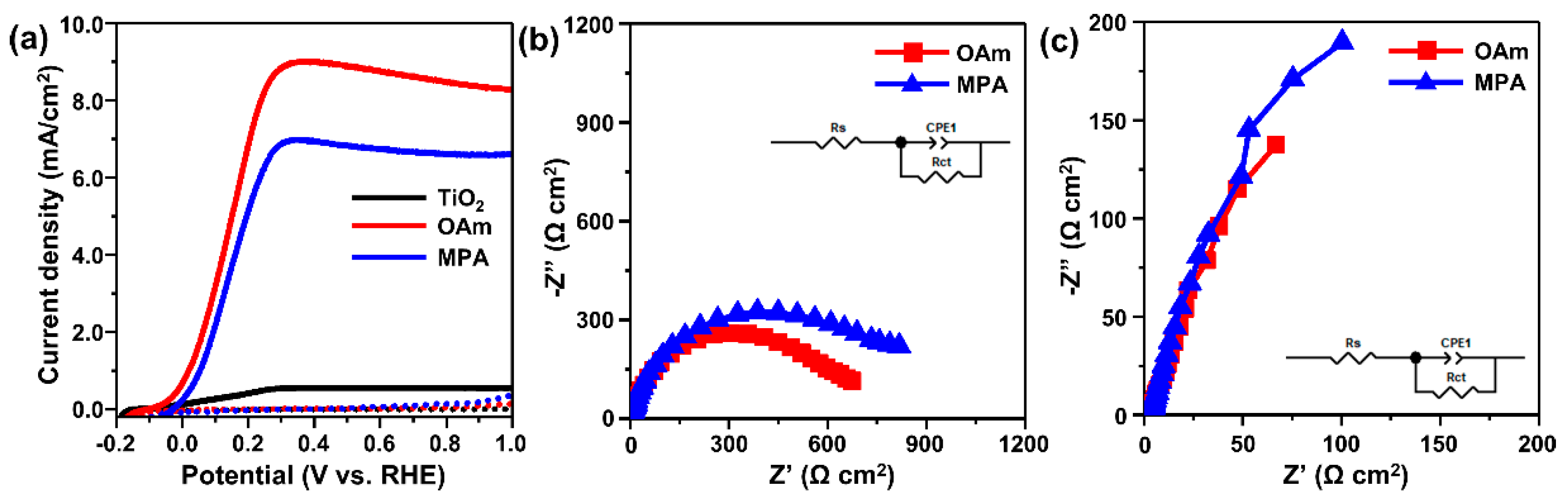

| Sample | Current Density (mA/cm2) | Dark Rs (Ω cm2) | Dark Rct (Ω cm2) | Light Rs (Ω cm2) | Light Rct (Ω cm2) |
|---|---|---|---|---|---|
| OAm-QD-photoanode | 8.236 | 4.25 | 660.8 | 3.21 | 1041 |
| MPA-QD-photoanode | 6.740 | 3.37 | 857.2 | 3.23 | 1180 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.I.; Jung, S.-M.; Kim, J.-Y.; Yang, J. Effects of Mono- and Bifunctional Surface Ligands of Cu–In–Se Quantum Dots on Photoelectrochemical Hydrogen Production. Materials 2022, 15, 6010. https://doi.org/10.3390/ma15176010
Park SI, Jung S-M, Kim J-Y, Yang J. Effects of Mono- and Bifunctional Surface Ligands of Cu–In–Se Quantum Dots on Photoelectrochemical Hydrogen Production. Materials. 2022; 15(17):6010. https://doi.org/10.3390/ma15176010
Chicago/Turabian StylePark, Soo Ik, Sung-Mok Jung, Jae-Yup Kim, and Jiwoong Yang. 2022. "Effects of Mono- and Bifunctional Surface Ligands of Cu–In–Se Quantum Dots on Photoelectrochemical Hydrogen Production" Materials 15, no. 17: 6010. https://doi.org/10.3390/ma15176010
APA StylePark, S. I., Jung, S.-M., Kim, J.-Y., & Yang, J. (2022). Effects of Mono- and Bifunctional Surface Ligands of Cu–In–Se Quantum Dots on Photoelectrochemical Hydrogen Production. Materials, 15(17), 6010. https://doi.org/10.3390/ma15176010








