Pullulan Oxidation in the Presence of Hydrogen Peroxide and N-Hydroxyphthalimide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
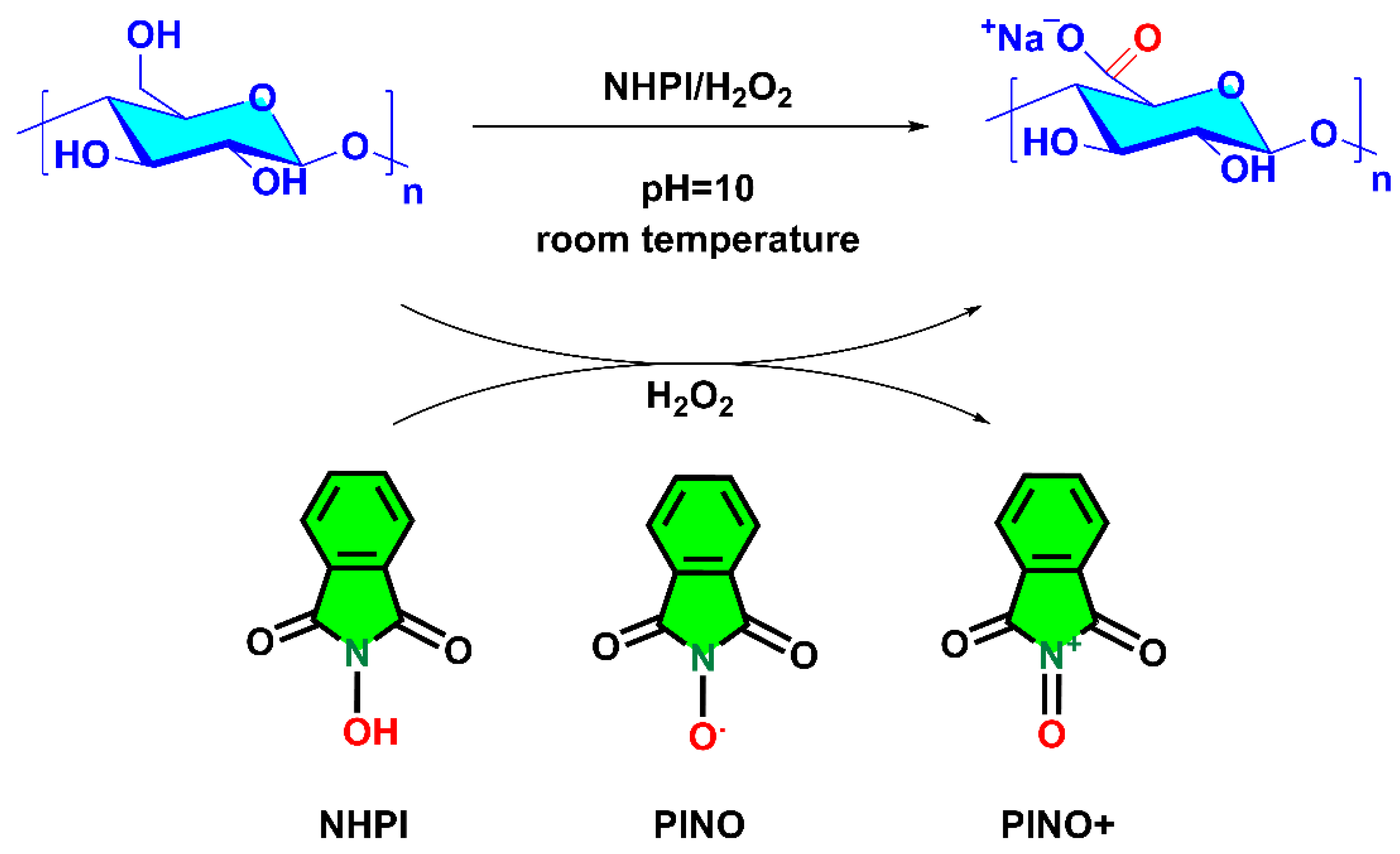
2.2. H2O2/NHPI-Mediated Oxidation of Pullulan
2.3. UV Measurements
2.4. Carboxyl Content Determination
2.5. Viscosimetric Measurements
2.6. Zeta-Potential (ζ) Measurements
2.7. FTIR Measurements
2.8. NMR Analyses
3. Results
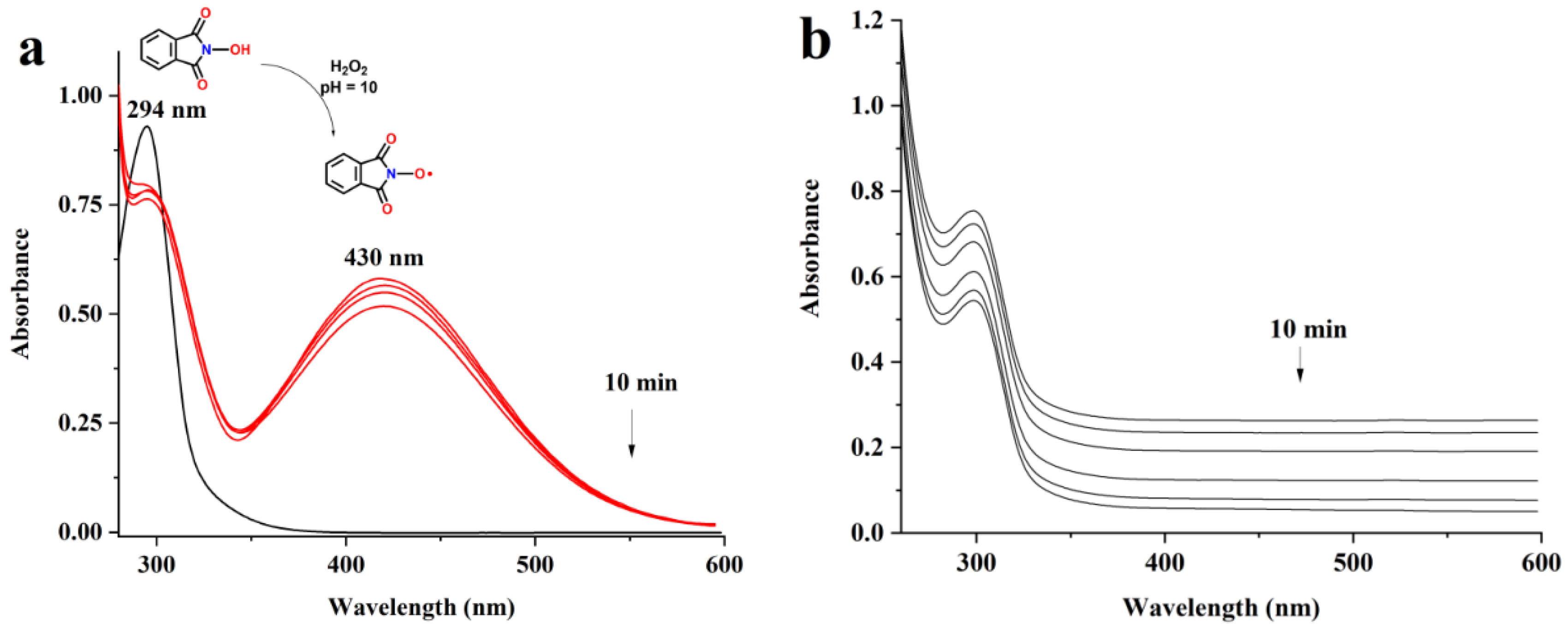
3.1. UV Measurements
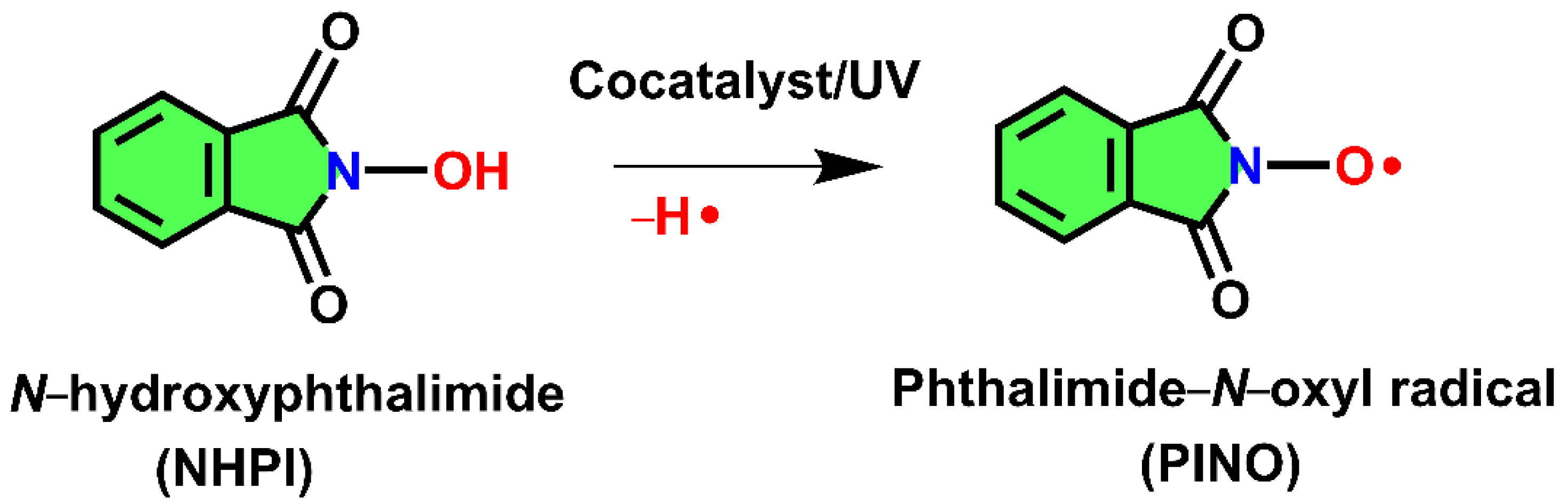
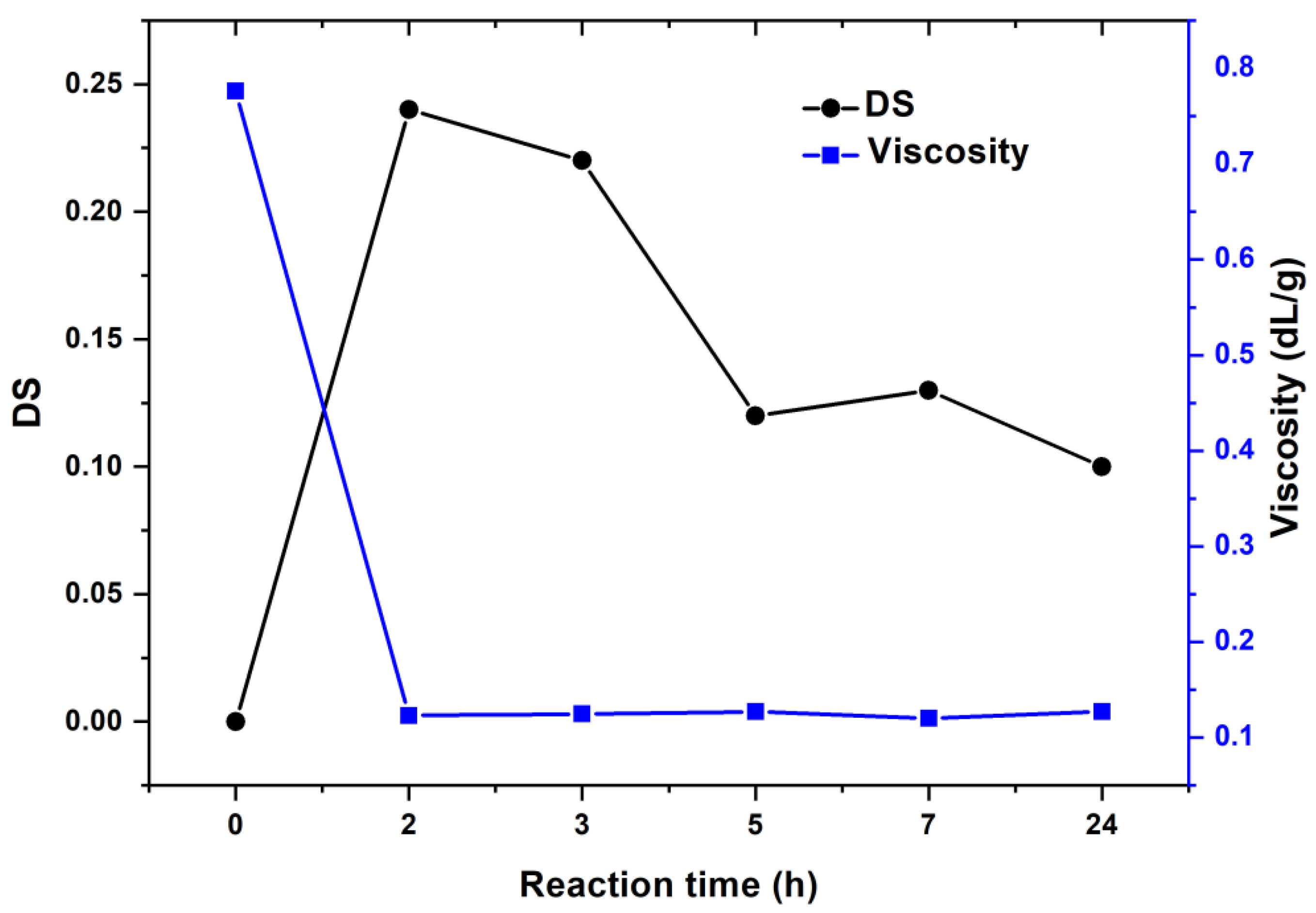
3.2. The Oxidation of Pullulan in the Presence of the H2O2/NHPI System
3.3. FTIR Analysis
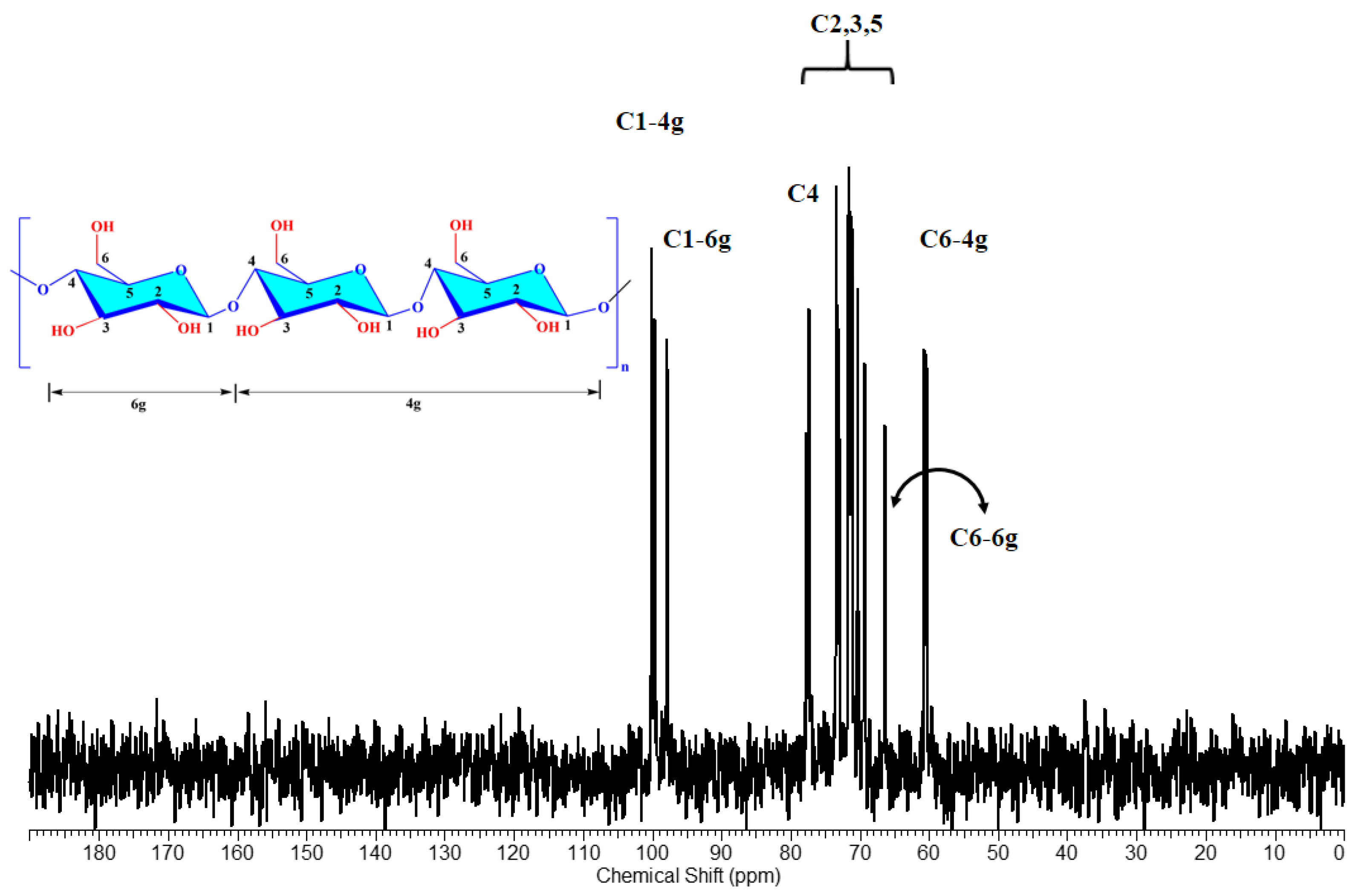
3.4. NMR Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pierre, G.; Punta, C.; Delattre, C.; Melone, L.; Dubessay, P.; Fiorati, A.; Pastori, N.; Galante, Y.M.; Michaud, P. TEMPO-mediated oxidation of polysaccharides: An ongoing story. Carbohydr. Polym. 2017, 165, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y. Key advances in the chemical modification of nanocelluloses. Chem. Soc. Rev. 2014, 43, 1519–1542. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Fu, S.; Lucia, L.A. The role of heteropolysaccharides in developing oxidized cellulose nanofibrils. Carbohydr. Polym. 2016, 144, 187–195. [Google Scholar] [CrossRef]
- Coseri, S.; Biliuta, G.; Simionescu, B.C.; Stana-Kleinschek, K.; Ribitsch, V.; Harabagiu, V. Oxidized cellulose—Survey of the most recent achievements. Carbohydr. Polym. 2013, 93, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Parveen, N.; Chowdhury, S.; Goel, S. Environmental impacts of the widespread use of chlorine-based disinfectants during the COVID-19 pandemic. Environ. Sci. Pollut. Res. 2022, 1, 1–19. [Google Scholar] [CrossRef] [PubMed]
- de Nooy, A.E.J.; Besemer, A.C.; van Bekkum, H. Highly selective nitroxyl radical-mediated oxidation of primary alcohol groups in water-soluble glucans. Carbohydr. Res. 1995, 269, 89–98. [Google Scholar] [CrossRef]
- Isogai, A.; Kato, Y. Preparation of polyuronic acid from cellulose by TEMPO-mediated oxidation. Cellulose 1998, 5, 153–164. [Google Scholar] [CrossRef]
- Coseri, S. Phthalimide-N-oxyl (PINO) radical, a powerful catalytic agent: Its generation and versatility towards various organic substrates. Catal. Rev.-Sci. Eng. 2009, 51, 218–292. [Google Scholar] [CrossRef]
- Biliuta, G.; Fras, L.; Drobota, M.; Persin, Z.; Kreze, T.; Stana-Kleinschek, K.; Ribitsch, V.; Harabagiu, V.; Coseri, S. Comparison study of TEMPO and phthalimide-N-oxyl (PINO) radicals on oxidation efficiency toward cellulose. Carbohydr. Polym. 2013, 91, 502–507. [Google Scholar] [CrossRef]
- Coseri, S. A New and Efficient Heterogeneous System for the Phthalimide N-Oxyl (PINO) Radical Generation. Eur. J. Org. Chem. 2007, 2007, 1725–1729. [Google Scholar] [CrossRef]
- Coseri, S.; Biliuta, G.; Simionescu, B.C. Selective oxidation of cellulose, mediated by: N-hydroxyphthalimide, under a metal-free environment. Polym. Chem. 2018, 9, 961–967. [Google Scholar] [CrossRef]
- Coseri, S.; Nistor, G.; Fras, L.; Strnad, S.; Harabagiu, V.; Simionescu, B.C. Mild and selective oxidation of cellulose fibers in the presence of N-hydroxyphthalimide. Biomacromolecules 2009, 10, 2294–2299. [Google Scholar] [CrossRef] [PubMed]
- Biliuta, G.; Fras, L.; Strnad, S.; Harabagiu, V.; Coseri, S. Oxidation of cellulose fibers mediated by nonpersistent nitroxyl radicals. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 4790–4799. [Google Scholar] [CrossRef]
- Biliuta, G.; Coseri, S. Magnetic cellulosic materials based on TEMPO-oxidized viscose fibers. Cellulose 2016, 23, 3407–3415. [Google Scholar] [CrossRef]
- Dobromir, M.; Biliuta, G.; Luca, D.; Aflori, M.; Harabagiu, V.; Coseri, S. XPS study of the ion-exchange capacity of the native and surface oxidized viscose fibers. Colloids Surf. A Physicochem. Eng. Asp. 2011, 381, 106–110. [Google Scholar] [CrossRef]
- Coseri, S.; Biliuta, G.; Zemljič, L.F.; Srndovic, J.S.; Larsson, P.T.; Strnad, S.; Kreže, T.; Naderi, A.; Lindström, T. One-shot carboxylation of microcrystalline cellulose in the presence of nitroxyl radicals and sodium periodate. RSC Adv. 2015, 5, 85889–85897. [Google Scholar] [CrossRef]
- Coseri, S.; Biliuta, G. Bromide-free oxidizing system for carboxylic moiety formation in cellulose chain. Carbohydr. Polym. 2012, 90, 1415–1419. [Google Scholar] [CrossRef]
- Biliuta, G.; Fras, L.; Harabagiu, V.; Coseri, S. Mild oxidation of cellulose fibers using dioxygen as ultimate oxidizing agent. Dig. J. Nanomater. Biostruct. 2011, 6, 293–299. [Google Scholar]
- Culica, M.E.; Kasperczyk, K.; Baron, R.I.; Biliuta, G.; Macsim, A.M.; Lazea-Stoyanova, A.; Orlinska, B.; Coseri, S. Recyclable Polymer-Supported N-Hydroxyphthalimide Catalysts for Selective Oxidation of Pullulan. Materials 2019, 12, 3585. [Google Scholar] [CrossRef]
- Li, Z.; Meng, C.; Zhou, J.; Li, Z.; Ding, J.; Liu, F.; Yu, C. Characterization and control of oxidized cellulose in ramie fibers during oxidative degumming. Text. Res. J. 2017, 87, 1828–1840. [Google Scholar] [CrossRef]
- Li, Z.; Yu, C.; Zhao, Q. A Method of Ramie Fiber Degummed with Multiple Feeding. Chinese Patent 103233279A, 7 August 2013. [Google Scholar]
- Meng, C.; Liu, F.; Li, Z.; Yu, C. The cellulose protection agent used in the oxidation degumming of ramie. Text. Res. J. 2016, 86, 1109–1118. [Google Scholar] [CrossRef]
- Yang, S.; Yu, C.; Zhang, B.; Zhang, P.; Bi, X.; Li, J.; Zhang, W. Highly efficient and low pollution catalytic oxidation of ramie degumming by NHPI. Ind. Crops Prod. 2022, 186, 115189. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Kennedy, J.F. Pullulan and Pullulan Derivatives as Promising Biomolecules for Drug and Gene Targeting. Carbohydr. Polym. 2015, 123, 190–207. [Google Scholar] [CrossRef]
- Cheng, K.C.; Demirci, A.; Catchmark, J.M. Pullulan: Biosynthesis, Production, and Applications. Appl. Microbiol. Biotechnol. 2011, 92, 29–44. [Google Scholar] [CrossRef]
- da Silva Perez, D.; Montanari, S.; Vignon, M.R. TEMPO-mediated oxidation of cellulose III. Biomacromolecules 2003, 4, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Meng, C.; Yu, C. Analysis of oxidized cellulose introduced into ramie fiber by oxidation degumming. Text. Res. J. 2015, 85, 2125–2135. [Google Scholar] [CrossRef]
- Erkselius, S.; Karlsson, O.J. Free radical degradation of hydroxyethyl cellulose. Carbohydr. Polym. 2005, 62, 344–356. [Google Scholar] [CrossRef]
- Guo, T.X.; Zhao, Y.; Ma, S.C.; Liu, S.T. Decomposition Characteristics of Hydrogen Peroxide in Sodium Hydroxide Solution. Adv. Mater. Res. 2013, 610–613, 359–362. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, S. Degumming of ramie bast fibers by Ca2+-activated composite enzyme. J. Text. Inst. 2013, 104, 78–83. [Google Scholar] [CrossRef]
- Li, Z.; Yu, C. Effect of peroxide and softness modification on properties of ramie fiber. Fibers Polym. 2014, 15, 2105–2111. [Google Scholar] [CrossRef]
- Long, X.; Xu, C.; Du, J.; Fu, S. The TAED/H2O2/NaHCO3 system as an approach to low-temperature and near-neutral pH bleaching of cotton. Carbohydr. Polym. 2013, 95, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Zou, M.; Li, X.; Zhao, J.; Qu, Y. An effective degumming enzyme from Bacillus sp. Y1 and synergistic action of hydrogen peroxide and protease on enzymatic degumming of ramie fibers. Biomed Res. Int. 2013, 2013, 212315. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Study on Selective Oxidation of Cellulose. Master’s Thesis, Zhejiang University, Hangzhou, China, 2014. [Google Scholar]
- Coseri, S.; Bercea, M.; Harabagiu, V.; Budtova, T. Oxidation vs. Degradation in Polysaccharides: Pullulan—A Case Study. Eur. Polym. J. 2016, 85, 82–91. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, X.; Xu, J.; Shi, M.; Wang, F.; Chen, C.; Xu, J. Depolymerization of Cellulose to Glucose by Oxidation–Hydrolysis. Green Chem. 2015, 17, 1519–1524. [Google Scholar] [CrossRef]
- Rånby, B.G.; Marchessault, R.H. Inductive Effects in the Hydrolysis of Cellulose Chains. J. Polym. Sci. 1959, 36, 561–564. [Google Scholar] [CrossRef]
- Yeasmin, S.; Yeum, J.H.; Yang, S.B. Fabrication and characterization of pullulan-based nanocomposites reinforced with montmorillonite and tempo cellulose nano fibril. Carbohydr. Polym. 2020, 240, 116307. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.T.; Moraes, R.M.; Alves, G.M.; Lacerda, T.M.; Santos, J.C.; Santos, A.M.; Medeiros, S.F. Synthesis of amphiphilic pullulan-graft-poly(ε-caprolactone) via click chemistry. Int. J. Biol. Macromol. 2020, 145, 701–711. [Google Scholar] [CrossRef]
- Singh, R.S.; Saini, G.K.; Kennedy, J.F. Downstream processing and characterization of pullulan from a novel colour variant strain of Aureobasidium pullulans FB-1. Carbohydr. Polym. 2009, 78, 89–94. [Google Scholar] [CrossRef]
- Chen, G.; Zhu, Y.; Zhang, G.; Liu, H.; Wei, Y.; Wang, P.; Wang, F.; Xian, M.; Xiang, H.; Zhang, H. Optimization and characterization of pullulan production by a newly isolated high-yielding strain Aureobasidium melanogenum. Prep. Biochem. Biotechnol. 2019, 49, 557–566. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Zhao, S.; Zhou, Q.; Xin, X.; Chen, L. Statistical optimization of medium for pullulan production by Aureobasidium pullulans NCPS2016 using fructose and soybean meal hydrolysates. Molecules 2018, 23, 1334. [Google Scholar] [CrossRef]
- Bercea, M.; Biliuta, G.; Avadanei, M.; Baron, R.I.; Butnaru, M.; Coseri, S. Self-healing hydrogels of oxidized pullulan and poly(vinyl alcohol). Carbohydr. Polym. 2019, 206, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Kacuráková, M.; Capek, P.; Sasinková, V.; Wellner, N.; Ebringerová, A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Kačuráková, M.; Mathlouthi, M. FTIR and laser-Raman spectra of oligosaccharides in water: Characterization of the glycosidic bond. Carbohydr. Res. 1996, 284, 145–157. [Google Scholar] [CrossRef]
- Cael, J.J.; Koenig, J.L.; Blackwell, J. Infrared and raman spectroscopy of carbohydrates: Part IV. Identification of configuration- and conformation-sensitive modes for D-glucose by normal coordinate analysis. Carbohydr. Res. 1974, 32, 79–91. [Google Scholar] [CrossRef]
- Jiang, W.; Zhou, X. Enzymatic preparation of oxidized viscose fibers-based biosorbent modified with ε-polylysine for dyes removal and microbial inactivation. Int. J. Biol. Macromol. 2021, 166, 509–520. [Google Scholar] [CrossRef]
- Wright, W.W.; Laberge, M.; Vanderkooi, J.M. Surface of Cytochrome c: Infrared Spectroscopy of Carboxyl Groups. Biochemistry 1997, 36, 14724–14732. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef]
- Arnosti, C.; Repeta, D.J. Nuclear Magnetic Resonance Spectroscopy of Pullulan and Isomaltose: Complete Assignment of Chemical Shifts. Starch-Stärke 1995, 47, 73–75. [Google Scholar] [CrossRef]
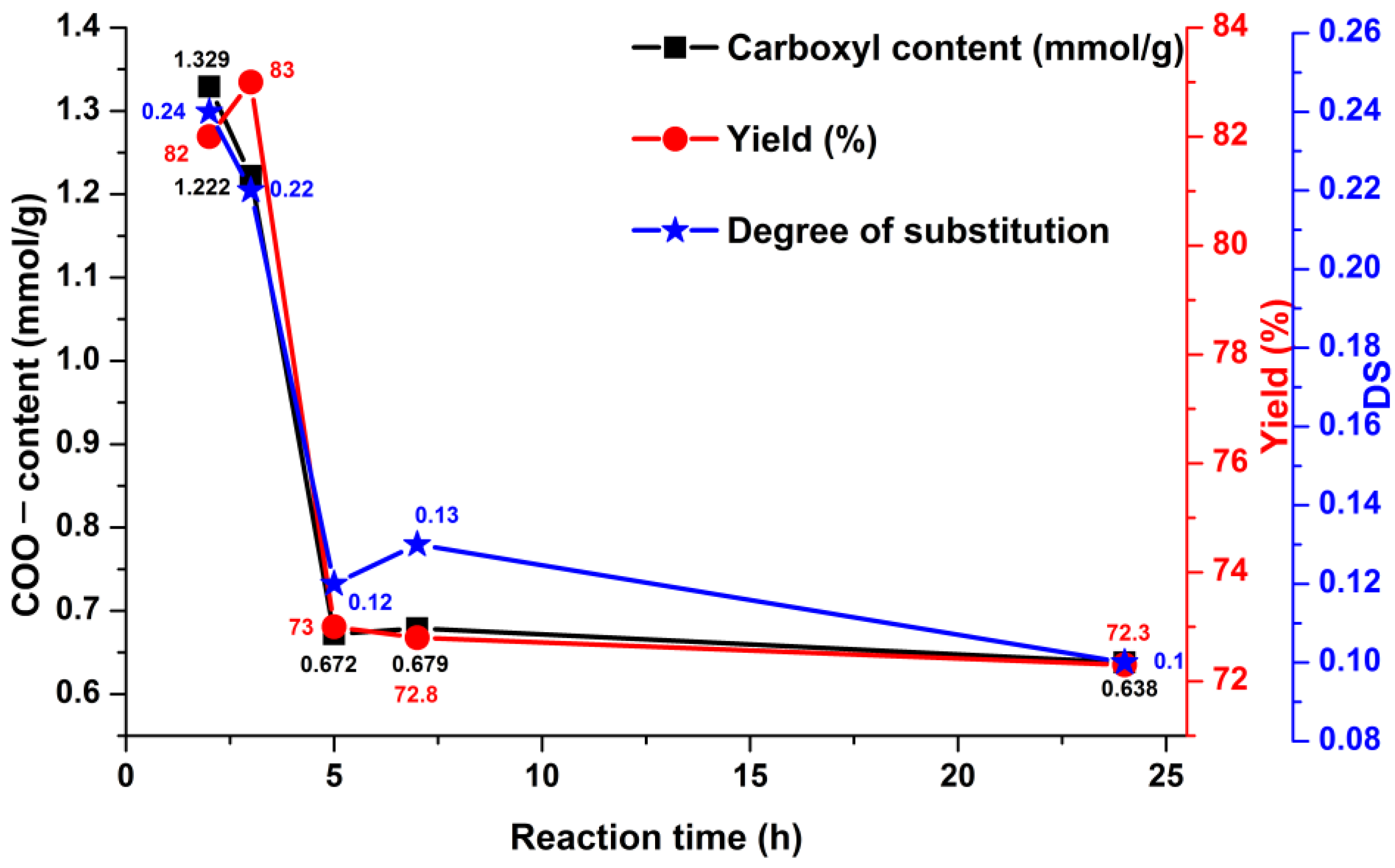


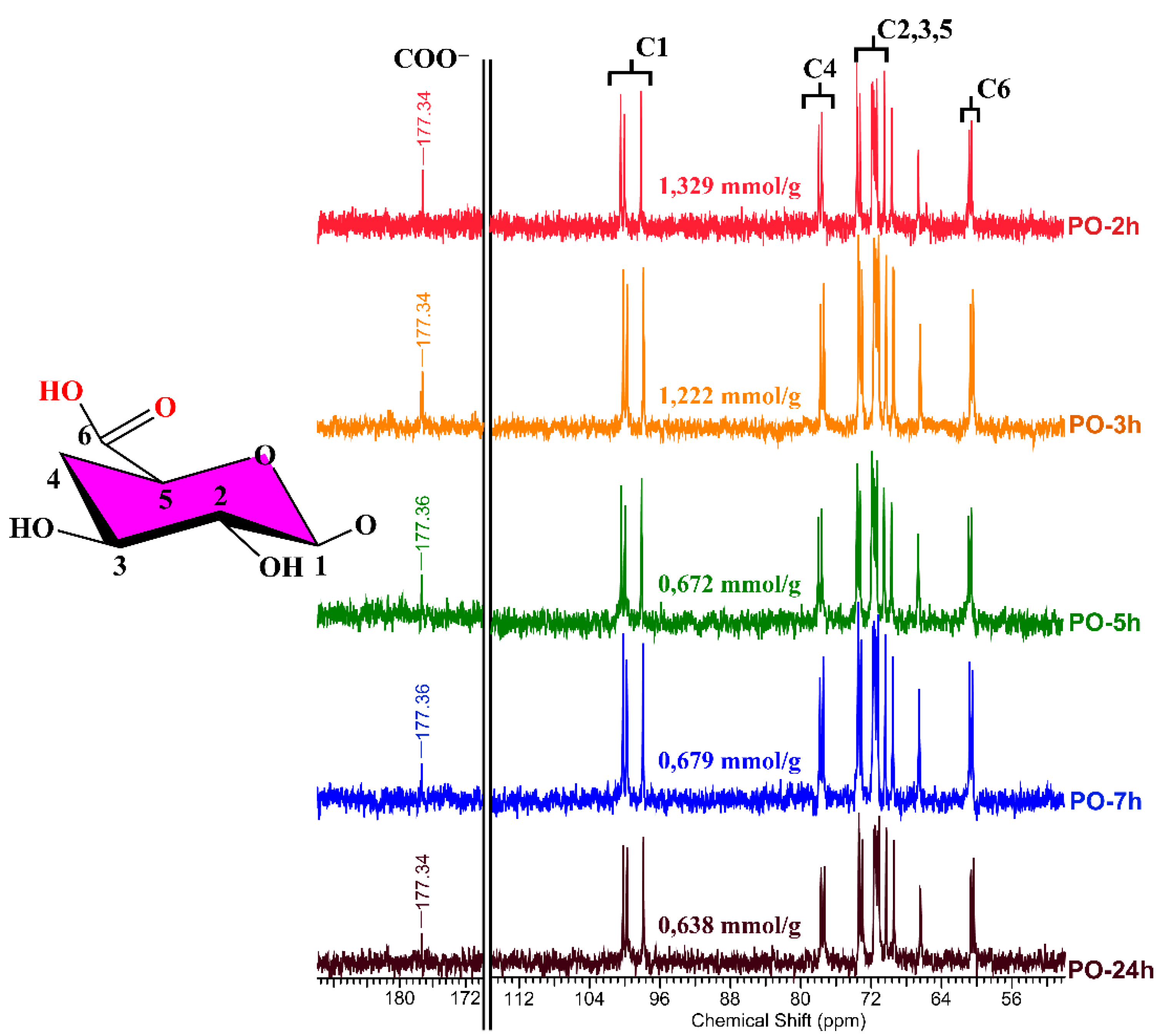
| Run | Reaction Time (h) | Amount of Negatively Charged Groups COO− (mmol/g) | Mass Yield (%) |
|---|---|---|---|
| PO-2 h | 2 | 1.33 | 83 |
| PO-3 h | 3 | 1.22 | 82 |
| PO-5 h | 5 | 0.70 | 77 |
| PO-5 h * | 5 | 2.17 | 70 |
| PO-7 h | 7 | 0.67 | 73 |
| PO-24 h | 24 | 0.63 | 72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biliuta, G.; Baron, R.I.; Coseri, S. Pullulan Oxidation in the Presence of Hydrogen Peroxide and N-Hydroxyphthalimide. Materials 2022, 15, 6086. https://doi.org/10.3390/ma15176086
Biliuta G, Baron RI, Coseri S. Pullulan Oxidation in the Presence of Hydrogen Peroxide and N-Hydroxyphthalimide. Materials. 2022; 15(17):6086. https://doi.org/10.3390/ma15176086
Chicago/Turabian StyleBiliuta, Gabriela, Raluca Ioana Baron, and Sergiu Coseri. 2022. "Pullulan Oxidation in the Presence of Hydrogen Peroxide and N-Hydroxyphthalimide" Materials 15, no. 17: 6086. https://doi.org/10.3390/ma15176086
APA StyleBiliuta, G., Baron, R. I., & Coseri, S. (2022). Pullulan Oxidation in the Presence of Hydrogen Peroxide and N-Hydroxyphthalimide. Materials, 15(17), 6086. https://doi.org/10.3390/ma15176086







