Growth of Nitrogen Incorporated Ultrananocrystalline Diamond Coating on Graphite by Hot Filament Chemical Vapor Deposition
Abstract
:1. Introduction
2. Materials and Experimental Methods
2.1. Seeding of Natural Graphite (NG) Surface with Nanocrystalline Diamond Particles
2.2. HFCVD Controlled Process Parameters for Growing N-UNCD Films
3. Experimental Results
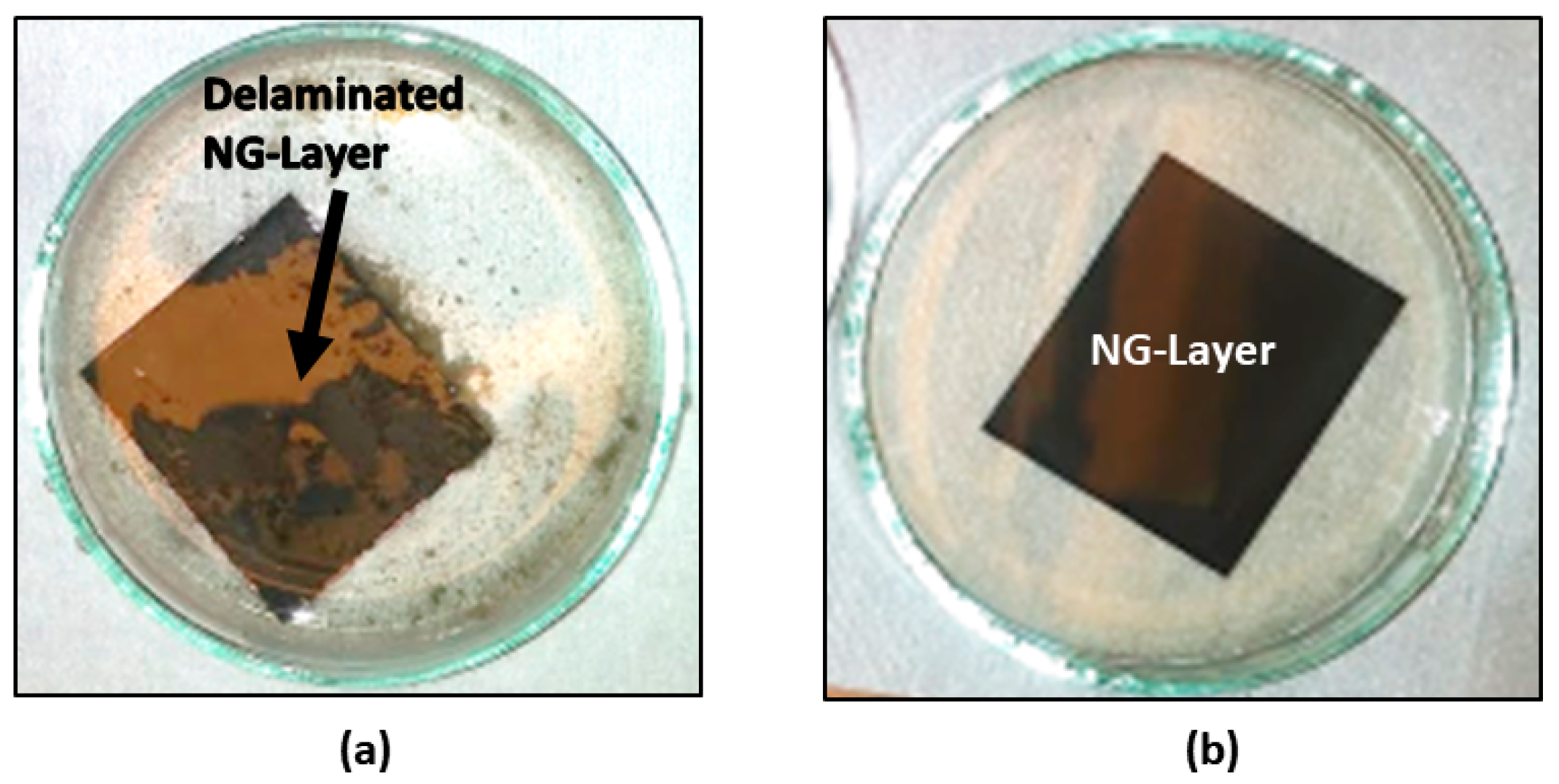
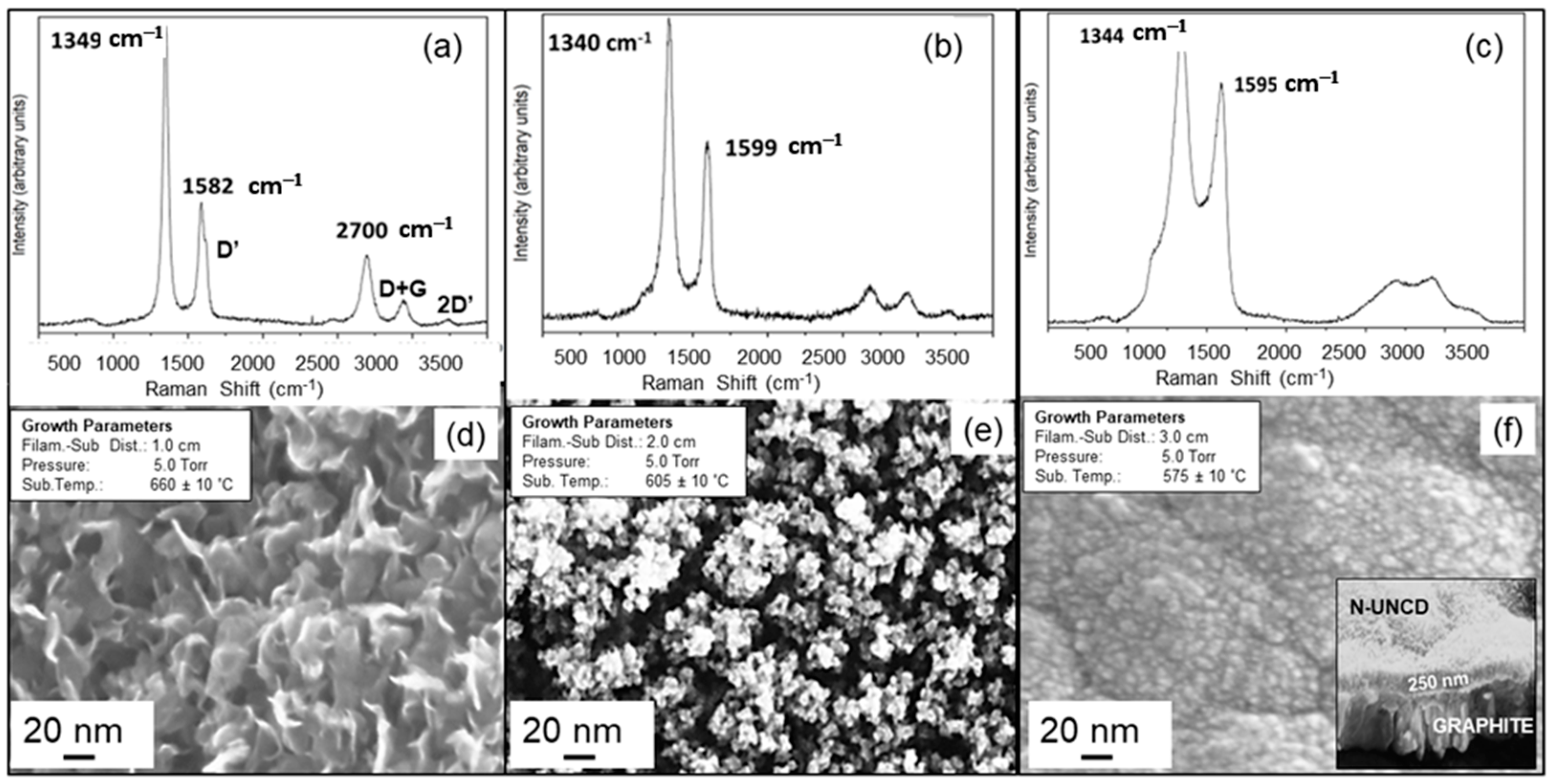
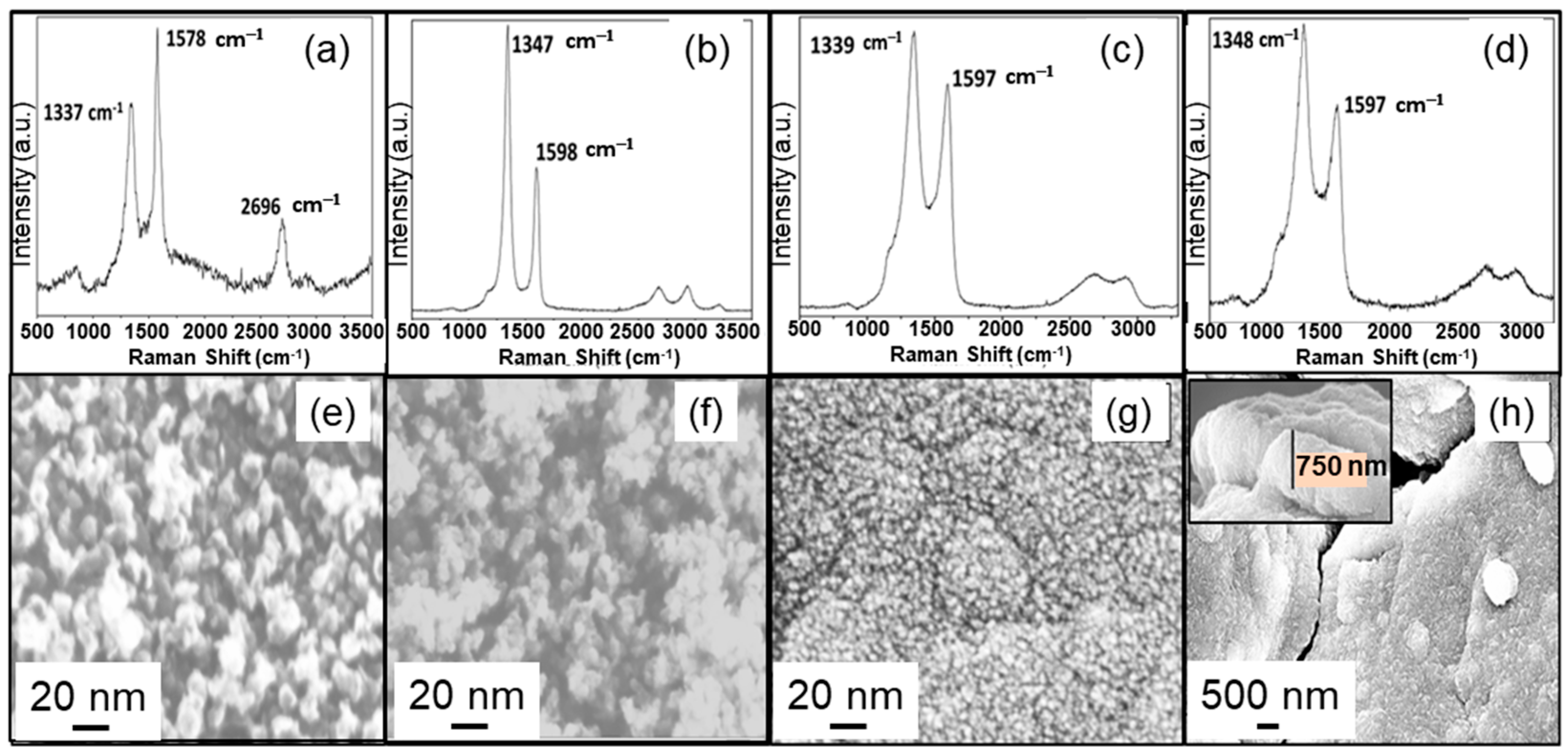
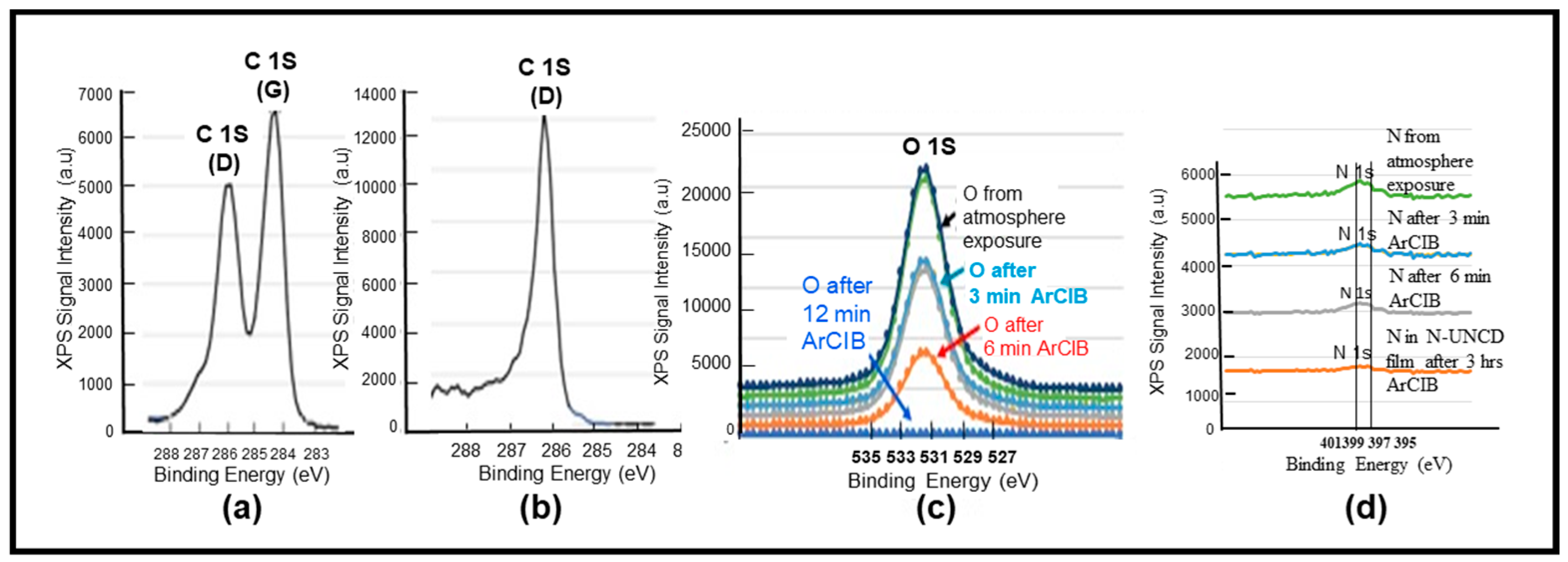
3.1. Characterization of Chemical and Surface Morphological Structures of Virgin NG/Cu Foil Anode
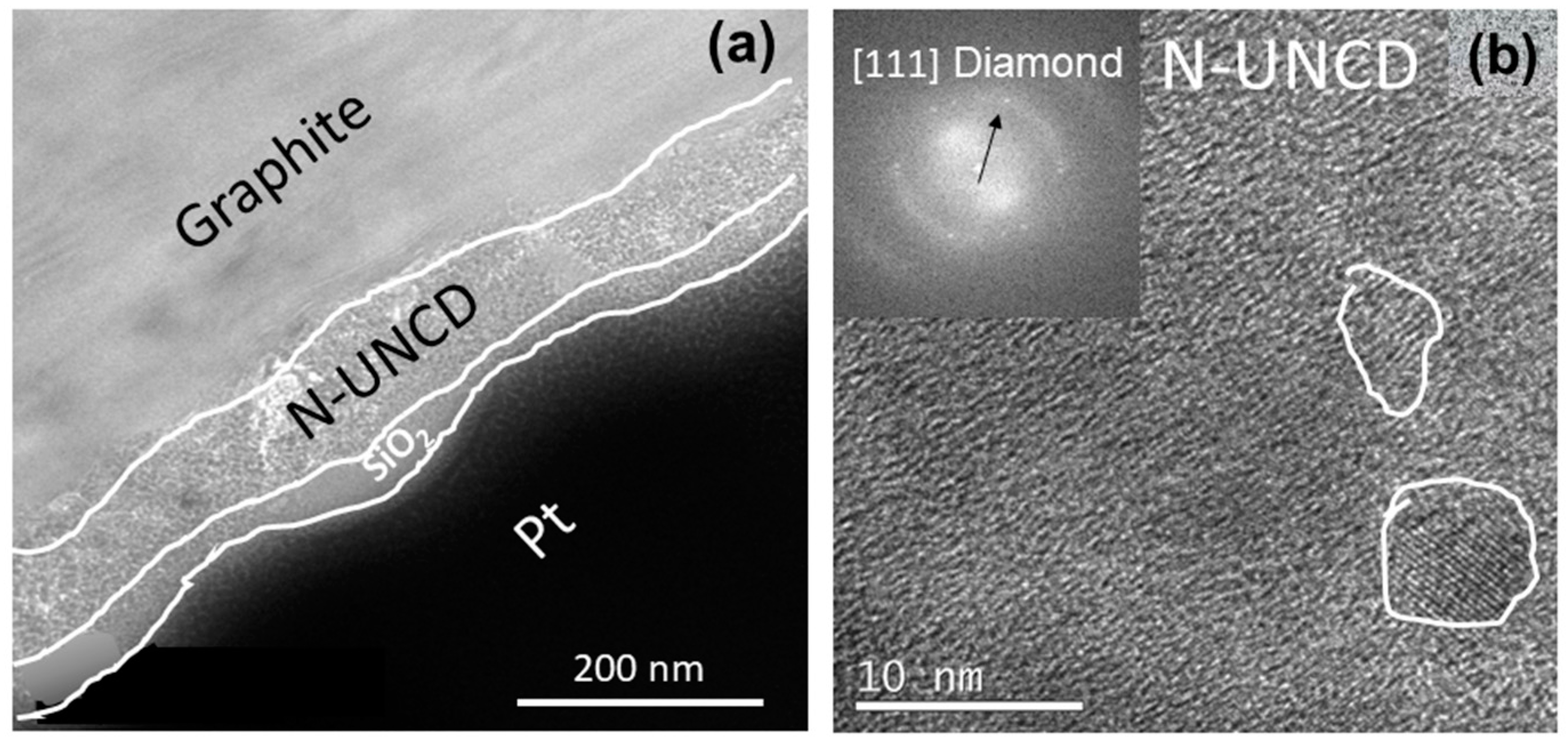
3.2. Characterization of Chemical and Surface Morphological Structures of N-UNCD Film Grown on Seeded NG Layer on Cu Foil Anode
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chan, C.K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2008, 3, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Magasinski, A.; Dixon, P.; Hertzberg, B.; Kvit, A.; Ayala, J.; Yushin, G. Erratum: High-performance lithium-ion anodes using a hierarchical bottom-up approach. Nat. Mater. 2010, 9, 461. [Google Scholar] [CrossRef]
- Dahn, J.R.; Zheng, T.; Liu, Y.; Xue, J.S. Mechanisms for lithium insertion in carbonaceous materials. Science 1995, 270, 590–593. [Google Scholar] [CrossRef]
- Birrell, J.; Carlisle, J.A.; Auciello, O.; Gruen, D.M.; Gibson, J.M. Morphology and electronic structure in nitrogen-doped ultrananocrystalline diamond. Appl. Phys. Lett. 2002, 81, 2235–2237. [Google Scholar] [CrossRef]
- Cheng, Y.-W.; Lin, C.-K.; Chu, Y.-C.; Abouimrane, A.; Chen, Z.; Ren, Y.; Liu, C.-P.; Tzeng, Y.; Auciello, O. Electrically Conductive Ultrananocrystalline Diamond-Coated Natural Graphite-Copper Anode for New Long Life Lithium-Ion Battery. Adv. Mater. 2014, 26, 3724–3729. [Google Scholar] [CrossRef]
- Auciello, O.; Sumant, A.V. Status review of the science and technology of ultrananocrystalline diamond (UNCDTM) films and application to multifunctional devices. Diam. Relat. Mater. 2010, 19, 699–718. [Google Scholar] [CrossRef]
- Kakanakova-Georgieva, A.; Giannazzo, F.; Nicotra, G.; Cora, I.; Gueorguiev, G.K.; Persson, P.O.; Pécz, B. Material proposal for 2D indium oxide. Appl. Surf. Sci. 2021, 548, 149275. [Google Scholar] [CrossRef]
- Villarreal, D.; Wittel, F.P.; Rajan, A.; Wittel, P.; Alcantar-Pena, J.; Auciello, O.; de Obaldia, E. Effect of nitrogen flow on the growth of nitrogen ultrananocrystalline diamond (N-UNCD) films on Si/SiO2/HfO2 substrate. In Proceedings of the 2019 7th International Engineering, Sciences and Technology Conference (IESTEC), Panama City, Panama, 9–11 October 2019; pp. 78–84. [Google Scholar] [CrossRef]
- Peña, J.J.A.; Montes, J.; Arellano-Jimenez, M.; Aguilar, J.O.; Berman-Mendoza, D.; García, R.; Yacaman, M.; Auciello, O. Low temperature hot filament chemical vapor deposition of Ultrananocrystalline Diamond films with tunable sheet resistance for electronic power devices. Diam. Relat. Mater. 2016, 69, 207–213. [Google Scholar] [CrossRef]
- Lin, Q.; Chen, S.; Ji, Z.; Huang, Z.; Zhang, Z.; Shen, B. A novel growth model for depositing ultrananocrystalline diamond films in CH4/H2 chemistry. Surf. Coat. Technol. 2021, 419, 127280. [Google Scholar] [CrossRef]
- Saito, R. Resonance Raman Spectroscopy of Graphene and Carbon Nanotubes. In Handbook of Carbon Nanomaterials; World Scientific: Singapore, 2019; pp. 113–142. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Childres, I.; Jauregui, L.A.; Park, W.; Caoa, H.; Chena, Y.P. Raman spectroscopy of graphene and related materials. New Dev. Phot. Mater. Res. 2013, 1, 403–418. [Google Scholar]
- Fuentes-Fernandez, E.; Alcantar-Peña, J.; Lee, G.; Boulom, A.; Phan, H.; Smith, B.; Nguyen, T.; Sahoo, S.; Ruiz-Zepeda, F.; Arellano-Jimenez, M.; et al. Synthesis and characterization of microcrystalline diamond to ultrananocrystalline diamond films via Hot Filament Chemical Vapor Deposition for scaling to large area applications. Thin Solid Films 2016, 603, 62–68. [Google Scholar] [CrossRef]
- Hiramatsu, M.; Kondo, H.; Hori, M. Graphene Nanowalls. In New Progress on Graphene Research; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Tirado, P.; Alcantar, J.; de Obaldia, E.; Garcia, R.; Auciello, O. Effect of the Gas Chemistry, Total Pressure, and Microwave Power on the Grain Size and Growth Rate of Polycrystalline Diamond Films Grown by Microwave Plasma Chemical Vapor Deposition Technique. In Proceedings of the 2019 7th International Engineering, Sciences and Technology Conference (IESTEC), Panama City, Panama, 9–11 October 2019; pp. 85–91. [Google Scholar] [CrossRef]
- De Obaldía, E.; Alcantar-Peña, J.; Wittel, F.; Veyan, J.; Gallardo-Hernadez, S.; Koudriavtsev, Y.; Berman-Mendoza, D.; Auciello, O. Study of atomic hydrogen concentration in grain boundaries of polycrystalline diamond thin films. Appl. Sci. 2021, 11, 3990. [Google Scholar] [CrossRef]
- Alcantar-Peña, J.J.; de Obaldia, E.; Tirado, P.; Arellano-Jimenez, M.J.; Aguilar, J.E.O.; Veyan, J.F.; Yacaman, M.J.; Koudriavtsev, Y.; Auciello, O. Polycrystalline diamond films with tailored micro/nanostructure/doping for new large area film-based diamond electronics. Diam. Relat. Mater. 2019, 91, 261–271. [Google Scholar] [CrossRef]
- Veyan, J.-F.; de Obaldia, E.; Alcantar-Peña, J.J.; Montes-Gutierrez, J.; Arellano-Jimenez, M.J.; Yacaman, M.J.; Auciello, O. Argon atoms insertion in diamond: New insights in the identification of carbon C 1s peak in X-ray photoelectron spectroscopy analysis. Carbon 2018, 134, 29–36. [Google Scholar] [CrossRef]
- Peña, J.J.A.; de Obaldia, E.; Montes-Gutierrez, J.; Kang, K.; Arellano-Jimenez, M.J.; Aguilar, J.E.O.; Suchy, G.P.; Berman-Mendoza, D.; Garcia, R.; Yacaman, M.J.; et al. Fundamentals towards large area synthesis of multifunctional Ultrananocrystalline diamond films via large area hot filament chemical vapor deposition bias enhanced nucleation/bias enhanced growth for fabrication of broad range of multifunctional devices. Diam. Relat. Mater. 2017, 78, 1–11. [Google Scholar] [CrossRef]
- Mallik, A.K.; Mendes, J.C.; Rotter, S.Z.; Bysakh, S. Detonation Nanodiamond Seeding Technique for Nucleation Enhancement of CVD Diamond—Some Experimental Insights. Adv. Ceram. Sci. Eng. 2014, 3, 36. [Google Scholar] [CrossRef]
- Shen, B.; Lin, Q.; Chen, S.; Ji, Z.; Huang, Z.; Zhang, Z. High-rate synthesis of ultra-nanocrystalline diamond in an argon-free hot filament chemical vapor deposition atmosphere for tribological films. Surf. Coat. Technol. 2019, 378, 124999. [Google Scholar] [CrossRef]
- Rowland, R.S.; Taylor, R. Intermolecular nonbonded contact distances in organic crystal structures: Comparison with distances expected from van der Waals Radii. J. Phys. Chem. 1996, 100, 7384–7391. [Google Scholar] [CrossRef]
- Zefirov, Y.V.; Zorky, P.M. New applications of van der Waals radii in chemistry. Russ. Chem. Rev. 1995, 64, 415–428. [Google Scholar] [CrossRef]
- Barbosa, D.; Hammer, P.; Trava-Airoldi, V.; Corat, E. The valuable role of renucleation rate in ultrananocrystalline diamond growth. Diam. Relat. Mater. 2012, 23, 112–119. [Google Scholar] [CrossRef]

| Sample | Substrate Surface Temperature ± 10 °C | Filament Substrate Distance (cm) | Pressure (Torr) | Growth Time (h) |
|---|---|---|---|---|
| 1 | 660 | 1.0 | 10 | 2.0 |
| 2 | 605 | 2.0 | 5 | 2.0 |
| 3 | 575 | 3.0 | 5 | 2.0 |
| 4 | 625 | 3.0 | 5 | 2.0 |
| 5 | 675 | 3.0 | 5 | 2.0 |
| 6 | 650 | 2.0 | 10 | 2.0 |
| 7 | 650 | 2.0 | 10 | 4.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villarreal, D.; Sharma, J.; Arellano-Jimenez, M.J.; Auciello, O.; de Obaldía, E. Growth of Nitrogen Incorporated Ultrananocrystalline Diamond Coating on Graphite by Hot Filament Chemical Vapor Deposition. Materials 2022, 15, 6003. https://doi.org/10.3390/ma15176003
Villarreal D, Sharma J, Arellano-Jimenez MJ, Auciello O, de Obaldía E. Growth of Nitrogen Incorporated Ultrananocrystalline Diamond Coating on Graphite by Hot Filament Chemical Vapor Deposition. Materials. 2022; 15(17):6003. https://doi.org/10.3390/ma15176003
Chicago/Turabian StyleVillarreal, Daniel, Jyoti Sharma, Maria Josefina Arellano-Jimenez, Orlando Auciello, and Elida de Obaldía. 2022. "Growth of Nitrogen Incorporated Ultrananocrystalline Diamond Coating on Graphite by Hot Filament Chemical Vapor Deposition" Materials 15, no. 17: 6003. https://doi.org/10.3390/ma15176003
APA StyleVillarreal, D., Sharma, J., Arellano-Jimenez, M. J., Auciello, O., & de Obaldía, E. (2022). Growth of Nitrogen Incorporated Ultrananocrystalline Diamond Coating on Graphite by Hot Filament Chemical Vapor Deposition. Materials, 15(17), 6003. https://doi.org/10.3390/ma15176003








